Abstract
Aim:
This paper systematically reviews clinical trials investigating the effectiveness of cognitive behavioural therapy for insomnia and pain in patients with chronic non-malignant pain.
Method:
A systematic search of MEDLINE, PSYCINFO, EMBASE, CINHAL and Cochrane library and register of trials was conducted.
Results:
Essential components of cognitive behavioural therapy for insomnia were included in all studies except for the cognitive restructuring component, which was not considered an intervention in one study. Interventions were provided by adequately trained clinicians. Significant within-group effect sizes (> 1) were observed in the intervention groups as compared with the control groups. Improvements were noted in sleep latency, sleep efficiency and wake after sleep onset times. Although improvements were noted in pain experienced by the participants, this was not a significant finding.
Conclusions:
These clinical trials demonstrate that cognitive behavioural therapy for insomnia is effective as an intervention for insomnia in individuals suffering from chronic non-malignant pain. Although pain and disturbed sleep are linked, cognitive behavioural therapy for insomnia alone may not be an effective solution for addressing chronic non-malignant pain. Trials of cognitive behavioural therapy for insomnia on a variety of chronic pain patients with disturbed sleep and with long-term follow-up are required to ascertain whether cognitive behavioural therapy for insomnia is an effective intervention to reduce pain and to add to increasing evidence that it is an effective intervention for insomnia in the chronic pain population.
Keywords: Chronic non-malignant pain, cognitive behavioural therapy, insomnia, sleep
Introduction
Chronic non-malignant pain is defined as non-malignant pain that exists for 3 or more months or pain that lasts beyond the period of expected healing.1 The majority of those who suffer from chronic pain report poor sleep.2 Insomnia is a problem with sleep initiation and sleep maintenance and is also associated with daytime neurocognitive impairment. It has a higher prevalence in the chronic pain population than in the general population.3,4 The well-established inter-relationship between disturbed sleep and pain severity is also related to mood disturbance, poor sleep hygiene, behavioural priming (previous experience influencing behaviour), cognitive distortions and other cognitive behavioural disturbances.4,5 There is strong evidence for the effectiveness of cognitive behavioural therapy in treating pain and insomnia.6,7 Cognitive behavioural therapy for insomnia (CBT-I) belongs to a family of psychological interventions that focus on treating insomnia and is a multi-component package that includes stimulus control, sleep restriction, sleep hygiene, sleep education, cognitive restructuring and/or relaxation training. CBT-I improves understanding and clarifies misconceptions individuals have about sleep and insomnia. Components of CBT-I aim to improve sleep habits, address sleep–wake schedule and reduce the incidence of behaviours that interfere with sleep continuity and build-up of sleep pressure. In addition, cognitive restructuring and relaxation helps reduce dysfunctional beliefs and anxiety around sleep.
Considering the relationship between sleep and pain, it can be suggested that treating pain can improve sleep and vice versa. If CBT-I is effective in treating primary insomnia and insomnia comorbid with other conditions, is it effective in treating insomnia comorbid with chronic pain? Does it indirectly reduce pain by improving sleep? On the other hand, if pain disrupts sleep and sleep restriction is associated with enhanced pain perception,4,5 does the presence of chronic pain interfere with the response to CBT-I in the chronic pain population? Or does sleep restriction, an essential component of CBT-I, increase pain? These questions becomes more relevant in current practice for treating insomnia after considering the proposed changes in the Diagnostic and Statistical Manual of Mental Disorders – V for the diagnostic criteria of insomnia, in which primary insomnia and insomnia related to another mental/medical disorder will be replaced with insomnia disorder with specification of clinically comorbid conditions, removing the long prevalent differentiation of insomnia based on aetiology.8
Not many researchers have looked at the efficacy of CBT-I in chronic pain patients on symptoms of insomnia and pain. This review systematically searches and appraises trials exploring this aspect.
Methodology
Objectives
The aim of this review is to explore if CBT-I is an effective treatment of insomnia co-morbid with chronic non-malignant pain and whether treating insomnia with CBT-I in this group modifies pain. This paper, therefore, systematically reviews and appraises randomised controlled clinical trials that specifically assess the effectiveness of CBT-I in individuals with chronic non-malignant pain and co-morbid insomnia, comparing the effectiveness of this intervention with other non-pharmacological treatments or usual care. The outcomes of interest are limited to change in symptoms of insomnia as the primary outcome and pain perception as the secondary outcome. Thus, the objectives can be summarised as follows:
Is CBT-I effective in improving symptoms of insomnia in individuals who have insomnia co-morbid with chronic pain?
Is CBT-I effective in improving pain in individuals who have insomnia co-morbid with chronic pain?
Search strategy
Studies were identified by searching electronic databases; scanning references of review articles and eligible studies; and contacting authors of studies that appeared to meet the criteria but where full text was not available, i.e. conference abstracts or thesis abstracts (Figure 1). However, authors were not contacted for studies that were available as full text for raw data due to the limitations of this assignment.
Figure 1.
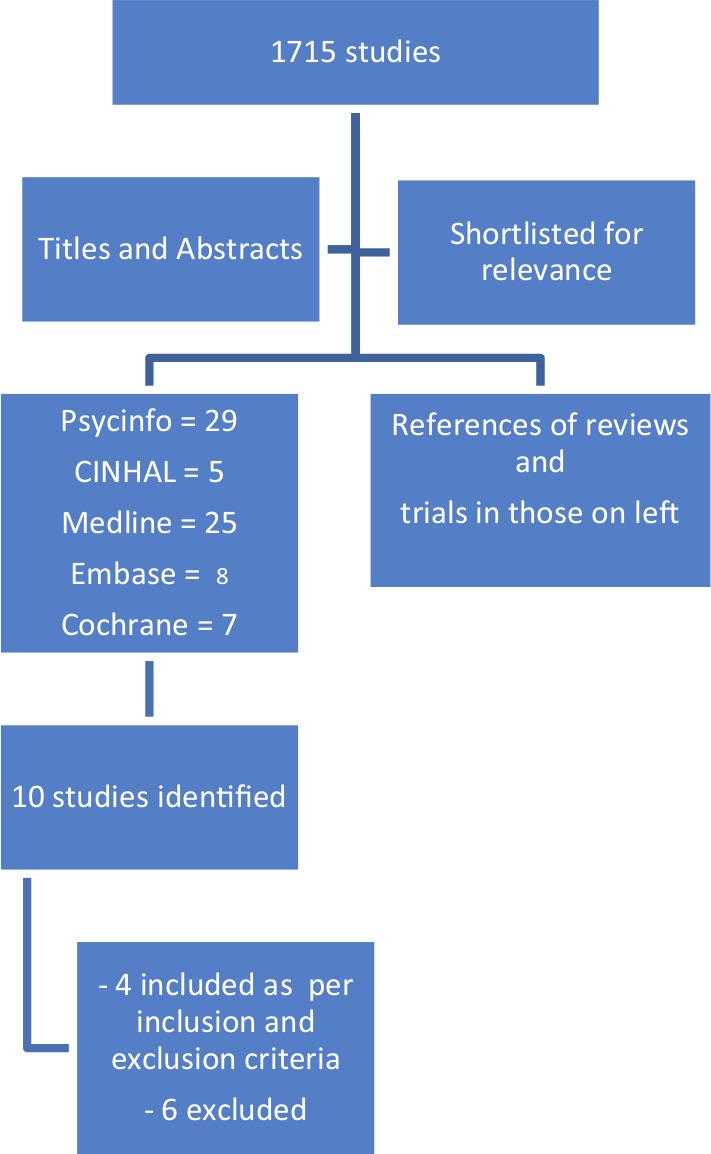
Flowchart of search methodology.
