Abstract
Background:
To investigate the association between the red cell distribution width (RDW) and mortality in patients with acute pancreatitis (AP), and to assess the ability of RDW to predict mortality in AP patients.
Materials and Methods:
This retrospective cohort study included 120 patients (50 males and 70 females) with AP who were admitted to the First Affiliated Hospital of Zhongshan Medical University from January 2011 to October 2013. Demographic data and laboratory measures including RDW were obtained from medical records of each patient. A receiver operating characteristic (ROC) curve analysis was used to assess RDW values to predict the death of AP patients.
Results:
The serum concentration levels of total Ca2+ (P = 0.007) and albumin (P < 0.001), and the white blood cell counts (P = 0.005) were significantly lower, and the mortality rate (P < 0.001) and body mass index (P < 0.001) were significantly higher (P = <0.001, <0.001) in patients with RDW values of >13.4% than in patients with RDW values of ≤13.4%. RDW values were negatively correlated with the serum concentration levels of albumin (r = −0.212, P = 0.012) and total Ca2+ (r = −0.206, P = 0.033), and were positively correlated with the patient's age (r = 0.201, P = 0.035). ROC analysis showed that the AUC for the RDW value was 0.894 (P < 0.001, 95% confidence interval = 0.823-0.966), and the optimal cut-off value to predict death was 14.35 (sensitivity = 88.2%, specificity = 91.8%).
Conclusion:
Red cell distribution width is a potentially new and sensitive predictor of mortality in patients with AP.
Keywords: Acute pancreatitis, mortality, red cell distribution width
INTRODUCTION
Acute pancreatitis (AP) is a sudden inflammation of the pancreas induced by activation of pancreatic enzymes by a variety of causes such as alcohol and gallstones. AP is one of the most common surgical diseases that causes severe acute abdomens. Recently, the incidence of AP has increased sharply,[1] and the total mortality rate of AP is approximately 3.8% to 7%in China.[2] AP can be categorized into mild AP and severe AP. In severe AP, there is an extensive systemic inflammatory response due to entry of pancreatic enzymes into the blood and subsequent induction of damage in distant organs.[3] Following tissue damage, a large amount of inflammatory mediators and cytokines are released and cause systemic complications such as systemic inflammatory response syndrome and multiple organ dysfunction syndrome (MODS). The mortality rate for severe AP can be as high as approximately 40-70%.[2,4] Therefore, early identification of AP patients with a high risk of mortality and appropriate evaluation of the severity of AP is important for early medical treatment of AP in order to reduce the mortality rate of severe AP. Currently, the Acute Physiology and Chronic Health Evaluation II score has been used for evaluation and prediction of AP severity.[5,6,7] The Ranson criteria have to be performed 48 h after the onset of AP and thus, may not be useful for early diagnosis and prediction of AP severity. In addition, since the two scoring systems involve many tests and are cumbersome to operate, they are not convenient for clinical practice. Therefore, it is important to identify a simple, easy, and sensitive marker for predicting the mortality associated with AP.
Red cell distribution width (RDW), performed as part of a routine blood test, is a quantitative measurement of variability in the size of peripheral red blood cells (RBCs), thus reflecting the heterogeneity of RBCs. Because the changes in the morphology and size of circulating RBCs is often associated with the occurrence and development of blood diseases, RDW is used for the morphological classification of anemia and differential diagnosis of microcytic anemia.[8,9] Several studies have shown that RDW is significantly associated with inflammatory markers such as C-reactive protein, interleukin 6, and fibrinogen.[8,10] Furthermore, it has been reported that RDW can serve as a predictor of the risk of mortality in community-dwelling elderly patients, critically ill patients, and patients with cardiovascular diseases, acute respiratory difficulty, and community-acquired pneumonia.[11,12,13,14,15,16]
In this retrospective cohort study, we measured RDW values in 120 AP patients. The purpose of this study was to assess the ability of RDW values for predicting the severity of AP and mortality of AP patients. We found that RDW was a sensitive predictor of mortality in patients with AP. Our study suggests that RDW may be used as a novel marker for predicting the mortality of AP patients.
