Abstract
The incidence of diabetes mellitus (DM) is increasing rapidly and it is expected to increase by 2030. Other than currently available therapeutic options, there are a lot of herbal medicines, which have been recommended for its treatment. Herbal medicines have long been used for the treatment of DM because of the advantage usually having no or less side-effects. Most of these plants have antioxidant activities and hence, prevent or treat hard curable diseases, other than having the property of combating the toxicity of toxic or other drugs. In this review other than presenting new findings of DM, the plants, which are used and have been evaluated scientifically for the treatment of DM are introduced.
Keywords: Diabetes mellitus, herbal drugs, diabetic nephropathy
INTRODUCTION
Diabetes mellitus (DM) is a group of metabolic disorders in which the blood sugar is higher than normal level either because the production of insulin is not enough (type 1 DM) or the cells do not properly respond to the insulin (type 2 DM).[1]
According to a report from World Health Organization, about 220 million people have type 2 DM. Its incidence is increasing rapidly, and it is expected to increase to more than 365 million by 2030.[2] DM occurs throughout the world. However, it is more common in the more developed countries. It is noteworthy that the highest increase in prevalence is expected to occur in Africa and Asia.[3] The increase in incidence in developing countries follows the trend of urbanization and lifestyle changes, perhaps most importantly a “Western-style” diets.[3]
Other than currently available therapeutic options, there are a lot of herbal medicines, which have been recommended for the treatment of DM,[4,5] hyperlipidemia[4,5,6,7] and other cardiovascular risk factors.[4,5,6,7,8,9]
Herbal medicines have long been used for the treatment of DM. This is because such herbal plants have hypoglycemic properties and other beneficial effects. Herbal medicines have the advantage of usually having no or less side-effects.[10,11] Most of these plant have antioxidant activities[12,13] and hence, prevent or treat hard curable diseases, other than having the property of combating the toxicity of toxic[14,15] or other drugs.[16,17,18,19] In this review other than presenting new findings of DM the plants which are used for the treatment of DM are introduced.
DIFFERENT FORMS OF DIABETES MELLITUS
There are several types of DM, three main types of them are type 1, type 2 and gestational diabetes. Type 1 diabetes mellitus “juvenile diabetes or insulin-dependent diabetes mellitus” results from the pancreas failure to produce insulin, and requires the patients to use insulin. Type 2 DM “adult-onset diabetes or noninsulin-dependent diabetes mellitus results from insulin resistance, a condition in which cells cannot use insulin properly. Gestational diabetes occurs when pregnant women develop a high blood glucose level without a previous diagnosis of diabetes. This kind of diabetes may precede the development of type 2 DM. Other forms of DM include steroid diabetes induced by high doses of glucocorticoids, congenital diabetes, which is due to genetic defects of insulin secretion, cystic fibrosis-related diabetes, and several forms of monogenic diabetes.[1,2,3,4,5,6,7]
DIABETES MELLITUS COMPLICATIONS
The patients with DM are at increased risk of complications such as peripheral vascular disease, retinopathy, nephropathy, neuropathy, coronary heart disease. The exact causes of type 2 diabetes are still need to be clear.[1,2,3,4,5,6,7] DM increases the risk of complications, which may develop after 10-20 years, however, may be the first symptom in patients who have not diagnosed before that time. The major long-term complications are related to damage to blood vessels. DM approximately doubles the risk of cardiovascular diseases. The main “macro-vascular” diseases are peripheral vascular disease, angina, myocardial infarction and stroke.[1,2,3,4,5,6,7,20]
Diabetes mellitus damages the capillaries causing micro-angiopathy. Diabetic retinopathy, which affects blood vessel in the eye retina, causes visual symptoms including reduced vision and blindness.[21,22,23] Diabetic nephropathy usually leads to changes in the kidney tissue, loss of progressively larger amounts of protein in the urine and chronic kidney disease.[21,22,23,24]
Diabetic neuropathy commonly causes tingling, numbness and pain in the feet. It also increases the risk of skin damage due to altered sensation. Vascular complications in the legs contribute to the risk of diabetes-related foot problems such as diabetic foot ulcers that might be difficult to treat and occasionally require amputation.[5,21,22,23,24]
Compared to the subjects without diabetes, those with the disease have about 1.5-fold greater rate of deficit in cognitive function, and herbal medicines with hypoglycemic activities have been shown to counteract this complication.[21,22,23,24,25,26]
DIABETES MELLITUS PATHOGENESIS
The cause of diabetes depends on the type of DM. Type 1 is, at least in part, inherited. It may also be triggered by certain toxins or infections. In patients susceptibility to some of these triggers a genetic element has been traced to particular HLA genotypes. However, even in patients genetically susceptible, type 1 DM usually requires an environmental trigger. In contrast to type 1 DM in which its onset is unrelated to lifestyle, type 2 DM is primarily due to lifestyle factors other than genetics. The most important lifestyle factors, which are known to be involved in the development of type 2 DM include: Urbanization, poor diet, lack of physical activity, stress, and obesity or body mass index of >30.[1,2,3,4]
Dietary factors also seem to have influence on development of type 2 DM. Consumption of drinks sweetened in excess increases the risk of type 2 DM. Trans fatty acids and saturated fats also increase the risk. In contrast monounsaturated and polyunsaturated fat decrease the risk.[1,5,27,28] Lack of exercise dramatically increases the risk of cases.[1,5,27,28]