The NHS library search engine was used to search four databases, namely Medline, EMBASE, PSYCINFO and CINHAL. The Cochrane library and Cochrane register of trials was searched to identify trials and systematic reviews on this topic and references of these studies were searched to identify relevant studies. The search strategy is depicted in Table 1. The search was restricted to English-language articles and published studies. Grey literature was not searched. A time limit was placed from 1990 to 2012 on EMBASE but the other databases did not have a time limit. No age limit was specified. The search was conducted between 12 and 16 June 2012.
Table 1.
Search strategy.
| Database | Search criteria | Number of studies | |
|---|---|---|---|
| 1 | CINHALUnrestricted search | 1. exp INSOMNIA/ OR exp SLEEP DEPRIVATION/ | 3385 |
| 2. (INSOMNIA OR DYSSOMNIA OR (SLEEP FRAGMENTATION) OR (poor sleep) OR (sleep quality) OR (broken sleep) OR (disturbed sleep) OR (sleep onset)).ti,ab | 5119 | ||
| 3. 1 and 2 | 7145 | ||
| 4. ((cognitive behavioural therapy) Or (cognitive behavioral therapy) OR (cognitive therapy) OR behavior* OR (behaviour*) OR CBT OR (psychological therapy) OR psychotherapy OR (sleep restriction) OR (bed restriction) OR (sleep hygiene) Or (stimulus control)).ti,ab | 93,951 | ||
| 5. Pain.ti,ab | 79,644 | ||
| 6. 3 AND 4 AND 5 | 148 | ||
| 2 | PSYCINFOUnrestricted search | 1. exp BACK PAIN/ OR exp CHRONIC PAIN/ OR exp “COMPLEX REGIONAL PAIN SYNDROME (TYPE I)”/ OR exp MYOFASCIAL PAIN/ OR exp NEUROPATHIC PAIN/ OR exp PAIN/ OR exp PAIN MANAGEMENT/ OR exp PAIN MEASUREMENT/ OR exp PAIN PERCEPTION/ OR exp PAIN THRESHOLDS/ OR exp SOMATOFORM PAIN DISORDER/ | 46,788 |
| 2. exp INSOMNIA/ | 3391 | ||
| 3. (insomnia OR dyssomnia OR (sleep fragmentation) OR sleep OR (fragmented sleep) OR (sleep disturbance) OR (sleep quality) OR (non-restorative sleep) OR (unrefreshing sleep) OR (broken sleep)).ti,ab | 41,609 | ||
| 4. 2 OR 3 | 41,692 | ||
| 5. exp COGNITIVE BEHAVIOR THERAPY/ OR exp COGNITIVE THERAPY/ OR exp GROUP PSYCHOTHERAPY/ OR exp BEHAVIOR THERAPY/ | 50,218 | ||
| 6. ((cognitive behavioural therapy) Or (cognitive behavioral therapy) OR (cognitive therapy) OR behavior* OR (behaviour*) OR CBT OR (psychological therapy) OR psychotherapy OR (sleep restriction) OR (bed restriction) OR (sleep hygiene) Or (stimulus control)).ti,ab | 678,835 | ||
| 7. 5 OR 6 | 692,321 | ||
| 8. 1 AND 4 AND 7 | 272 | ||
| 3 | MEDLINE | 1. exp ABDOMINAL PAIN/ OR exp PAIN MANAGEMENT/ OR exp PAIN MEASUREMENT/ OR exp ACUTE PAIN/ OR exp BACK PAIN/ OR exp BREAKTHROUGH PAIN/ OR exp CHEST PAIN/ OR exp CHRONIC PAIN/ OR exp COMPLEX REGIONAL PAIN SYNDROMES/ OR exp LOW BACK PAIN/ OR exp MUSCULOSKELETAL PAIN/ OR exp NECK PAIN/ OR exp NOCICEPTIVE PAIN/ OR exp PAIN/ OR exp PAIN CLINICS/ OR exp PAIN PERCEPTION/ OR exp PAIN THRESHOLD/ OR exp PELVIC PAIN/ OR exp PLEASURE-PAIN PRINCIPLE/ OR exp SHOULDER PAIN/ | 303,067 |
| 2. [exp COGNITIVE THERAPY/ OR exp BEHAVIOR THERAPY/ OR exp SLEEP INITIATION AND MAINTENANCE DISORDERS/] OR [((cognitive behavioural therapy) Or (cognitive behavioral therapy) OR (cognitive therapy) OR behavior* OR (behaviour*) OR CBT OR (psychological therapy) OR psychotherapy OR (sleep restriction) OR (bed restriction) OR (sleep hygiene) Or (stimulus control)).] | 740,306 | ||
| 3. exp SLEEP INITIATION AND MAINTENANCE DISORDERS/ | 7896 | ||
| 4. ((insomnia) OR (dyssomnia) OR (sleep fragmentation) OR sleep OR (fragmented sleep) OR (sleep disturbance) OR (sleep quality) OR (non-restorative sleep) OR (unrefreshing sleep) OR (broken sleep)).ti,ab | 94,012 | ||
| 5. 3 OR 4 | 95,715 | ||
| 6. 1 AND 2 AND 5 [Publication Year 1990-Current] | 839 | ||
| 4 | EMBASE | 1. exp ABDOMINAL PAIN/ OR exp CHRONIC PAIN/ OR exp COMPLEX REGIONAL PAIN SYNDROME/ OR exp DISCOGENIC PAIN/ OR exp GASTROINTESTINAL PAIN/ OR exp HEADACHE AND FACIAL PAIN/ OR exp HIP PAIN/ OR exp INTRACTABLE PAIN/ OR exp LEG PAIN/ OR exp LIMB PAIN/ OR exp LOW BACK PAIN/ OR exp MUSCULOSKELETAL CHEST PAIN/ OR exp MUSCULOSKELETAL PAIN/ OR exp MYOFASCIAL PAIN/ OR exp NECK PAIN/ OR exp NEUROPATHIC PAIN/ OR exp NOCICEPTIVE PAIN/ OR exp NONCARDIAC CHEST PAIN/ OR exp PAIN/ OR exp PAIN ASSESSMENT/ OR exp PAIN CLINIC/ OR exp PAIN PARAMETERS/ OR exp PAIN RECEPTOR/ OR exp PAIN THRESHOLD/ OR exp PELVIS PAIN SYNDROME/ OR exp POSTTRAUMATIC PAIN/ OR exp PSYCHOGENIC PAIN/ OR exp RADICULAR PAIN/ OR exp REFERRED PAIN/ OR exp RETROSTERNAL PAIN/ OR exp SCROTAL PAIN/ OR exp UPPER ABDOMINAL PAIN/ OR exp VISCERAL PAIN/ | 687,726 |
| 2. [exp INSOMNIA/] OR [((dyssomnia) OR (sleep fragmentation) OR sleep OR (fragmented sleep) OR (sleep disturbance) OR (sleep quality) OR (non-restorative sleep) OR (unrefreshing sleep) OR (broken sleep) OR (sleep initiation AND maintenance disorder*)).ti,ab] | 139,158 | ||
| 3. {((cognitive behavioural therapy) Or (cognitive behavioral therapy) OR (cognitive therapy) OR behavior* OR (behaviour*) OR CBT OR (psychological therapy) OR psychotherapy OR (sleep restriction) OR (bed restriction) OR (sleep hygiene) Or (stimulus control)).ti,ab [Limit to: Publication Year 1990-Current]} OR {exp COGNITIVE THERAPY/ OR exp BEHAVIOR THERAPY/} | 690,319 | ||
| 4. 1 AND 2 AND 3 [Limit to: Human and English Language and Exclude MEDLINE Journals and (Records From Embase) and (Clinical Trials Clinical Trial or Randomized Controlled Trial or Controlled Clinical Trial or Multicenter Study) and Publication Year 1990–Current] | 209 | ||