MATERIALS AND METHODS
Patients
This retrospective cohort study included 120 consecutive patients (50 males and 70 females) with AP who were admitted to the First Affiliated Hospital of Sun Yat-sen University from January 2011 to October 2013. The average age was 51.2 ± 18.5 years. AP was diagnosed based on typical clinical presentations including acute persistent abdominal pain, plasma amylase level of more than 3 times the upper normal threshold (110 IU/L), and/or abnormal pancreatic morphology on computed topographic images. AP was classified into mild acute pancreatitis (MAP) and severe acute pancreatitis (SAP). MAP is characterized by the absence of organ failure and the absence of local or systemic complications. SAP is characterized by persistent single or multiple organ failure, and usually accompanied by one or more local complications.[3] Patients’ information such as age, sex, body mass index (BMI), and complications were extracted from hospital records. Patients with incomplete clinical data such as routine blood tests and biochemical detection index were excluded from the study. Patients with the following diseases were excluded:
Malignant tumors with chemotherapy and radiotherapy;
Organ transplantation;
Severe liver diseases;
Severe kidney failure;
Anemia; and
Severe infection in the previous 1-month.
Sixteen patients died during the follow-up period of 3 months via outpatient service review. The median of survival time was 6 days (range, 2-81 days). This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University, and was performed in accordance with the Helsinki Declaration. Written informed consent was obtained from each participant at the time of enrollment.
Laboratory measurement
Patients fasted overnight, and blood was collected within 24 h after admission, and used for routine blood tests and biochemical tests. Routine blood tests were performed using a Sysmex XE 200 hematology analyzer (Sysmex Corp., Kobe, Japan), and biochemical parameters were measured using an Abbott Aeroset 200 automatic biochemistry analyzer (Abbott Diagnostics, Illinois, USA).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, USA). Quantitative data with a normal distribution are expressed as mean and standard deviation. Quantitative data without a normal distribution are expressed as median and interquartile range. One-way analysis of variance or Mann-Whitney U-tests were used to compare the difference between groups. Categorical data were expressed as frequency and percentage and analyzed using Chi-square tests or Kruskal-Wallis H-tests. Pearson correlation tests were used to analyze the association between RDW and laboratory results. A receiver operating characteristic (ROC) curve was used to assess the discriminating performance of RDW to predict death in AP and the cut-off values for RDW with sensitivity and specificity were calculated. A P < 0.05 was considered as statistically significant.
RESULTS
Clinical characteristics
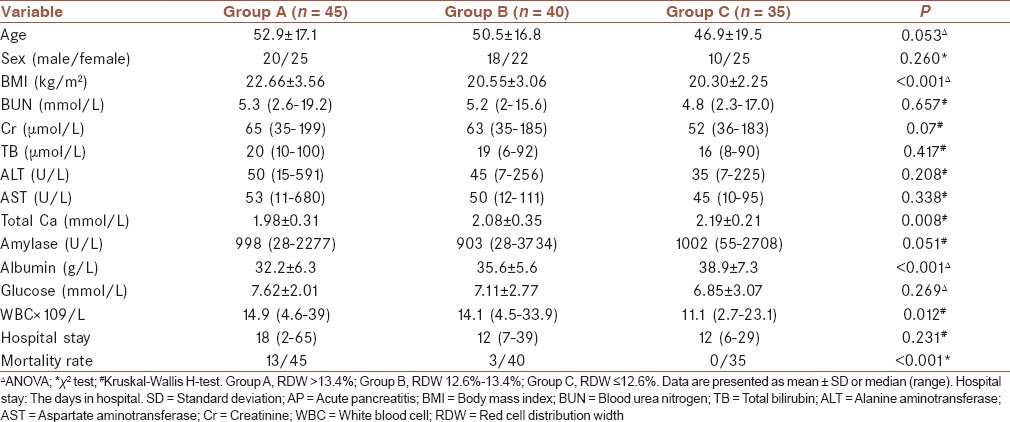
Eighty-nine patients had mild AP, and 31 patients had severe AP. Five patients with severe AP developed necrotizing pancreatitis, and twelve patients with severe AP developed MODS. The causes of AP included biliary tract diseases (n = 75), alcoholism (n = 18), high-fat diet (n = 17), drugs (n = 5), and other (n = 5). The AP patients were categorized into three groups according to the RDW values: Group A (RDW >13.4%), Group B (12.6% >RDW ≤13.4%), and Group C (RDW ≤12.6%). Table 1 summarizes the demographic and clinical characteristics of patients among Groups A, B, and C. There were no significant differences in the age, sex, or length of hospital stay among the three groups. However, the BMI was significantly higher in Group A than in Groups B and C (P < 0.001). For laboratory tests, there were no significant differences in blood urea nitrogen (BUN), creatinine (Cr), total bilirubin (TB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), amylase, or glucose (Glu) among the three groups. The serum concentration levels of total Ca2+ (P = 0.035) and albumin (<0.001) were significantly lower in Group A than in Groups B and C. The white blood cell (WBC) count was significantly lower in Group C than in Groups A (P = 0.045) and B (P < 0.001). The mortality rate was significantly higher in Group A than in Groups B (P = 0.012) and C (P = 0.001).