DIABETES MELLITUS MANAGEMENT
There is no known cure for DM except in very specific situations. Management of DM concentrates mostly on keeping blood sugar to normal levels as possible, which is usually accomplished with exercise, diet, and use of appropriate medications.[1,5,27,28]
The complications of diabetes are less common and less severe in patients who have well-managed blood sugar levels. Therefore, patient participation is vital. The goal of treatment is keeping an HbA1C level of 6.5%, however, it should not be less than that.[1,5,27,28,29] Attention should also be paid to other factors which may accelerate the deleterious effects of diabetes, including elevated cholesterol level, obesity, high blood pressure, smoking, and lack of regular exercise.[27,28,29]
Several lines of medications are used in the treatment of MD [Table 1]. The current used therapies for type-2 DM include sulfonylureas, biguanides, inhibitors of a-glucosidase, thiazolidinediones, and inhibitors of dipeptidyl peptidase-4. Metformin is generally used as first line treatment for type 2 DM, as it has shown to decrease mortality rate.[30] When blood sugar is very high and insulin is used in type 2 diabetes, usually a long-acting drug is added initially, while continuing oral medications Type 1 DM is typically treated with synthetic insulin and usually a combination of regular and NPH insulin.[30]
Table 1.
Oral anti-diabetic drugs currently available for the treatment of diabetes mellitus
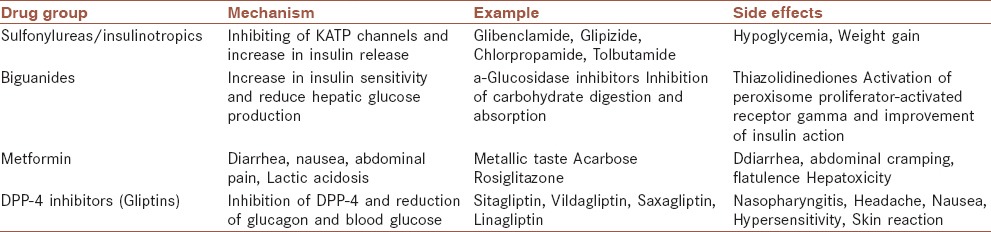
The available synthetic drugs for the treatment of DM mostly are expensive and produce serious side effects [Table 1]. Hence, safer and more effective anti-diabetic drugs are urgently needed. Nowadays medicinal plants with antioxidant activity have been on the focus of the researchers for their hypoglycemic activities[31] or for reduction of the side-effects of hypoglycemic drugs.[31,32,33]
ANTIOXIDANT AND OTHER THERAPIES
As the pathogenesis of DM involves oxidative stress, antioxidant therapies should have a potential value in its treatment. Many trials in animal models of diabetes and diabetic patients have attempted to determine the role of antioxidant therapy on prevention or treatment of diabetes complications.[32,33,34]
Furthermore, significant increase in endogenous prooxidant activity and decrease in antioxidants has been shown to contribute to the oxidative stress in diabetes. A marked decrease in glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) activities have been reported in diabetic animals.[30,31,32,33,34] Treatment with probucol, which has antioxidant activity resulted in a significant improvement in myocardial activities of catalase, SOD and GSHPx (antioxidant enzymes) providing evidence that diabetic cardiomyopathy was associated with an antioxidant deficit.[30,31,32,33,34,35] Overexpression of catalase in STZ-treated transgenic mice attenuated the onset of diabetic complications, indicating the therapeutic potential of catalase.[32,33,34,35,36]
Several pharmacologic agents effective in reducing diabetic mortalities have been shown to have antioxidant activities. For example, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, angiotensin-converting enzyme (ACE) inhibitors or statins have beneficial effects on diabetic patients[30,31,32,33,34,35,36,37] that may involve antioxidant effects. Interestingly, ACE inhibitors, which act partially to prevent the prooxidant effects of angiotensin II, were shown to prevent the onset of type 2 diabetes.[32,33,34,35,36,37,38] Vitamin E supplementation has been associated with a significant decline in protein oxidation, lipid peroxidation and enhancement in the antioxidant defense system. Vitamin E may promote beneficial effects on diabetic complications through the attenuation of oxidative stress.[35,36,37,38,39]
Peroxynitrite and other reactive species can also induce oxidative DNA damage. Inhibitors of specific components of ROS-sensitive signaling cascades such as CGP53353 and ruboxistaurin, which are specific inhibitors of protein kinase C, are able to attenuate hyperglycemia-induced vascular cell adhesion molecule-1 expression and nuclear factor-kappa-B activation in human aortic endothelial cells.[30,31,32,33,34,35,36,37,38,39,40]
Coenzyme Q10, a lipid-soluble antioxidant, has been shown to scavenge superoxide and improve endothelial function in diabetes. Caffeic acid phenethyl ester (CAPE), a flavonoid-like compound, has an ameliorating effect on oxidative stress in cardiac tissue via its antioxidant property, indicating that CAPE should be considered for preventing oxidative stress in the diabetic heart.[36,37,38,39,40,41]
Medicinal plants with antioxidant activities have also been shown to be protective in diabetic rats by scavenging oxygen free radicals and decreasing the expressions of intercellular cell adhesion molecule-1 protein.[35,36,37,38,39,40,41,42]
CLINICAL PERSPECTIVES OF ANTIOXIDANT THERAPY
Despite several experimental studies suggesting beneficial effects antioxidants in reduction of diabetes complications, results from clinical trials on beneficial effects of traditional antioxidants such as Vitamin E or C have been disappointing.[40,41,42,43] A meta-analysis of clinical trials, studying Vitamin E therapy suggests that the use of high-dose Vitamin E (greater than 400 IU/day) may actually increase mortality;[41,42,43,44] however, this finding has been questioned.[42,43,44,45] Zinc and melatonin in combination with a regularly used metformin have been shown to significantly reduce fasting glucose and glycated hemoglobin levels in patients with type 2 diabetes.[43,44,45,46] However, not all studies supported this notion. Several studies indicated no improvement in the glucose metabolism in either type 1 or type 2 diabetic patients after zinc treatment.[44,45,46,47]
These contradictory results may have emerged from a variety of factors, such as patient diversity and zinc speciation.