| 5 | Cochrane library | ((cognitive behavior*) OR (cognitive behaviour*)) AND (sleep) – title, abstract and keyword search, restricted to clinical trials | 247 |
Inclusion criteria
The inclusion criteria for the study were as follows:
Sleep and pain assessments should be primary or secondary outcomes and not incidental findings.
Studies should use validated patient-reported outcome tools to evaluate pain and sleep pre and post treatment.
Interventional studies with a control group.
The control group is a non-pharmacological intervention for sleep.
The participants should not have had a recent (within the past 3 months) change in the treatment of pain or sleep prior to participating in the study.
Exclusion criteria
The exclusion criteria were as follows:
Studies in which the participants have the presence of another sleep disorder other than insomnia in the study group, which is not effectively treated.
Studies in which malignant (cancer-related) pain participants are included.
Studies for which the control arm has a pharmacological intervention.
Studies for which measuring pain and insomnia outcomes are not part of the objectives and have been included in the analysis as a post-hoc finding.
Results
Results of the search
A total of 1715 studies were identified from the database search. The titles of these articles were read to identify those that looked at insomnia, cognitive behaviour therapy and pain, and this resulted in selecting 29 articles from PSYCINFO, five from CINHAL, 25 from MEDLINE, eight from EMBASE and seven from Cochrane respectively. Further, titles and abstracts of these studies were read. PSYCINFO, CINHAL, EMBASE, MEDLINE and Cochrane produced one, three, three, three and five studies, respectively. These included duplicates. Ten studies were shortlisted from the above search.
When read in full, six out of the ten articles did not meet all the inclusion and exclusion criteria or could not be included for other reasons (see Appendix 1). Four studies met all the inclusion and exclusion criteria.9–12 Findings of the four studies that met all the criteria are reported in Tables 2 and 3. Of the four studies selected, three studied chronic pain, either spinal/limb9,11 or arthritic in origin,12 whereas one studied fibromyalgia patients.10 One study extracted its data from a parent study done 4 years earlier and hence the latter study was used to supplement information for the objectives of the review.12,13
Table 2.
Characteristics of studies.
| Study | Sample characteristics | Interventions and controls | Treatment/control components |
|---|---|---|---|
| Currie et al. (2000)9 | 60 participants: 27 female and 33 male. Recruitment was from three local pain clinics. Average age 45.0 years. Average duration of pain 9.2 years and average duration of insomnia was 7.9 years. The nature of chronic pain was neck pain (20%), low back pain (72%), pelvic pain (2%) and lower limb pain (5%) | Two groups:– CBT-I– WLC |
CBT-I: Seven 2-hour weekly group sessions composed of sleep education, stimulus control, sleep restriction, relaxation training, sleep hygiene and cognitive therapy (using Morin’s (1993) approach) WLC: 10-min weekly phone calls along with completion of sleep diary |
| Edinger et al. (2005)10 | 47 participants, 45 of whom were female. Recruitment was via newspaper advertisements. Average age was 48.6 years. All patients had fibromyalgia as the chronic pain condition. 34% and 72% also had osteoarthritis and headaches, respectively. Average duration of fibromyalgia not given. Average insomnia duration was 9.9 years | Three groups:– CBT-I– Sleep hygiene– Usual care |
CBT-I: One session of 45–60 min plus five 15- to 30-min weekly individual sessions composed of sleep education, stimulus control and sleep restriction. Sleep hygiene: Sleep hygiene advice and adherence review weekly over six weekly sessions. Usual care: Weekly sessions for questionnaire completion and usual fibromyalgia care, no behavioural therapy |
| Jungquist et al. (2010)11 | 28 participants, 22 (78%) of whom were female. Subjects were recruited from a mean age of 48.7 years. All patients had chronic spinal pain [neck (32%), back (64%), thoracic spine (4%)]. Minimum 6 months chronic pain with 75% having pain for > 5 years | Two groups:– CBT-I– Control condition |
CBT-I: Eight weekly individual sessions that ranged between 30 and 90 minutes as per published manual – education, sleep restriction, stimulus control, sleep hygiene, cognitive therapy, relapse prevention. Control condition: Eight weekly sessions of 45–90 min, direct therapist contact, no interventions with interrogative review only |
| Vitiello et al. (2009)12 | 51 participants (45 female, 6 male), all over 65 with mean age 69.2 for intervention group and 66.5 for control group. All patients had osteoarthritis, the average duration of which was not known | Two groups: – CBT-I– SMW (attention control) |
CBT-I: Eight weekly group sessions of 2 hours each with an average group size of five participants (using Morin’s (1993) approach) composed of SC and SR emphasised in first three sessions, sleep education, sleep hygiene, cognitive restructuring and relaxation training. SMW: attentional control; included problem solving, goal setting, cognitive approach for stress and anxiety, interpersonal skills training and education about exercise enhancement |
CBT-I: cognitive behavioural therapy for insomnia; SC: stimulus control, SMW: stress management and wellness, attention control arm, SR: sleep restriction WLC, waiting list control.
