Abstract
Background:
Computed tomography (CT) scan is one the most useful devices in chest imaging. CT scan can be used in mediastinal abnormality, lungs, and pleural evaluations. According to the high prevalence and different causes of pulmonary nodules, we designed this study to evaluate the prevalence and the types of pulmonary nodules in noncancerous patients who underwent chest multi-detector CT (MDCT) scan.
Materials and Methods:
This was a cross-sectional study which was in our hospital to evaluate the prevalence of pulmonary nodules in noncancerous patients who underwent MDCT. A checklist was used for data collection containing number, location, size, and shape of pulmonary nodules if present in CT scan, and we also included patient's age and history of smoking. We analyzed the data with Statistical Program for Social Sciences software (version 18).
Results:
In this study, 115 patients (40%) had a pulmonary nodule. The mean number of a total nodule in each patient was 0.8 ± 0.07. Mean number of intra-parenchymal, sub pleural, and perivascular nodules were 0.34 ± 0.04, 0.31 ± 0.04, and 0.14 ± 0.02, respectively. The mean number of calcified nodules was 0.13 ± 0.02. There was no significant correlation between age and nodule characteristics (P > 0.05).
Conclusion:
The prevalence of pulmonary nodules was quite frequent in MDCT scan of noncancerous cases. So, it should not be overvalued in noncancerous cases.
Keywords: Solitary pulmonary nodule, thorax, tomography X-ray computed
INTRODUCTION
Solitary pulmonary nodule (SPN) is one of the common clinical problems. In most of the times, SPN is discovered incidentally on chest X-ray (CXR) or on computed tomography (CT) scan. Pulmonary nodule evaluation improved by multi-detector CT (MDCT) which is capable for rapid and thin section imaging and this ability can help with detection and characterization of nodules. MDCT is performed for many reasons such as screening, investigations for pulmonary embolism or cardiac function and search for metastases of other cancers than lung cancer.[1,2]
SPN is detected in 0.09-7% of chest radiographs. Patel et al. have reported that 7% of 1000 healthy individuals had 1-3 nodules in their CXR screening and they had also noted that missing lung cancer in CXR was frequent.[3]
In the lung cancer screening studies, the prevalence of SPN was from 8% to 51%.[4,5,6] On the other hand, failures of detection or interpretive errors which lead to incorrect findings can cause missed nodules.[2]
CT scan is more sensitive and more specific than CXR for detecting nodules.[7]
According to nodule management protocol of the NELSON Randomized Lung Cancer Screening Trial, predictor factors of malignancy in nodules are: Size, border, calcification, density, growth, and location.[8]
Larger lesions are more suspicious to malignancy. Malignancy probability in nodules which are smaller than 3 mm is about 0.2%, in 4-7 mm nodules is 0.9%, in 8-20 mm nodules is 18% and in nodules, which are larger than 20 mm is about 50%. Nodules which are larger than 30 mm should be considered malignant until proven otherwise.[9]
Smooth and discrete border, satellite nodules, calcification, presence of focal fat within a solid SPN, thin walled cavitating nodules are more common in benign lesions. On the other hand, fast growing and upper lobe location are more likely in malignant ones.[10,11,12]
The prevalence of malignancy in SPN is varying from 1% to 12% in the various studies.[1] Elderly patients, history of smoking, larger nodule size, female sex, and previous history of cancer can enhance the probability of malignancy in SPN.[13]
We designed this study to determine the prevalence of pulmonary nodules in noncancerous patients who underwent chest MDCT and to define the nodules size and location; because there are so prevalent in normal population and radiologists should consider this fact during reporting thoracic MDCTs. We also evaluate the correlations between risk factors and nodule characteristics and we recommend a guideline, according to previous reports.
MATERIALS AND METHODS
This was a cross-sectional study which was performed in Al-Zahra Hospital of Isfahan University of Medical Sciences from April to October 2013. All patients who were referred for thoracic MDCT scan for reasons other than malignancy evaluation were enrolled. Most common reasons were trauma patients, cases suspected to pulmonary infection, pulmonary thromboembolism, and evaluation for dyspnea that were enrolled to our study. We just exclude patients who did not sign our informed consent.
In this study, we enrolled all patients who underwent chest MDCT in Al-Zahra Hospital (referral academic MDCT center in Isfahan), without previous history of malignancy.
The entire chest MDCTs were performed with 64 MDCT scanner (made in GE company) reported daily by radiologists. Our sampling method was convenience time-based sequential, and we used a check list for our data collection. In our check list, we evaluate location,[14] number, size, border and calcification of nodules. The size of the detected nodules was calculated with computer electronic calipers. We also evaluate demographic information, history of smoking and patient's job.