Although initial studies have suggested that antioxidant supplementation might promote health, however, large clinical trials declared no benefit and even suggested that excess supplementation with certain antioxidants might be harmful.[40,41,42,43,44,45,46,47,48] From the literature review it might be concluded that supplementation with single antioxidant may not be beneficial, but the diets high in antioxidants (fruits and vegetables) are nearly always useful. The possible explanation is that, in fruits and vegetables there are mixture of antioxidants and it is well recognized that they work as a continuous chain, while supplementation is usually given using one or two substances. Therefore, the antioxidant chain is not completely available.[40,41,42,43,44,45,46,47,48] In this situation, after scavenging free radicals, if an antioxidant is not restored by the following suitable antioxidant in the chain, it begins to be a pro-oxidant. Hence, the final effect of such supplementations would be no effect or damaging.[40,41,42,43,44,45,46,47,48,49] Therefore, in antioxidant therapy complimentary antioxidants cannot always substitute the fruits and vegetables high in antioxidants. However, consumption of vegetable and fruits as well as medicinal plants with high antioxidant content is recommended.[40,41,42,43,44,45,46,47,48,49,50]
MEDICINAL PLANTS WITH ANTI-DIABETIC ACTIVITIES
The results of the studies suggest a trend towards the benefit of consuming vegetables and fruits consumption in DM.[48,49,50,51] Several studies examining dietary patterns and incidence of type 2 diabetes have also shown that vegetables and fruits are important components of the dietary patterns associated with a decreased risk of type 2 diabetes.[48,49,50,51,52]
A possible benefit of vegetables and fruit is from their antioxidant components and thus a contribution to reduction of systemic oxidative stress.[50,51,52,53] Vegetables and fruits have been shown to contain high concentrations of antioxidants, which might reduce the risk of diabetes especially type 2 DM. Vegetables and fruits are also good sources of α linolenic acid, an omega 3 polyunsaturated fatty acid.[49,50,51,52,53,54]
Medicinal plants also have played an important role in the management of DM, worldwide [Table 2]. Medicinal plants have a long history in the treatment of diseases. In traditional medicine, about 800 plants are used for the treatment of DM.[50,51,52,53,54,55]
Table 2.
Anti-diabetic plants
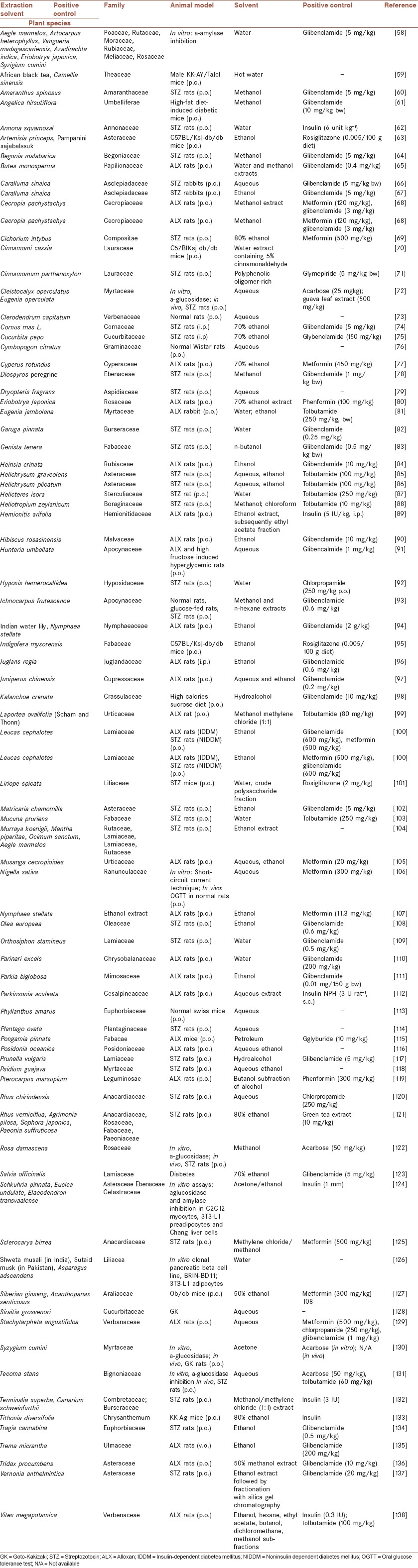
With rapid advancement of technologies and the increase in research on anti-diabetic plants, many new herbs and their active principles have been discovered which may lead us to develop novel anti-diabetic agents to supplement the current chemotherapies. Jung et al. (2006) reviewed the hypoglycemic effects of several plants with anti-diabetic properties, as well as the plants by-products discovered during 2001-2005 having anti-diabetic actions.[55,56,57] In this paper, the newly identified anti-diabetic plants (2005-2013) are summarized in Table 2, in which the reliable hypoglycemic plants are included. Although in many cases these agents have the same mechanism as synthetic agents act, however, some of them may act with a different way. These probable mechanisms should be evaluated when searching new agents and their mechanisms of actions.