Table 3.
Results of studies.
| Study | Screening tests | Measurement instruments | Results (pre and post treatment) and long-term follow-up |
|---|---|---|---|
| Currie et al. (2000)9 | 1. Initial telephone screening2. Face-to-face diagnostic interview for insomnia/rule out other sleep disorders and psychiatric disorders3. Structured interview for sleep disorders for DSM-III R (SIS-D).4. No polysomnography | Sleep: sleep diary, actigraphy, PSQI (for sleep quality)Pain: MPI-PSEmotional distress: BDIMedication use: MQI |
Primary outcomes
SE, SL and WASO showed an improvement post treatment and at follow-up (3 months) in the CBT-I group that was greater and significant than in the WLC group.The major improvement indicators for CBT-I were (as measured on sleep diary):SE: 72% to 85% to 84%SL: 54.7 min to 28.1 min to 27.8 minWASO: 88.9 min to 40.2 min to 40.6 minPSQI: 13.6 to 8.8 to 7.9Actigraphy activity levels were significantly reduced in the CBT group post treatment but not at follow-up. There was a significant reduction in activity levels in the CBT group compared with the WLC group at post treatment but not at follow-up.A significantly higher number of CBT group participants were classed as clinically improved in each of the three definitions compared with the WLC group but only five in the CBT group and none in the WLC group met all three criteria for significant improvement. Secondary outcomes There was no significant difference in pain scores on the MPI-PS scale post treatment or at follow-up in the CBT group as compared with the WLC group. There was a significant time effect across post treatment and at follow-up for both groups but no between-group difference and no increase in pain.BDI score changes were insignificant. |
| Edinger et al. (2005)10 | 1. Structured sleep and psychiatric interviews2. Tender point examination for FM3. PSG (baseline)4. Sleep log | Sleep logsActigraphyInsomnia severity scale (mean score)Medical Outcomes Survey 36-item Short Form Health Survey (mental composite score)MPQ (total score)BPI (total score)Profile of Mood States (total score)Therapy Evaluation Questionnaire | Primary outcomes SE, SL and TWT showed an improvement post treatment and at follow-up (6 months) in the CBT-I group that was greater and significant as compared with the UC group. On actigraphy, this significance was noticed only in sleep latency. However, SH therapy did not differ in any measure from either CBT-I group or UC group independently.The major improvement indicators for CBT-I (as measured on the sleep diary) were as follows:SE: 80.6% to 88% to 89%SL: 33 min to 17 min to 15.8 minTWT: 103.1 min to 59.8 min to 53.6 minA significantly higher number of CBT patients (57%) were classed as clinically improved as compared with SH (p = 0.05) and UC (p = 0.007) based on three definitions of clinical improvement. This improvement was noticed both in the sleep diary and on actigraphy findings.Secondary outcomesThough a significant group × time effect was observed for secondary outcomes including pain scores, no significant change was observed in the pain ratings on MPQ and BPI scores in the CBT group in comparison with the UC or SH group. However, a significant reduction in pain was noted for the SH group in comparison with the UC group on paired comparison |
| Jungquist et al. (2010)11 | 1. Physical examination including physical examination and urine analysis2. MINI Neuropsychiatric interview/urine drug screen3. PSG (baseline)4. Pain assessment using Mc Gill Pain Index | Sleep logs recording TST, WASO, SL, SE, NWAK, EMA.0–10 scales for fatigue/mood/pain/sleep quality/stress/alertness/concentrationISIPDIMPI – pain interference and pain severity subscale scores were usedBDIESSMFI | Primary outcomes Significant improvement was observed in SE, SL, WASO and NWAK in the CBT-I group as compared with the control group from pre treatment to post treatment. There was a trend of improvement in TST and early morning awakening.The major improvement indicators for CBT-I (as measured on the sleep diary) were as follows:SE: 75% to 94%SL: 37 min to 9 minWASO: 58 min to 12 minA positive treatment response (considered equivalent to clinically significant improvement of other studies) was seen in 78% of CBT-I participants as compared with 22% of controls, and, of the CBT-I participants, 42% achieved normal sleep efficiency (> 90%) post treatment.Secondary outcomesAmongst secondary outcomes, a significant improvement (p = 0.0318) in the pain interference subscale of the MPI was seen in the CBT-I group. No other parameters including pain severity, MPI, PDI or BDI showed any significant differences between the CBT-I group and control group |
| Vitiello et al. (2009)12 | 1. Initial telephone screening2. Home PSG (baseline screening)3. MMSE (> 24 were included)4. Brief symptom inventory to exclude individuals who exhibited behaviour that would interfere with study participation | Sleep log to measure SL, SE, TST and WASOSF-36 (bodily pain subscale).Short-form MPQGeriatric depression scale(These have been selected from several other standardised questionnaires used in the original research study, which also included PSQI) | Primary outcomes SE, SL and WASO showed an improvement in the CBT-I group post treatment that was greater than and significant as compared with the SMW (control) group. 1-year follow-up for CBT-I participants (including cross-over) showed significant improvement in SL, SE, WASO and TST. This was not compared with those who did not receive CBT-I.Major improvement indicators for CBT-I (as measured on the sleep diary) include:SE: 71% to 84%SL: 40.4 min to 23.5 minWASO: 62 min to 25 minPre-treatment to 1-year follow-up for CBT-I group including cross-over include:TST: 363 min to 390 minSE: 74.7% to 82.7%SL: 34.7 min to 23.7 minWASO: 49.1 min to 29.2 minClinical improvement was not measured.Secondary outcomesA significant improvement in pain was observed for CBT-I group as compared with the SMW group from pre to post treatment on the SF-36 pain subscale score, and a non-significant improvement trend was observed for the pain scores for this group on the MPQ. For 1-year follow-up for the entire CBT-I group, including cross-over, there was a significant improvement in pain scores on MPQ but not on the SF-36 pain subscale |
BDI, Beck’s Depression Inventory; BPI: Brief Pain Inventory; CBT-I: cognitive behavioural therapy for insomnia; DSM-III R (SIS D):; EMA: ; ESS: Epworth Sleepiness Scale; FM: ; ISI: Insomnia Severity Index; MFI: Multidimensional Fatigue Index; MMSE: ;MPI-PS: Multi-dimensional Pain Inventory Pain Severity Scale; MPQ: McGill Pain Questionnaire; MQI: Medication Quantification Index; NWAK: ; PDI: Pain Disability Index; PSG: ; PSQI:, SE: sleep efficiency; SH: sleep hygiene; SL: sleep latency; TST: total sleep time; TWT: total wake time; WASO: wake after sleep onset; WLC: waiting list control; UC: usual care;