The statistical procedure was done by Statistical Program for Social Sciences software version 18 (SPSS Inc., Chicago, IL). Student's t-test and Pearson correlations were used for data analyses. The significance level (P) was set at 0.05 and we present our findings using mean ± standard error. This study was approved by research committee of Islamic Azad University, Najafabad Branch and adopted from first author thesis (Thesis number: 15010101912032).
RESULTS
In this cross-sectional study, we enrolled 287 patients. The mean age of participants was 51.32 ± 10.95 years old. One hundred and fifty-nine (55.6%) participants were male, and 128 (44.5%) were female. Thirty-four patients (12.7%) were a smoker (all smokers were male). In our study, pulmonary nodules were detected in 115 patients (40%).
From these cases, 63 ones (21.9%) had intra-parenchymal nodule; 66 (23%) ones had sub pleural nodule, and perivascular nodules were diagnosed in 40 patients (13.9%).
The mean number of nodules in all participants was 0.8 ± 0.07.
Mean number of intra-parenchymal, subpleural and perivascular nodules in all participants was 0.34 ± 0.04, 0.31 ± 0.03, and 0.14 ± 0.02 respectively.
Thirty-two (11.1%) participants had calcified nodules. The mean number of calcified nodules was 0.13 ± 0.02 in all participants.
The mean number of smaller than 5 mm nodules was 0.59 ± 0.05. The mean size of these nodules was 3.5 ± 0.65 mm; these nodules were detected in 102 participants. The mean number of 5-10 mm nodules was 0.18 ± 0.03 and mean size was 6.67 ± 0.62 mm; 39 patients had 5-10 mm nodules. Eight patients had nodules which were larger than 10 mm. The mean size of these nodules was 11.24 ± 0.42 mm.
Pearson product moment correlations showed that there was direct correlation between age and total number of pulmonary nodules (P = 0.001, r = 0.421), Perivascular (P = 0.001, r = 0.336), subpleural (P = 0.001, r = 0.255), intra-parenchymal (P = 0.001, r = 0.177), and calcified nodules (P = 0.001, r = 0.408). There was direct correlation between age, number of smaller than 5 mm (P = 0.001, r = 0.222) and 5-10 mm nodules (P = 0.001, r = 0.088). Size of smaller than 5 mm nodules was correlated with age too (P = 0.013, r = 0.114).
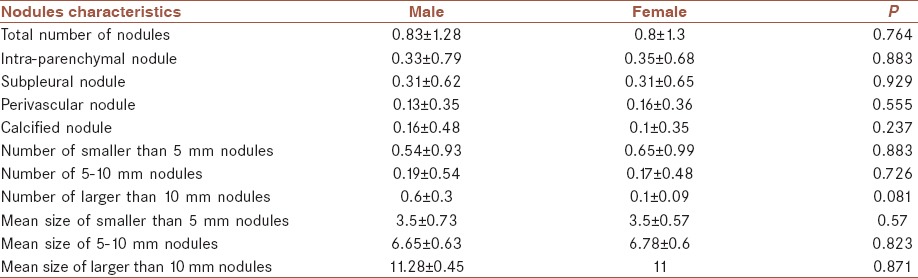
There was no significant difference between male and female participants in nodules characteristics, including location and size of them (P > 0.05). Table 1 shows the nodules characteristics in both male and females.
Table 1.
Nodules characteristics in both male and females
The mean number of a pulmonary nodule in smoker patients was 1.12 ± 0.06 (16 participants had nodules) and in nonsmoker patients was 0.76 ± 0.03. There was a significant difference between groups (P = 0.001).
Fifty-three patients in nonsmoker group had intra-parenchymal nodule; mean number was 1.47 ± 0.03 and in smoker group 10 patients had intra-parenchymal nodules. The mean number was 2 ± 0.05.
98 patients in nonsmoker group had subpleural nodule; the mean number was 0.77 ± 0.03 and in smoker group 16 patients had subpleural nodules. Mean number was 0.94 ± 0.03. Five patients in smoker group and 27 patients in nonsmoker group had calcified nodules.
Ninety-seven patients between nonsmokers had perivascular nodule; the mean number was 0.39 ± 0.04 and in smoker group 16 patients had perivascular nodules. Mean number was 0.19 ± 0.02.
In nonsmoker group, mean number of smaller than 5 mm nodules was 0.56 ± 0.03 and in the smoker group was 0.82 ± 0.06 (P = 0.199). Mean size in smoker group was 3.57 ± 0.06 and in nonsmoker group was 3.47 ± 0.04 (P = 0.57).
In nonsmoker group, mean number of 5-10 mm nodules was 0.17 ± 0.02 and in the smoker group was 0.26 ± 0.3 (P = 0.415). Mean size in smoker group was 6.5 ± 0.53 and in nonsmoker group was 6.68 ± 0.06 (P = 0.46).