DISCUSSION AND CONCLUSION
Medicinal plants have a long history in the treatment of diseases including DM.[50,51,52,53,54,55] The beneficial effects of medicinal plants in DM have been confirmed in several studies.
In this paper, the newly identified anti-diabetic plants (2005-2013) were summarized in Table 2. Although in many cases the mechanism actions of these agents were presented and it was shown that they may have the same mechanism as synthetic agents act, however, the exact mechanism action of these drugs are poorly established. Hence, more works are needed to realize the exact mechanisms of these plants.
A possible mechanism and benefit of medicinal plants is from their antioxidant activities. Most of medicinal plants with anti-diabetic property possess antioxidant activity.[50,51,52,53] In this regards, it has been confirmed that vegetables and fruits, in comparison to synthetic antioxidants, are more effective and are able to decrease the risk of DM.[48,49,50,51,52]
It has been shown that under stressful conditions free radicals are over-produced, inducing oxidative stress. Oxidative stress occurs when there is an imbalance between free radical formation and antioxidant defense capacity.[139,140,141,142,143] This oxidative stress usually causes or exacerbates chronic hard curable diseases such as diabetes,[144,145,146,147,148] hypertension,[149,150] cardiovascular[151,152,153] cancer,[154,155,156] cognitive diseases,[157,158,159,160] and pain[161,162,163,164,165] or exacerbation of some other diseases like infectious disorders.[166,167,168,169,170,171,172]
Although, in some cases, synthetic antioxidants have also been effective in reduction of DM, however, in contrast to natural antioxidants, synthetic antioxidants usually produce side effects such as toxicity. Hence, preparation of natural products with antioxidant activities with property to prevent and treat free radical-associated diseases is essential.[172,173,174] Other than the plants which were introduced here, a lot of other plants have antioxidant activities.[175,176,177,178,179,180,181]
These plants have drawn much attraction because they have protective or curative properties against most of hard curable diseases such as cognitive deficit, memory impairment, cancer, and cardiovascular diseases which have been attributed to their antioxidant activities.[182,183,184,185,186,187,188,189,190] Therefore, they also might be effective on DM.
AUTHOR'S CONTRIBUTIONS
HN, HSh, MRK contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AB, MRK contributed in the design of the work, Editing the final version, approval of the final version of the manuscript, and agreed for all aspects of the work. All authors wrote the manuscript equally.