Evaluation of selected papers
Quality assessment of the studies was done using the CONSORT checklist for reporting clinical trials, Cochrane risk of bias tool and the quality assessment scale for psychological interventions.14–16 Overall, all four clinical trials were of good quality. All studies had a small unexplained sample size. Jungquist et al.11 and Vitiello et al.12 described the randomisation and blinding process in detail.11,12 Apart from Vitiello et al., other studies did not clearly describe the study settings. All studies used the same therapists (who varied in skill level across the studies) for the experimental and control interventions/contact. Three studies used multi-faceted cognitive behavioural therapy,9,11,12 whereas Edinger et al.10 focused on the behavioural component (stimulus control and sleep restriction) of CBT-I. Jungquist et al.11 and Edinger et al.10 provided individual therapy whereas Currie et al.9 and Vitiello et al.12 provided group therapy in the intervention. Outcome measures are detailed later. There were no changes in outcomes along the duration of each study. Similarities between groups were controlled for,12 acknowledged10,11 or avoided.9 A clear description of the statistical methods used and their appropriateness for the purpose of the study was given in each study. The number of participants added up in all studies and losses were accounted for. There was a minor discrepancy in the parent study of Vitiello et al.12 where one participant was misrepresented in analysis, but this is unlikely to have affected the results.13 Although intention to treat was followed in principle by most, Edinger et al.10 did not include the baseline measures of those who did not complete treatment. Results were reported as means of improvement with standard deviations and also as effect sizes by Jungquist et al.11 and Vitiello et al.12 Although p-values were reported, none of the studies reported the changes in outcomes/effect sizes with its confidence intervals.
Assessment of patients
The total combined sample size for these studies was 186 participants. All participants were adults with the Vitiello et al.12 study restricted to those over 65 years. The International Classification of Sleep Disorders, Diagnostic and Statistical Manual of Mental Disorders – III and Diagnostic and Statistical Manual of Mental Disorders – IV TR were the main criteria for diagnosing insomnia in the studies selected. Two studies used structured interviews for insomnia.9,10 Pain was assessed using structured pain questionnaires11 and/or confirmation by specialists.9,10,12 All but Currie et al. used baseline polysomnography (PSG) to rule out other sleep disorders and Currie et al. relied on structured interviews to rule out other sleep disorders. All studies excluded significant psychiatric disorders and, apart from the Edinger et al. study, all excluded fibromyalgia patients. Vitiello et al. selected osteoarthritis as the chronic pain condition, Currie et al selected spinal/limb/pelvic pain, Edinger et al. selected fibromyalgia and Jungquist et al. selected spinal pain. All studies accounted for analgesics and sleep medications in tabular form and controlled for changes in medications during participation in the trial. Edinger et al. also analysed medication use by both groups and reported no significant differences. The duration of CBT-I was 8 weeks of 2-hourly weekly sessions in the Vitiello et al. study. This treatment was 8 weeks of weekly sessions varying from 30 minutes to 90 minutes in the Jungquist et al. study, 7 weeks of 2-hour weekly sessions in the Currie et al. study, and six weekly sessions with the first up to 60 minutes and remaining 15–30 minutes in duration in the Edinger et al. study.
Measurement of outcomes
Sleep latency, sleep efficiency, wake after sleep onset (WASO), total sleep time and total wake time were the major insomnia variables measured as outcomes in the four studies. These were primarily measured using sleep logs in all four studies. Actigraphy was also used by Edinger et al. and Currie et al. Currie et al. used Pittsburg Sleep Quality Index (PSQI) for sleep quality.17 Though the parent study of Vitiello et al. used PSQI for sleep quality, this was not reported for the osteoarthritis group used by Vitiello et al.13 Insomnia symptoms were measured using the insomnia symptom questionnaire5 and insomnia severity index.11 Pain was measured on various valid questionnaires, including the Multidimensional Pain Inventory (MPI),9,11 McGill Pain Questionnaire (MPQ),10 Short-form MPQ,12 Pain Disability Index (PDI),11 Brief Pain Inventory (BPI),10 SF-36 bodily pain subscale12 and a 0–10 Likert scale for pain.11 Other variables measured emotional distress, fatigue and other daytime consequences, but are beyond the scope of this review’s objectives.
Change in sleep and pain parameters
The effectiveness of CBT-I was examined in comparison with control groups that included waiting list controls (WLCs), usual treatment, sleep hygiene and attentional control, with Edinger et al. using three groups: experimental (CBT-I), sleep hygiene and usual care for fibromyalgia patients.
Currie et al. demonstrated significant improvements in sleep latency, sleep efficiency and WASO across time and in comparison with WLC in the CBT-I group.9 Sleep quality improved significantly in both groups post treatment, but at follow-up only the CBT-I group showed statistically significant improvement. Between-group analyses revealed that sleep quality in the CBT-I group was significantly better than in the WLC group across time.9 Edinger et al. demonstrated significant improvement in sleep efficiency, sleep latency and total wake time (from sleep logs) across time and in comparison with usual care in the CBT-I group.10 However, the sleep hygiene group did not differ from the other groups. For actigraphy, this significant improvement was noticed only for sleep latency. Edinger et al. also measured night-to-night variability across sleep variables and reported less variability in CBT-I group.10 This was not an outcome of interest for this review. Jungquist et al. reported a significant improvement in sleep efficiency, sleep latency, WASO and number of awakenings in CBT-I group as compared with the control group, as well as in sleep continuity in the insomnia severity index scores in the CBT-I group.11 Vitiello et al. demonstrated a significant improvement in sleep latency, WASO and sleep efficiency post treatment and at 1-year follow-up in the CBT-I group, and also an improvement in total sleep time at 1-year follow-up in the CBT-I group.12 This significance at follow-up also persisted when the CBT-I group included those who crossed over from control to CBT-I at the end-of-treatment phase of the study.