Table 1.
The demographic and clinical characteristics of AP patients
Correlation analysis
Red cell distribution width values were negatively correlated with the serum concentration levels of albumin (r = −0.212, P = 0.012) and total Ca2+ (r = −0.206, P = 0.033), and were positively correlated with the patient's age (r = 0.201, P = 0.035). No significantly correlation was found between RDW values and serum concentrations of BUN, Cr, TB, ALT, AST, and Glu, or WBC count (P > 0.05).
Association of red cell distribution width with the mortality rate in acute pancreatitis patients
Sixteen patients died during hospital stay and the follow-up period of 3 months. Twelve patients died of MODS and four patients died of infected pancreatic necrosis. Thirteen patients died within 2 weeks after admission and three patients died 2-3 weeks after discharge. The serum total Ca2+ (P < 0.001), total protein (P < 0.001), and albumin levels (P < 0.001) at admission were significantly lower in dead patients than those in alive patients (1.80 ± 0.34 mmol/L, 50.3 ± 5.68 g/L, and 28.9 ± 3.36 g/L, respectively, versus 2.20 ± 0.38 mmol/L, 60.3 ± 8.78 g/L, and 35.8 ± 6.75 g/L, respectively). The age (P < 0.001), serum concentrations of Cr (P < 0.001) and BUN (P < 0.001) and RDW values (P < 0.001) at admission were significantly higher in dead patients than in those alive (66.3 ± 10.1 years, 110 (range, 45-199) μmol/L), 7.8 (range, 7.6-16.5) mmol/L, and 14.31 ± 0.85%, respectively, versus 50.1 ± 14.1 years, 55 (range, 35-185) μmol/L, 4.7 (range, 2.0-19.2) mmol/L and 12.82 ± 0.95%, respectively).
Receiver operating characteristic curve analysis was used to evaluate the values for RDW to predict mortality in AP patients [Figure 1]. The area under the curve (AUC) and the optimal cut-off value were calculated. The AUC for the RDW value was 0.894 (P < 0.001, 95% confidence interval = 0.823-0.966). The optimal cut-off value to predict deaths was 14.35 (sensitivity = 88.2%, specificity = 91.8%).
Figure 1.