ACKNOWLEDGMENT
Thanks to Research Deputy of Shahrekord University of Medical Sciences for support. There is no grant for this work.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
REFERENCES
- 1.Shoback D, Gardner DG, editors. 9th ed. Ch. 17. New York: McGraw-Hill Medical; 2011. Greenspan's Basic & Clinical Endocrinology. [Google Scholar]
- 2.Nasri H. On the occasion of the world diabetes day 2013; diabetes education and prevention; a nephrology point of view. J Renal Inj Prev. 2013;2:31–2. doi: 10.12861/jrip.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahbazian H. World diabetes day; 2013. J Renal Inj Prev. 2013;2:123–4. doi: 10.12861/jrip.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasri H, Rafieian-Kopaei M. Herbal medicine and diabetic kidney disease. J Nephropharmacol. 2013;2:1–2. [PMC free article] [PubMed] [Google Scholar]
- 5.Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathol. 2013;2:20–7. doi: 10.5812/nephropathol.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasri H, Sahinfard N, Rafieian M, Rafieian S, Shirzad M, Rafieian-kopaei M. Effects of Allium sativum on liver enzymes and atherosclerotic risk factors. J HerbMed Pharmacol. 2013;2:23–8. [Google Scholar]
- 7.Tamadon MR, Baradaran A, Rafieian-Kopaei M. Antioxidant and kidney protection; differential impacts of single and whole natural antioxidants. J Renal Inj Prev. 2014;3:41–2. doi: 10.12861/jrip.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolouian R, T Hernandez G. Prediction of diabetic nephropathy: The need for a sweet biomarker. J Nephropathol. 2013;2:4–5. doi: 10.5812/nephropathol.8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasri H, Yazdani M. The relationship between serum LDL-cholesterol, HDL-cholesterol and systolic blood pressure in patients with type 2 diabetes. Kardiol Pol. 2006;64:1364–8. [PubMed] [Google Scholar]
- 10.Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Plarmacol. 2013;2:21–2. [Google Scholar]
- 11.Mogharabi M, Abdollahi M, Faramarzi MA. Safety concerns to application of graphene compounds in pharmacy and medicine. Daru. 2014:22–23. doi: 10.1186/2008-2231-22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafieian-Kopaei M, Baradaran A, Rafieian M. Plants antioxidants: From laboratory to clinic. J Nephropathol. 2013;2:152–3. doi: 10.12860/JNP.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mardani S, Nasri H, Hajian S, Ahmadi A, Kazemi R, Rafieian-Kopaei M. Impact of Momordica charantia extract on kidney function and structure in mice. J Nephropathol. 2014;3:35–40. doi: 10.12860/jnp.2014.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasri H. Impact of diabetes mellitus on parathyroid hormone in hemodialysis patients. J Parathyr Dis. 2013;1:9–11. [Google Scholar]
- 15.Rahimi Z, Mansouri Zaveleh O, Rahimi Z, Abbasi A. AT2R-1332 G: A polymorphism and diabetic nephropathy in type 2 diabetes mellitus patients. J Renal Inj Prev. 2013;2:97–101. doi: 10.12861/jrip.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardalan MR, Rafieian-Kopaei M. Is the safety of herbal medicines for kidneys under question? J Nephropharmacol. 2013;2:11–2. [PMC free article] [PubMed] [Google Scholar]
- 17.Roshan B, Stanton RC. A story of microalbuminuria and diabetic nephropathy. J Nephropathol. 2013;2:234–40. doi: 10.12860/JNP.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kafeshani M. Ginger, micro-inflammation and kidney disease. J Renal Endocrinol. 2015;1:e04. [Google Scholar]
- 19.Nasri H, Behradmanesh S, Ahmadi A, Rafieian-Kopaei M. Impact of oral Vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol. 2014;3:29–33. doi: 10.12860/jnp.2014.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asadi-Samani M, Bahmani M, Rafieian-Kopaei M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pac J Trop Med. 2014;7(Suppl 1):22–28. doi: 10.1016/S1995-7645(14)60199-1. [DOI] [PubMed] [Google Scholar]
- 21.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafieian-Kopaei M, Nasri H. Vitamin D therapy in diabetic kidney disease. J Nephropharmacol. 2014;3:3–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki M. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med. 2014;7(Suppl 1):348–354. doi: 10.1016/S1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- 24.Rouhi H, Ganji F. Effects of N-acetyl cysteine on serum lipoprotein (a) and proteinuria in type 2 diabetic patients. J Nephropathol. 2013;2:61–6. doi: 10.5812/nephropathol.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafieian-Kopaei M, Behradmanesh S, Kheiri S, Nasri H. Association of serum uric acid with level of blood pressure in type 2 diabetic patients. Iran J Kidney Dis. 2014;8:152–4. [PubMed] [Google Scholar]
- 26.Ardalan MR, Sanadgol H, Nasri H, Baradaran A, Tamadon MR, Rafieian-Kopaei R. Impact of Vitamin D on the immune system in kidney disease. J Parathyr Dis. 2013;1:17–20. [Google Scholar]
- 27.Ajabshir S, Asif A, Nayer A. The effects of Vitamin D on the renin-angiotensin system. J Nephropathol. 2014;3:41–3. doi: 10.12860/jnp.2014.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasri H. Correlation of serum magnesium with serum levels of 25-hydroxyvitamin D in hemodialysis patients. J Parathyr Dis. 2014;2:11–3. [Google Scholar]