Two studies measured clinical significance.9,10 Currie et al.9 defined clinical improvement (good sleepers) as sleep latency or WASO < 30 min, sleep efficiency ≥ 85% and PSQI < 6. A significantly higher number (p < 0.05) of CBT-I participants met either criteria as compared with controls, but only 16% of CBT-I participants met all three criteria. None of the WLC participants met all three criteria. The above improvements persisted at follow-up. Edinger et al.10 adopted a slightly different set of criteria for good sleepers, i.e. total sleep time ≥ 6.5 h, total wake time < 60 min (45 min for actigraphy) and sleep efficiency ≥ 85%. Here, 57% of CBT-I participants as compared with 17% sleep hygiene and 0% usual treatment participants met either criteria for good sleepers post treatment. The findings were similar for actigraphy (43% CBT-I, 7% sleep hygiene, 0% usual treatment participants).
Jungquist et al.11 demonstrated a significant improvement in pain interference subscale of the MPI for the experimental group. Vitiello et al. demonstrated a significant improvement in the SF-36 pain subscale across post treatment for the experimental group (p = 0.01). Vitiello et al. also reported a significant improvement in the MPQ score across follow-up for the CBT-I group, including crossovers.12 They also reported a non-significant improvement trend for pain on the MPQ at post treatment for the CBT-I group (p = 0.029).12 Edinger et al. reported a significant improvement in pain (MPQ and BPI scores) in the sleep hygiene group as compared with the usual control group (p = 0.02 and p = 0.04). This difference did not exist for the CBT-I group.9 Though Currie et al. showed that pain ratings across the whole sample were significantly lower post treatment and at follow-up (p = 0.001), there were no significant differences between the CBT-I and control group.8
Within-group and between-group effect sizes of each study for sleep efficiency, sleep latency and WASO are given in Table 4. These consistently improved across all four studies in the CBT-I group in comparison with the control group. A weighted mean calculation was done to measure the overall effect size for each variable. However, these effect sizes should be looked at with caution because of the heterogeneity of these studies. The effect sizes for pain measures in Currie et al., Edinger et al. and Vitiello et al. were insignificant (i.e. small, < 0.4), although mean scores for all showed a trend towards improvement in pain for the CBT-I group as compared with the control.9,10,12 Over a 1-year follow-up in the CBT-I group, including cross-overs, Vitiello et al. reported a significant improvement in MPQ scores, although the effect size remained small.12 Jungquist et al. observed a between-group medium effect size for the MPQ pain-interference scale and PDI (effect size > 0.6), whereas the score on the MPI pain intensity subscale and on the average daily pain scale was < 0.6.11
Table 4.
Effect sizes for individual studies.
| Variable | Group | Cohen’s d effect size | |
|---|---|---|---|
| Within group [effect size] (95% CI) | Between group [effect size] (95% CI) | ||
| Sleep latency | |||
| Currie et al. (2000)9 | CBT-I | [−0.95] (−1.47 to −0.44)* | [1.04] (1.8 to 0.2)* |
| WLC | [0.28] (−0.24 to 0.8) | ||
| Edinger et al. (2005)10 | CBT-I | [−0.79] (−1.4 to −0.1)* | [0.7] (−0.43 to 1.92) |
| UC | [−0.2] (−1 to 0.6) | ||
| Jungquist et al. (2010)11 | CBT-I | [−2] (−2.8 to −1.2)* | [1.5] (0.19 to 2.8)* |
| Control | [0] (−0.9 to +0.9) | ||
| Vitiello et al. (2009)12 | CBT-I | [−0.7] (−1.3 to −0.17)* | [0.51] (−1.3 to 0.3) |
| SMW | [−0.1] (−0.6 to 0.4) | ||
| Sleep efficiency | |||
| Currie et al. (2000)9 | CBT-I | [0.9] (0.4 to 1.5)* | [0.9] (0.2 to 1.5)* |
| WLC | [0] (0.5 to −0.5) (0.07) | ||
| Edinger et al. (2005)10 | CBT-I | [0.9] (0.2 to 1.5)* | [0.67] (−0.2 to 1.6) |
| UC | [0.28] (−0.5 to 1.1) | ||
| Jungquist et al. (2010)11 | CBT-I | [2] (1.2 to 2.8)* | [2] (0.9 to 3.1)* |
| Control | [0.1] (−0.7 to 1.1) | ||
| Vitiello et al. (2009)12 | CBT-I | [1.24] (0.6 to 1.8)* | [0.6] (0.002 to 1.2)* |
| SMW | [0.35] (−0.17 to 0.8) | ||
| Wake after sleep onset | |||
| Currie et al. (2000)9 | CBT-I | [−0.8] (−1.3 to −0.3)* | [0.65] (−0.13 to 1.4) |
| WLC | [−0.13] (−0.6 to 1.3) | ||
| Edinger et al. (2005)10 | CBT-I | [−1.3] (−2 to −0.6)* | [0.66] (−0.5 to 1.9) |
| UC | [−0.40] (−1.2 to 0.43) | ||
| Jungquist et al. (2010)11 | CBT-I | [−1.4] (−2.1 to −0.7)* | [1.23] (−0.69 to 3.17) |
| Control | [−0.1] (−1 to 0.8) | ||
| Vitiello et al. (2009)12 | CBT-I | [−1] (−1.6 to −0.4)* | [0.62] (−0.2 to 1.5) |
| SMW | [−0.2] (−0.8 to 0.2) | ||
Indicates significant results where the 95% CI does not cross 0. Cohen’s d of > 0.2, > 0.5 and > 0.8 are considered small, medium and large effect sizes, respectively. A negative value indicates a reduction (or improvement) in sleep latency or wake after sleep onset. Between-group effect sizes are presented as positive for improvement.
CBT-I: cognitive behavioural therapy for insomnia; SMW: stress management and wellness; UC: usual care; WLC: waiting list control
Discussion
The main limitations of this review are that the sample sizes used in the studies are small and the pain populations quite variable. The results indicate that CBT-I is effective in improving insomnia symptoms in individuals with co-morbid chronic pain. Table 5 reports weighted means of the effect sizes of each variable for all the studies combined. As the four studies are heterogeneous, it is not ideal that the effect sizes of the four studies be combined using weighted means (i.e. fixed-effects model). However, these results are still meaningful and provide useful information about the effectiveness of CBT-I in improving insomnia in a broad range of chronic pain patients. The combined within-group effect sizes of CBT-I for improvement in sleep latency, sleep efficiency and WASO is high, all > 1. This is in contrast to the weighted mean of the effect sizes for the control arm, i.e. all < 0.2. The between-group effect sizes of the three variables compared the post-treatment effect size for the intervention and control group. The weighted mean for this shows that the largest effect size was for sleep latency followed by sleep efficiency and WASO, with the latter two variables in the medium range. A quality appraisal of the four studies suggests that the Jungquist et al. study, which shows the largest effect sizes for all sleep variables, also has the highest score on the quality assessment tool for psychological interventions and Cochrane risk of bias tool.11,14,15
Table 5.