In nonsmoker group, mean number of larger than 10 mm nodules was 0.04 ± 0.01 and in the smoker group was 0.03 ± 0.01 (P = 0.056). Mean size in smoker group was 11 and in nonsmoker group was 11.28 ± 0.03 (P = 0.54).
DISCUSSION
This was a cross-sectional study which was performed in noncancerous patients who underwent MDCT.
In our study, 115 (40%) of participants had a pulmonary nodule. Mean number of a nodule in each patient was 0.8 ± 0.07. Mean number of intra-parenchymal nodule was 0.34 ± 0.04, subpleural nodule was 0.31 ± 0.03, and perivascular nodule was 0.14 ± 0.02. There was no significant difference between male and female participants in nodules characteristics. Mean number of a pulmonary nodule in smoker patients was 1.12 ± 0.06 and in nonsmoker patients was 0.76 ± 0.03. There was a significant difference between groups.
Swensen et al. in 2003 evaluated 1520 American participants how were a smoker and older than 50 years old in Mayo Clinic. Their patients had at least history of 20 pack/year smoking. Two thousand eight hundred and thirty-two noncalcified nodules were diagnosed in 1049 patients. Forty participants are diagnosed as lung cancer patients. One thousand seven hundred and thirty-five nodules were smaller than 4 mm (61%), 950 were between 4 and 8 mm (34%), 136 were between 8 and 30 mm (5%) and 11 were larger than 20 mm (0.4%).[15] In our study, 34 patients were a smoker and 16 participants (47%) had pulmonary nodules. This difference in prevalence of pulmonary nodules in smoker patients could be explained by larger sample size in Swensen et al. study.
Henschke et al. evaluated 1000 patients how were a smoker or had a history of smoking for at least 10 pack/year. Twenty-three percent had noncalcified, and 2.7% had malignant nodules.[16] In our study, 5 (14.7%) smoker patients had calcified nodules. This difference may be because of our smaller sample size.
Gómez-Sáez et al. in 2014 in Spain had evaluated 25,529 participants how were older than 35 years old. These patients were referred to two hospitals for chest imaging. Two thousand four hundred and ninety-seven patients underwent chest CT scan. Prevalence of pulmonary nodules between these patients was 17% and other participants underwent CXR and pulmonary nodules were diagnosed in 2.1% of these patients. They had reported that age, male sex and respiratory disease are significantly correlated with pulmonary nodules.[17] We found more pulmonary nodules in male patients too. Slice thickness in Gómez-Sáez et al. study was 1.25 mm on the other hand in our study was 0.3 mm. Because of this difference we found more pulmonary nodules in our patients.
Tammemägi et al. in 2013 had reported that 37.9% of their patients who were enrolled to National Lung Screening Trial study had pulmonary nodules. This result is agreed with our findings.[18]
McWilliams et al. in 2013 had reported that the prevalence of SPN in high-risk population is 73.7%.[13] As they had evaluated high-risk patients, their reported prevalence is higher than ours.
According to Fleischner Society recommendations patients who have smaller than 4 mm pulmonary nodules without risk factor don't need follow-up. But if they have risk factor CT at 12 months is recommended, and if there was no change, no more follow-up is needed. In patients with 4-6 mm nodules without risk factor work-up is the same as patients with smaller than 4 mm nodules with the risk factor. But if patients have risk factor Initial follow-up CT should be considered at 6-12 months and then 18-24 months follow-up CT if there was no change. In patients with 6-8 mm nodules recommended workup is the same as patients with 4-6 mm pulmonary nodules with risk factor and if patient has risk factor initial follow-up CT at 3-6 months, then 9-12 months and 24 months if no change is recommended. Finally in patients how have larger than 8 mm nodules with or without risk factor, follow-up with CT at 3, 9 and 24 months, dynamic contrast enhanced CT, considering positron emission tomography scan ± biopsy is recommended.
van't Westeinde et al. in 2008 had reported that in larger than 4-5 mm nodules consistency, margin and shape should be considered. They also reported that number, site and presence or absence of growth and volume doubling time are important factors in patient's therapeutic plan. In their study growth in pulmonary nodules is defined as a volume doubling time in 400 days or less, according to nodules volume. Their approach to smaller than 4 mm nodules was the same as Fleischner Society recommendation. For solid, smooth or attached indeterminate noncalcified pulmonary nodules, which are between 5 and 10 mm they recommended an annual CT scan, whilst for purely intra-parenchymal nodules a 3-month CT scan should be considered for growth evaluation.