- 29.Nasri H, Behradmanesh S, Ahmadi A, Baradaran A, Nasri P, Rafieian-Kopaei M. Association of serum lipids with level of blood pressure in type 2 diabetic patients. J Renal Inj Prev. 2014;3:43–6. doi: 10.12861/jrip.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasri H, Rafieian-Kopaei M. Metformin improves diabetic kidney disease. J Nephropharmacol. 2012;1:1–2. [PMC free article] [PubMed] [Google Scholar]
- 31.Ayodhya S, Kusum S, Saxena A. Hypoglycaemic activity of different extracts of various herbal plants. Int J Res Ayurveda Pharm. 2010:1–212. [Google Scholar]
- 32.Abdollahi M, Farshchi A, Nikfar S, Seyedifar M. Effect of chromium on glucose and lipid profiles in patients with type 2 diabetes; a meta-analysis review of randomized trials. J Pharm Pharm Sci. 2013;16:99–114. doi: 10.18433/j3g022. [DOI] [PubMed] [Google Scholar]
- 33.Rafieian-Kopaei M, Nasri H. Ginger and diabetic nephropathy. J Renal Inj Prev. 2013;2:9–10. doi: 10.12861/jrip.2013.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasri H, Rafieian-Kopaei M. Tubular kidney protection by antioxidants. Iran J Public Health. 2013;42:1194–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Ardalan MR, Sanadgol H, Nasri H, Baradaran A, Tamadon MR, Rafieian-Kopaei R. Vitamin D therapy in diabetic kidney disease; current knowledge on a public health problem. J Parathyr Dis. 2014;2:15–7. [Google Scholar]
- 36.Turdi S, Li Q, Lopez FL, Ren J. Catalase alleviates cardiomyocyte dysfunction in diabetes: Role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci. 2007;81:895–905. doi: 10.1016/j.lfs.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Rafieian-Kopaie M. Metformin and renal injury protection. J Renal Inj Prev. 2013;2:91–2. doi: 10.12861/jrip.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eurich DT, Majumdar SR, Tsuyuki RT, Johnson JA. Reduced mortality associated with the use of ACE inhibitors in patients with type 2 diabetes. Diabetes Care. 2004;27:1330–4. doi: 10.2337/diacare.27.6.1330. [DOI] [PubMed] [Google Scholar]
- 39.Rafieian-Kopaei M, Baradaran A. Combination of metformin with other antioxidants may increase its renoprotective efficacy. J Renal Inj Prev. 2013;2:35–6. doi: 10.12861/jrip.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahimi Z. ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol. 2012;1:143–51. doi: 10.5812/nephropathol.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavafi M. Complexity of diabetic nephropathy pathogenesis and design of investigations. J Renal Inj Prev. 2013;2:59–62. doi: 10.12861/jrip.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru. 2012:20–56. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of Vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: A randomized controlled trial. Am J Clin Nutr. 2009;90:429–37. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage Vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 45.Greenland S. Weaknesses of Bayesian model averaging for meta-analysis in the study of Vitamin E and mortality. Clin Trials. 2009;6:42–6. doi: 10.1177/1740774509103251. [DOI] [PubMed] [Google Scholar]
- 46.Fikree M, Hanafi B, Hussain ZA, Masuadi EM. Glycemic control of type 2 diabetes mellitus. Bahrain Med Bull. 2006;28:1–6. [Google Scholar]
- 47.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–15. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 48.Rafieian-Kopaei M, Baradaran A, Rafieian M. Oxidative stress and the paradoxical effects of antioxidants. J Res Med Sci. 2013:18–629. [PMC free article] [PubMed] [Google Scholar]
- 49.Hajivandi A, Amiri M. World kidney day 2014: Kidney disease and elderly. J Parathyr Dis. 2014;2:3–4. [Google Scholar]
- 50.Rafieian-Kopaei M, Baradaran A. Teucrium polium and kidney. J Renal Inj Prev. 2013;2:3–4. doi: 10.12861/jrip.2013.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamadon MR, Ardalan MR, Nasri H. World kidney day 2013; acute renal injury; a global health warning. J Parathyr Dis. 2013;1:27–8. [Google Scholar]
- 52.Rafieian-Kopaie M, Nasri H. Silymarin and diabetic nephropathy. J Renal Inj Prev. 2012;1:3–5. doi: 10.12861/jrip.2012.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: Implications for metabolism and disease. Biol Rev Camb Philos Soc. 2005;80:155–69. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- 54.Nasri H, Behradmanesh S, Maghsoudi AR, Ahmadi A, Nasri P, Rafieian-Kopaei M. Efficacy of supplementary Vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J Renal Inj Prev. 2014;3:31–4. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 56.Asgari A. Herbal medicines and kidney; friends or foes? J Nephropharmacol. 2014;3:5–6. [PMC free article] [PubMed] [Google Scholar]
- 57.Kotowaroo MI, Mahomoodally MF, Gurib-Fakim A, Subratty AH. Screening of traditional antidiabetic medicinal plants of Mauritius for possible alpha-amylase inhibitory effects in vitro. Phytother Res. 2006;20:228–31. doi: 10.1002/ptr.1839. [DOI] [PubMed] [Google Scholar]
- 58.Shoji Y, Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Arch Pharm Res. 2006;29:786–94. doi: 10.1007/BF02974080. [DOI] [PubMed] [Google Scholar]
- 59.Sangameswaran B, Jayakar B. Anti-diabetic, anti-hyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn. On streptozotocin-induced diabetic rats. J Nat Med. 2008;62:79–82. doi: 10.1007/s11418-007-0189-9. [DOI] [PubMed] [Google Scholar]