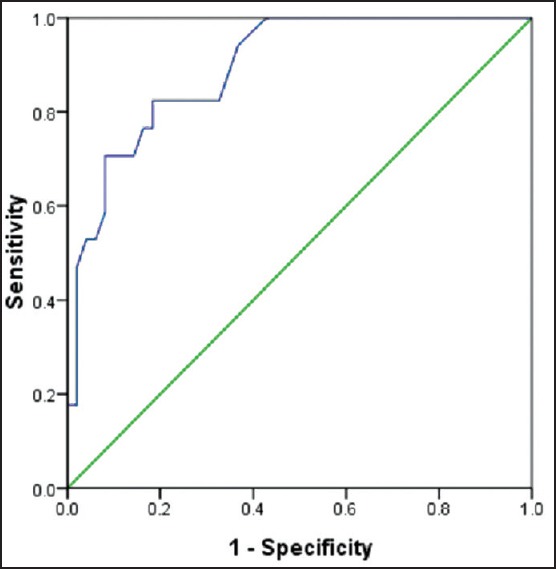
The receiver operating characteristic curve of red cell distribution width values for predicting death in patients with acute pancreatitis
DISCUSSION
Acute pancreatitis involves both local inflammatory lesions and systemic pathological damage. Appropriate evaluation of the severity of AP is important for early and effective medical treatment for AP. Approximately 70-80% of AP is mild AP, which can be treated. In contrast, severe AP develops rapidly and most patients with severe AP die of MODS within 1-week after disease onset, while others die of infected pancreatic necrosis 1-week after disease onset.[4,17] Similarly, we found that of the 16 patients that died during our study period, 12 AP patients died of MODS and four patients died of infected pancreatic necrosis. Thirteen patients died within 2 weeks and three patients died 2 weeks. Therefore, early diagnosis and appropriate treatment is critical for treating patients with severe AP and improving the survival rate of AP.
Currently, no single prognostic index is available for acutely evaluating the severity of AP in the clinic. Disease occurrence and mortality is often predicted by combined use of clinical data, imaging, and biochemical analysis. However, approximately 20-30% of severe AP is misdiagnosed.[18] Searching for an ideal marker for predicting the severity of AP is important for early identification of severe AP. The ideal marker for predicting mortality of AP patients should be predictive in the early stage of AP disease, economic, objective, repeatable, simple, noninvasive, sensitive, and specific. RDW, which is a routinely reported parameter in the complete blood count test, has been reported to predict the mortality of AP with a low sensitivity of 47.6% and a specificity of 96.3%.[18] In the present study, we examined the RDW values in 120 AP patients and found that the mortality rate was significantly higher in patients with RDW >13.4% than those with RDW <13.4%. Our study suggests that the severity of AP is greater and patient mortality increased with higher RDW values. In addition, we further assessed the ability of RDW values to predict death in AP patients using ROC curve analysis. We found that the optimal cut-off value for RDW to predict death was 14.35 with a sensitivity of 88.2% and a specificity of 91.8%. The sensitivity (88.3%) identified in this study is greatly higher than that (47.6) reported by Senol et al.,[19] and the specificity (91.8%) is slightly lower that that (96.3%) reported by Senol et al.[19] Our study suggests that RDW can be used a sensitive marker for predicting the mortality of AP patients.
It has been reported that changes in RDW is associated with the inflammation status of the disease, which may explain why patients with higher RDW values have a higher mortality rate. It has been proposed that inflammation promotes deaths of RBCs or inhibits the maturation of RBCs, which is associated with an increase in RDW.[20,21,22] Some inflammatory mediators influence bone marrow function and iron metabolism and suppress erythropoietin-induced maturation of RBCs.[21,22] Therefore, RDW values reflect the inflammation status of AP and thus, may be used for predicting the severity of AP. In addition, Gao et al. have reported that BMI is associated with the severity of AP.[23] Consistent with a previous study by Gao et al.,[23] we found that AP patients with RDW values >13.4% had a significantly higher BMI than those with RDW values <13.4%.
In summary, we evaluated the ability of RDW values for predicting the death of AP patients and found that RDW was a good prognostic predictor of death in AP patients. Increased RDW can be used as a new indicator of mortality in patients with AP. In addition, a cut-off RDW value of 14.35 with a sensitivity of 88.2% and a specificity of 91.8 was identified. These results indicate that RDW is convenient, economic, and sensitive monitoring method for helping clinicians predict death in AP patients. RDW values in combination with other scoring systems will be useful for properly evaluating the severity of AP.