Growing lesions which have volume doubling time <400 days needs further work-up and diagnosis.[19]
According to previous studies pulmonary nodules which are smaller than 5 mm and patient does not have any risk factor such as the history of smoking, age <60 years and history of malignancy do not need any work-up. But if patients have any of these risk factors CT scan at 12 months is recommended. On the other hand in larger than 10 mm nodules with or without risk factor further evaluations seems to be needed as it was mentioned in the introduction larger lesions are more suspicious to be malignant.[20]
Designing multi-center studies and long-term follow-up of noncancerous patients with pulmonary nodules seems to be needed.
As shown in our study, the prevalence of pulmonary nodules was quite frequent in MDCT scan of noncancerous cases. So, it should not be overvalued in noncancerous cases.
AUTHOR'S CONTRIBUTIONS
AA and ATo provided assistance in design. AT and AA participated in data collection. ATo and AA prepared the manuscript. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENTS
All of CT scan employers of AI-Zahra Hospital which this study was performed there.
Footnotes
Source of Support: Nil
Conflicts of Interest: No conflict of interests.
REFERENCES
- 1.Murrmann GB, van Vollenhoven FH, Moodley L. Approach to a solid solitary pulmonary nodule in two different settings-“Common is common, rare is rare”. J Thorac Dis. 2014;6:237–48. doi: 10.3978/j.issn.2072-1439.2013.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko JP. Lung nodule detection and characterization with multi-slice CT. J Thorac Imaging. 2005;20:196–209. doi: 10.1097/01.rti.0000171625.92574.8d. [DOI] [PubMed] [Google Scholar]
- 3.Patel VK, Naik SK, Naidich DP, Travis WD, Weingarten JA, Lazzaro R, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: Part 1: Radiologic characteristics and imaging modalities. Chest. 2013;143:825–39. doi: 10.1378/chest.12-0960. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi G, Maisonneuve P, De Pas TM, Bellomi M. Does lung cancer screening with low-dose CT remain promising despite disappointing DANTE results? Am J Respir Crit Care Med. 2010;182:720–1. doi: 10.1164/ajrccm.182.5.720. [DOI] [PubMed] [Google Scholar]
- 5.Henschke CI, Yankelevitz DF, Naidich DP, McCauley DI, McGuinness G, Libby DM, et al. CT screening for lung cancer: Suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231:164–8. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 6.Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, Writing Committee, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: The Lung Screening Study of the National Cancer Institute. Chest. 2004;126:114–21. doi: 10.1378/chest.126.1.114. [DOI] [PubMed] [Google Scholar]
- 7.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369:920–31. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;54:177–84. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Mehta HJ, Ravenel JG, Shaftman SR, Tanner NT, Paoletti L, Taylor KK, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145:464–72. doi: 10.1378/chest.13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marten K, Engelke C, Seyfarth T, Grillhösl A, Obenauer S, Rummeny EJ. Computer-aided detection of pulmonary nodules: Influence of nodule characteristics on detection performance. Clin Radiol. 2005;60:196–206. doi: 10.1016/j.crad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Henschke CI, Yankelevitz DF, Yip R, Reeves AP, Farooqi A, Xu D, et al. Lung cancers diagnosed at annual CT screening: Volume doubling times. Radiology. 2012;263:578–83. doi: 10.1148/radiol.12102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuvelmans MA, Oudkerk M, de Bock GH, de Koning HJ, Xie X, van Ooijen PM, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol. 2013;23:1836–45. doi: 10.1007/s00330-013-2799-9. [DOI] [PubMed] [Google Scholar]
- 13.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–9. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das M, Ley-Zaporozhan J, Gietema HA, Czech A, Mühlenbruch G, Mahnken AH, et al. Accuracy of automated volumetry of pulmonary nodules across different multislice CT scanners. Eur Radiol. 2007;17:1979–84. doi: 10.1007/s00330-006-0562-1. [DOI] [PubMed] [Google Scholar]
- 15.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226:756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 16.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Sáez N, González-Álvarez I, Vilar J, Hernández-Aguado I, Domingo ML, Lorente MF, et al. Prevalence and variables associated with solitary pulmonary nodules in a routine clinic-based population: A cross-sectional study. Eur Radiol. 2014;24:2174–82. doi: 10.1007/s00330-014-3249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–36. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AN, Al-Jahdali HH, Irion KL, Arabi M, Koteyar SS. Solitary pulmonary nodule: A diagnostic algorithm in the light of current imaging technique. Avicenna J Med. 2011;1:39–51. doi: 10.4103/2231-0770.90915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van't Westeinde SC, de Koning HJ, Xu DM, Hoogsteden HC, van Klaveren RJ. How to deal with incidentally detected pulmonary nodules less than 10 mm in size on CT in a healthy person. Lung Cancer. 2008;60:151–9. doi: 10.1016/j.lungcan.2008.01.020. [DOI] [PubMed] [Google Scholar]