Weighted mean effect sizes for all studies.
| Weighted mean ES | SL | SE | WASO |
|---|---|---|---|
| CBT-I | 1.68 | 1.16 | 1.04 |
| Control | 0.16 | 0.18 | 0.18 |
| Between group | 0.87 | 0.75 | 0.68 |
Weighted means are presented for the combined effect sizes of the four studies for each of the three sleep variables.
Although pain severity, as measured in the four studies, showed a trend towards improvement in pain, not all studies showed a statistically significant improvement on treatment with CBT-I. The lack of significant improvement in pain can be explained in several ways. A larger sample size could have possibly made the observed trend of improvement in pain more significant in each study. It is also likely that the short duration of follow-up in the Currie et al. study and no follow-up post-treatment in the Jungquist et al. study could have influenced the overall significance of the findings. This is possible because chronic pain resolution after improvement in sleep takes longer, unlike the immediate effects observed in sleep parameters. Vitiello et al. followed up participants for 1 year and reported a significant improvement in the MPQ scores in those who received CBT-I unlike changes found post treatment. This is an indication that perhaps long-term follow-up with a larger sample size would give more meaningful results for pain improvement with CBT-I. However, the effect sizes for improvement in pain in the latter study were small (~ 0.3). This suggests that, although there was a significant improvement in pain scores with CBT-I on follow-up, the size of the change was not very meaningful when translated to actual patient improvement. Jungquist et al. have provided follow-up data for their study separately and report that, although the sleep improvements following CBT-I in chronic pain patients are sustained for at least 6 months post treatment, the effects on subjective improvement in pain remain less convincing.18
After considering the inter-relationship of sleep and pain, it would be natural to assume that an improvement in sleep parameters would result in a significant improvement in pain. Why this was not observed could be explained at a biological and psychological level. More than one-third of the participants in both groups were prescribed analgesics with around 70% in the Edinger et al. study prescribed analgesics or antidepressants. This would be an expected situation for those with chronic pain. Although all studies controlled for this confounding factor, the use of these agents per se could be a reason why pain did not improve despite improvement in macro-aspects of sleep. Analgesics such as antidepressants and opioids have varied effects on sleep macro-architecture and also affect the micro-architecture (e.g. rapid eye movement suppression by antidepressants and opioids).19,20 Little is known about the impact of these changes on the analgesic effects of commonly used analgesics. It is likely that the improvement in sleep noted in the sleep macro-architecture by CBT-I was not accompanied by an improvement in the micro-architecture, and hence resulted in minimal improvement in pain. Although this is a speculation, it is also a possibility. In addition to this, it is likely that long-term changes brought about by a chronic pain condition are irreparable by only improvement in sleep.11
From a psychological perspective, poor sleepers with chronic pain are also more likely to have dysfunctional beliefs about sleep that are similar to those found in individuals with primary insomnia.21 Chronic pain patients endorse dysfunctional beliefs about sleep and pain and attribute their poor sleep to pain, hence failing to use effective sleep improving measures.21 A Cochrane review on CBT for chronic pain reports that CBT for pain has a small to medium effect size in reducing pain.6 This effect size is similar to that of CBT-I on pain, as noted above. Haynes has argued that because of the limited effectiveness of cognitive behavioural approaches to pain management in these two situations, there is little beyond mild benefits to achieve from CBT as far as chronic pain is concerned.22 However, considering that chronic pain patients have dysfunctional beliefs about pain and sleep, which contribute to maintaining both, it is worthwhile to suggest that a cognitive approach that addresses cognitive distortions for sleep and pain combined with a behavioural approach for both may have an additive effect and perhaps increase the effectiveness of CBT for poor sleepers with chronic pain.
Since this literature review was conducted, other researchers have studied the impact of CBT-I on sleep and pain parameters in the chronic pain population. Two studied the effect of a combined model of CBT that addresses both pain and insomnia in comparison with either CBT for insomnia, pain and control23 or CBT for pain and a control group.24 Another reports the long-term follow-up data of the Jungquist et al. study included in this review.18 These studies address some of the questions raised by this systematic review. A further clinical trial looking at the effect of treatment of insomnia on pain has been recently completed in the UK, but the findings have so far not been reported.
Conclusion
Only four studies were identified and all were heterogeneous. This limits the conclusions that can be drawn from this review. However, it demonstrates that CBT-I produces statistically and clinically significant improvement in insomnia in patients with comorbid chronic pain. It also suggests that CBT-I may contribute to some improvement in pain; however, the lack of significance of these findings makes it difficult to state this with confidence. From the practice perspective, the effectiveness of CBT-I for this group suggests that it should not be overlooked as an option for poor sleepers with chronic pain. From the research perspective, future studies should continue to explore:
- The effectiveness of CBT-I in poor sleepers with both chronic pain and psychiatric illness in view of the fact that this will make the results more generalisable to the real-world chronic pain population where emotional difficulties are highly prevalent.
- Developing a CBT model that addresses both pain and sleep and investigate its effectiveness in this group for both sleep and pain. More recently, researchers have studied the effectiveness of a hybrid CBT model aimed at addressing both insomnia and pain in this population.23–25
- Future clinical trials should include a larger sample size, study diverse chronic pain populations and evaluate long-term follow-up.
Appendix
Appendix 1.
Excluded studies with reasons of exclusion.
| Study title | Author | Reason for exclusion |
|---|---|---|
| Behavioral management of sleep disturbances secondary to chronic pain | Morin et al., 1989 | Case series |
| Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: A pilot, randomized controlled trial | Miro et al., 2011 | Pain and sleep are not primary and secondary outcomes. Primary outcome is executive functioning. Amongst sleep outcomes, only sleep quality was measured, although sleep latency and wake after sleep onset was measured at baseline |
| A placebo controlled test of cognitive-behavioral therapy for co-morbid insomnia in older adults | Rybarczyk et al., 2005 | Parent study of study (Vitiello et al) already reported |
| Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain | Currie et al., 2002 | Study uses data from the Currie et al study (not independent) and does not measure sleep and pain outcomes |
| The role of self-help CBT-I in the management of insomnia symptoms associated with chronic disease | Morgan, 2011 | Abstract only. The author was contacted and informed us that the full study has been submitted for publication. Once the study has been published, the details will be forwarded. Hence, this is an unpublished study |
| Psychological treatment of secondary insomnia | Lichstein et al., 2000 | Does not measure pain and sleep as primary and secondary outcomes |
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: The author declares that there is no conflict of interest.