- 60.Leu YL, Chen YW, Yang CY, Huang CF, Lin GH, Tsai KS, et al. Extract isolated from Angelica hirsutiflora with insulin secretagogue activity. J Ethnopharmacol. 2009;123:208–12. doi: 10.1016/j.jep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 61.Ajikumaran Nair S, Shylesh BS, Gopakumar B, Subramoniam A. Anti-diabetes and hypoglycaemic properties of Hemionitis arifolia (Burm.) Moore in rats. J Ethnopharmacol. 2006;106:192–7. doi: 10.1016/j.jep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Jung UJ, Baek NI, Chung HG, Bang MH, Yoo JS, Jeong TS, et al. The anti-diabetic effects of ethanol extract from two variants of Artemisia princeps Pampanini in C57BL/KsJ-db/db mice. Food Chem Toxicol. 2007;45:2022–9. doi: 10.1016/j.fct.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Pandikumar P, Babu NP, Ignacimuthu S. Hypoglycemic and antihyperglycemic effect of Begonia malabarica Lam. in normal and streptozotocin induced diabetic rats. J Ethnopharmacol. 2009;124:111–5. doi: 10.1016/j.jep.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Somani R, Kasture S, Singhai AK. Antidiabetic potential of Butea monosperma in rats. Fitoterapia. 2006;77:86–90. doi: 10.1016/j.fitote.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Krisanapun C, Peungvicha P, Temsiririrkkul R, Wongkrajang Y. Aqueous extract of Abutilon indicum Sweet inhibits glucose absorption and stimulates insulin secretion in rodents. Nutr Res. 2009;29:579–87. doi: 10.1016/j.nutres.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Habibuddin M, Daghriri HA, Humaira T, Al Qahtani MS, Hefzi AA. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on streptozotocin-induced diabetic rabbits. J Ethnopharmacol. 2008;117:215–20. doi: 10.1016/j.jep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Aragão DM, Guarize L, Lanini J, da Costa JC, Garcia RM, Scio E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 2010;128:629–33. doi: 10.1016/j.jep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Kamtchouing P, et al. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol. 2007;110:434–8. doi: 10.1016/j.jep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–23. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 70.Park SH, Ko SK, Choi JG, Chung SH. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch Pharm Res. 2006;29:256–64. doi: 10.1007/BF02969402. [DOI] [PubMed] [Google Scholar]
- 71.Mai TT, Chuyen NV. Anti-hyperglycemic activity of an aqueous extract from flower buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci Biotechnol Biochem. 2007;71:69–76. doi: 10.1271/bbb.60373. [DOI] [PubMed] [Google Scholar]
- 72.Adeneye AA, Adeleke TI, Adeneye AK. Hypoglycemic and hypolipidemic effects of the aqueous fresh leaves extract of Clerodendrum capitatum in Wistar rats. J Ethnopharmacol. 2008;116:7–10. doi: 10.1016/j.jep.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 73.Shamsi F, Asgari S, Rafieian R, Kazemi S. Effects of Cornus mas L. on blood glucose, insulin and histopathology of pancreas in alloxan-induced diabetic rats. J Isfahan Med Sch. 2011;29:927–37. [Google Scholar]
- 74.Kazemi S, Asgari S, Moshtaghian SJ, Rafieian-Kopaei M, Mahzooni P. Preventive effect of Pumpkin (Cucurbita pepo L.) on diabetic index and histopathology of pancreas in Alloxan-induced diabetes in rats. J Isfahan Med Sch. 2011;28:872–81. [Google Scholar]
- 75.Adeneye AA, Agbaje EO. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J Ethnopharmacol. 2007;112:440–4. doi: 10.1016/j.jep.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 76.Raut NA, Gaikwad NJ. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia. 2006;77:585–8. doi: 10.1016/j.fitote.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47:2679–85. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 78.Khookhor O, Bolin Q, Oshida Y, Sato Y. Effect of Mongolian plants on in vivo insulin action in diabetic rats. Diabetes Res Clin Pract. 2007;75:135–40. doi: 10.1016/j.diabres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 79.Li WL, Wu JL, Ren BR, Chen J, Lu CG. Pharmacological studies on anti-hyperglycemic effect of folium eriobotryae. Am J Chin Med. 2007;35:705–11. doi: 10.1142/S0192415X07005193. [DOI] [PubMed] [Google Scholar]
- 80.Sharma SB, Nasir A, Prabhu KM, Murthy PS. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol. 2006;104:367–73. doi: 10.1016/j.jep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 81.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107:285–90. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 82.Rauter AP, Martins A, Lopes R, Ferreira J, Serralheiro LM, Araújo ME, et al. Bioactivity studies and chemical profile of the antidiabetic plant Genista tenera. J Ethnopharmacol. 2009;122:384–93. doi: 10.1016/j.jep.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Okokon JE, Umoh EE, Etim EI, Jackson CL. Antiplasmodial and antidiabetic activities of ethanolic leaf extract of Heinsia crinata. J Med Food. 2009;12:131–6. doi: 10.1089/jmf.2008.0116. [DOI] [PubMed] [Google Scholar]
- 84.Aslan M, Orhan DD, Orhan N, Sezik E, Yeºilada E. A study of antidiabetic and antioxidant effects of Helichrysum graveolens capitulums in streptozotocin-induced diabetic rats. J Med Food. 2007;10:396–400. doi: 10.1089/jmf.2006.293. [DOI] [PubMed] [Google Scholar]