AUTHOR'S CONTRIBUTIONS
ML contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. DW, JY, JZ, SZ and BW contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENT
We thank Medjaden bioscience limited Corporation for helping in proofreading and editing the English of final manuscript.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: Impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539–48. doi: 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Cui Z, Zhang J, Li H, Zhang D, Miao B, et al. Early predictive factors of in hospital mortality in patients with severe acute pancreatitis. Pancreas. 2010;39:114–5. doi: 10.1097/MPA.0b013e3181b65dd5. [DOI] [PubMed] [Google Scholar]
- 3.Branch of Digestive Diseases of Chinese Medical Association. Guidelines for diagnosis and treatment of acutepancreatitis (draft) Zhonghua Nei Ke Za Zhi. 2004;43:236–8. [Google Scholar]
- 4.Isaji S, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN Guidelines for the management of acute pancreatitis: Surgical management. J Hepatobiliary Pancreat Surg. 2006;13:48–55. doi: 10.1007/s00534-005-1051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–5. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 6.Yeung YP, Lam BY, Yip AW. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:294–9. [PubMed] [Google Scholar]
- 7.Chatzicostas C, Roussomoustakaki M, Vlachonikolis IG, Notas G, Mouzas I, Samonakis D, et al. Comparison of Ranson, APACHE II and APACHE III scoring systems in acute pancreatitis. Pancreas. 2002;25:331–5. doi: 10.1097/00006676-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Fornal M, Wizner B, Cwynar M, Królczyk J, Kwater A, Korbut RA, et al. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin Hemorheol Microcirc. 2014;56:325–35. doi: 10.3233/CH-131745. [DOI] [PubMed] [Google Scholar]
- 9.Aslan D, Gümrük F, Gürgey A, Altay C. Importance of RDW value in differential diagnosis of hypochrome anemias. Am J Hematol. 2002;69:31–3. doi: 10.1002/ajh.10011. [DOI] [PubMed] [Google Scholar]
- 10.Hu ZD, Chen Y, Zhang L, Sun Y, Huang YL, Wang QQ, et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clin Chim Acta. 2013;425:202–5. doi: 10.1016/j.cca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: A meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–65. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–23. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunziker S, Celi LA, Lee J, Howell MD. Red cell distribution width improves the simplified acute physiology score for risk prediction in unselected critically ill patients. Crit Care. 2012;16:R89. doi: 10.1186/cc11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makhoul BF, Khourieh A, Kaplan M, Bahouth F, Aronson D, Azzam ZS. Relation between changes in red cell distribution width and clinical outcomes in acute decompensated heart failure. Int J Cardiol. 2013;167:1412–6. doi: 10.1016/j.ijcard.2012.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Hong N, Oh J, Kang SM, Kim SY, Won H, Youn JC, et al. Red blood cell distribution width predicts early mortality in patients with acute dyspnea. Clin Chim Acta. 2012;413:992–7. doi: 10.1016/j.cca.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Braun E, Domany E, Kenig Y, Mazor Y, Makhoul BF, Azzam ZS. Elevated red cell distribution width predicts poor outcome in young patients with community acquired pneumonia. Crit Care. 2011;15:R194. doi: 10.1186/cc10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnovale A, Rabitti PG, Manes G, Esposito P, Pacelli L, Uomo G. Mortality in acute pancreatitis: Is it an early or a late event? JOP. 2005;6:438–44. [PubMed] [Google Scholar]
- 18.Guo ZH, Hao JY. The review of acute pancreatitis scoring system. Chin J Clin Hepatol. 2011;27:1170–3. [Google Scholar]
- 19.Senol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med. 2013;31:687–9. doi: 10.1016/j.ajem.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Song CS, Park DI, Yoon MY, Seok HS, Park JH, Kim HJ, et al. Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig Dis Sci. 2012;57:1033–8. doi: 10.1007/s10620-011-1978-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Zhang H, Wang Q, Liu J, Sun J. Clinical usefulness of measuring red cell volume distribution width to diagnosis heart failure in acute coronary syndrome patients. Int J Lab Med. 2012;20:2437–9. [Google Scholar]
- 22.Fukuta H, Ohte N, Mukai S, Saeki T, Asada K, Wakami K, et al. Elevated plasma levels of B-type natriuretic Peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J. 2009;50:301–12. doi: 10.1536/ihj.50.301. [DOI] [PubMed] [Google Scholar]
- 23.Gao H, Wang L, Yao P. Relationship of body mass index blood glucose and serum triacylglycerol with severity of acute pancreafitis. Clin J Gastroenterol. 2012;17:27–9. [Google Scholar]