Multiple-choice questions
- Which of the following components of cognitive behavioural therapy for insomnia has the weakest evidence for clinical effectiveness as an individual component for treating primary insomnia?
- Sleep hygiene
- Sleep restriction
- Stimulus control
- Cognitive restructuring
- Relaxation training
- Which of the following are not proposed mechanisms of sleep disturbance impacting on pain?
- Hypersensitivity
- Modulation of the ascending pain pathway
- Modulation of the descending pain pathway
- Cognitive distortions
- Increase in pain threshold
- Poor sleepers with chronic pain have dysfunctional beliefs about sleep. Similarly, dysfunctional beliefs about sleep are also found in all of the following except:
- Primary insomnia
- Anxiety
- Depression
- Chronic fatigue syndrome
- Acute stress
- Which of the following agents, also used as an analgesic, is least likely to impact on sleep?
- Amitryptiline
- Tramadol
- Methadone
- Ibuprofen
- Nabilone
Answers:
1. a
2. e
3. e
4. d
References
- 1. Covington EC, Mathews M. Chronic non malignant pain. In: Carrey WD. (ed.) Current clinical medicine. The Cleveland clinic. Philadelphia, PA: Saunders Elsevier; 2009, pp. 989–996. [Google Scholar]
- 2. Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain 1998; 14: 311–314. [DOI] [PubMed] [Google Scholar]
- 3. Moldofsky H. Sleep and pain. Sleep Med Rev 2001; 5(5): 387–398. [DOI] [PubMed] [Google Scholar]
- 4. Smith MT, Haythornwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8(2): 119–132. [DOI] [PubMed] [Google Scholar]
- 5. Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain – a conceptual model. Curr Pain Headache Rep 2009; 13: 447–454. [DOI] [PubMed] [Google Scholar]
- 6. Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2009; CD007407. [DOI] [PubMed] [Google Scholar]
- 7. Wang MY, Wang SY, Tsai PS. Cognitive behavioural therapy for primary insomnia. A systematic review. J Adv Nurs 2005; 50(5): 553–564. [DOI] [PubMed] [Google Scholar]
- 8. Reynolds CF, III, Redline S. The DSM-V sleep–wake disorders nosology: an update and an invitation to the sleep community. Sleep 2010; 33(1): 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Currie SR, Wilson KG, Pontefract AJ, et al. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol 2000; 68: 407–416. [DOI] [PubMed] [Google Scholar]
- 10. Edinger JD, Wohlgemuth WK, Krystal AD, et al. Behavioral insomnia therapy for fibromyalgia patients. A randomized clinical trial. Arch Intern Med 2005; 165: 2527–2535. [DOI] [PubMed] [Google Scholar]
- 11. Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, et al. The efficacy of cognitive behavioural therapy for insomnia in patients with chronic pain. Sleep Med 2010; 11: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vitiello MV, Rybarczyk B, Von Korf M, Stepanski EJ. Cognitive behavioural therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009; 5(4): 355–362. [PMC free article] [PubMed] [Google Scholar]
- 13. Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo controlled test of cognitive-behavioral therapy for co-morbid insomnia in older adults. J Consult Clin Psychol 2005; 73(6): 1164–1174. [DOI] [PubMed] [Google Scholar]
- 14. Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. Available at: http://www.trialsjournal.com/content/pdf/1745-6215-11-32.pdf (2010, accessed 12 July 2012). [DOI] [PMC free article] [PubMed]
- 15. Higgins JPT, Altman DG, Gøtzsche PC, Peter Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yates SL, Morley S, Eccleston C, et al. A scale for rating the quality of psychological trials for pain. Pain 2005; 117(3): 314–325. [DOI] [PubMed] [Google Scholar]
- 17. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. (1989) The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practise. Psychiatry Res 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 18. Jungquist CR, Tra Y, Smith MT, et al. The durability of cognitive behavioral therapy for insomnia in patients with chronic pain. Sleep Disord 2012; Article ID 679648. 10.1155/2012/679648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharpley AL, Cowen PJ. Effects of pharmacological treatments on sleep of depressed patients. Biol Psychiatry 1995; 37: 85–98. [DOI] [PubMed] [Google Scholar]
- 20. Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007; 11: 35–46. [DOI] [PubMed] [Google Scholar]
- 21. Ashworth PCH, Davidson KM, Espie CA. Cognitive behavioural factors associated with sleep quality in chronic pain patients. Behav Sleep Med 2010; 8: 28–39. [DOI] [PubMed] [Google Scholar]
- 22. Haynes PL. Is CBT-I effective for pain? J Clin Sleep Med 2009; 5(4): 362–364. [PMC free article] [PubMed] [Google Scholar]
- 23. Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain and insomnia: a pilot study. Behav Res Ther 2012; 50(11): 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Korff M, Vitiello MV, McCurry SM, et al. Group interventions for co-morbid insomnia and osteoarthritis pain in primary care: the lifestyles cluster randomised trial design. Contemp Clin Trials 2012; 33(4): 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang NKY, Goodchild CE, Salkovskis PM. Hybrid cognitive behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav Res Ther 2012; 50(12): 814–821. [DOI] [PubMed] [Google Scholar]
Suggested reading
- 1. Currie SR, Wilson KG, Curran D. Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain. J Behav Med 2002; 25(2): 135–153. [DOI] [PubMed] [Google Scholar]
- 2. Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S. (eds.) Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley, 2008, pp. 187–241. [Google Scholar]
- 3. Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging 2000; 15: 232–240. [DOI] [PubMed] [Google Scholar]
- 4. Miro E, Lupianez J, Martinez MP, Sanchez AI, Diaz-Piedra C, Guzman MA, Buela-Casal G. Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: a pilot, randomized controlled trial. J Health Psychol 2011; 16(5): 770–782. [DOI] [PubMed] [Google Scholar]
- 5. Morgan K. The role of self-help CBT-I in the management of insomnia symptoms associated with chronic disease. Sleep Biol Rhythms 2011; 9(4): 232. [Google Scholar]
- 6. Morin CM, Kowatch RA, Wade JB. Behavioral management of sleep disturbances secondary to chronic pain. J Behav Ther Exp Psychiatry 1989; 20: 295–302. [DOI] [PubMed] [Google Scholar]
- 7. Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioural treatment programs for co-morbid geriatric insomnia. Psychol Aging 2002; 17(2): 288–298. [PubMed] [Google Scholar]
- 8. Vitiello MV. Cognitive behavioural therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. Sleep Biol Rhythms 2011; 9(4): 232–253. [PMC free article] [PubMed] [Google Scholar]