- 85.Aslan M, Deliorman Orhan D, Orhan N, Sezik E, Yesilada E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J Ethnopharmacol. 2007;109:54–9. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Kumar G, Banu GS, Murugesan AG, Pandian MR. Hypoglycaemic effect of Helicteres isora bark extract in rats. J Ethnopharmacol. 2006;107:304–7. doi: 10.1016/j.jep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 87.Murugesh K, Yeligar V, Dash DK, Sengupta P, Maiti BC, Maity TK. Antidiabetic, antioxidant and antihyperlipidemic status of Heliotropium zeylanicum extract on streptozotocin-induced diabetes in rats. Biol Pharm Bull. 2006;29:2202–5. doi: 10.1248/bpb.29.2202. [DOI] [PubMed] [Google Scholar]
- 88.Kamtchouing P, Kahpui SM, Dzeufiet PD, Tédong L, Asongalem EA, Dimo T. Anti-diabetic activity of methanol/methylene chloride stem bark extracts of Terminalia superba and Canarium schweinfurthii on streptozotocin-induced diabetic rats. J Ethnopharmacol. 2006;104:306–9. doi: 10.1016/j.jep.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesh S, Thilagavathi J, Shyam Sundar D. Anti-diabetic activity of flowers of Hibiscus rosasinensis. Fitoterapia. 2008;79:79–81. doi: 10.1016/j.fitote.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Adeneye AA, Adeyemi OO. Further evaluation of antihyperglycaemic activity of Hunteria umbellata (K. Schum) Hallier f. seed extract in experimental diabetes. J Ethnopharmacol. 2009;126:238–43. doi: 10.1016/j.jep.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 91.Ojewole JA. Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [‘African Potato’] aqueous extract in mice and rats. J Ethnopharmacol. 2006;103:126–34. doi: 10.1016/j.jep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Subash-Babu P, Ignacimuthu S, Agastian P. Insulin secretagogue effect of Ichnocarpus frutescence leaf extract in experimental diabetes: A dose-dependent study. Chem Biol Interact. 2008;172:159–71. doi: 10.1016/j.cbi.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Rajagopal K, Sasikala K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats. Singapore Med J. 2008;49:137–41. [PubMed] [Google Scholar]
- 94.Chakrabarti R, Damarla RK, Mullangi R, Sharma VM, Vikramadithyan RK, Rajagopalan R. Insulin sensitizing property of Indigofera mysorensis extract. J Ethnopharmacol. 2006;105:102–6. doi: 10.1016/j.jep.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 95.Asgary S, Parkhideh S, Solhpour A, Madani H, Mahzouni P, Rahimi P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J Med Food. 2008;11:533–8. doi: 10.1089/jmf.2007.0611. [DOI] [PubMed] [Google Scholar]
- 96.Ju JB, Kim JS, Choi CW, Lee HK, Oh TK, Kim SC. Comparison between ethanolic and aqueous extracts from Chinese juniper berries for hypoglycaemic and hypolipidemic effects in alloxan-induced diabetic rats. J Ethnopharmacol. 2008;115:110–5. doi: 10.1016/j.jep.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 97.Kamgang R, Mboumi RY, Fondjo AF, Tagne MA, N'dillé GP, Yonkeu JN. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (Crassulaceae) J Nat Med. 2008;62:34–40. doi: 10.1007/s11418-007-0179-y. [DOI] [PubMed] [Google Scholar]
- 98.Momo CE, Oben JE, Tazoo D, Dongo E. Antidiabetic and hypolipidaemic effects of a methanol/methylene-chloride extract of Laportea ovalifolia (Urticaceae), measured in rats with alloxan-induced diabetes. Ann Trop Med Parasitol. 2006;100:69–74. doi: 10.1179/136485906X78517. [DOI] [PubMed] [Google Scholar]
- 99.Bavarva JH, Narasimhacharya AV. Leucas cephalotes regulates carbohydrate and lipid metabolism and improves antioxidant status in IDDM and NIDDM rats. J Ethnopharmacol. 2010;127:98–102. doi: 10.1016/j.jep.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, Bai X, Liu Y, Tian L, Zhou J, Zhou Q, et al. Anti-diabetic effects of water extract and crude polysaccharides from tuberous root of Liriope spicata var. prolifera in mice. J Ethnopharmacol. 2009;122:205–9. doi: 10.1016/j.jep.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 101.Cemek M, Kaga S, Simsek N, Büyükokuroglu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med. 2008;62:284–93. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 102.Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia. 2008;79:539–43. doi: 10.1016/j.fitote.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Narendhirakannan RT, Subramanian S, Kandaswamy M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin Exp Pharmacol Physiol. 2006;33:1150–7. doi: 10.1111/j.1440-1681.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 104.Adeneye AA, Ajagbonna OP, Ayodele OW. Hypoglycemic and antidiabetic activities on the stem bark aqueous and ethanol extracts of Musanga cecropioides in normal and alloxan-induced diabetic rats. Fitoterapia. 2007;78:502–5. doi: 10.1016/j.fitote.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–24. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 106.Dhanabal SP, Raja MK, Ramanathan M, Suresh B. Hypoglycemic activity of Nymphaea stellata leaves ethanolic extract in alloxan induced diabetic rats. Fitoterapia. 2007;78:288–91. doi: 10.1016/j.fitote.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Eidi A, Eidi M, Darzi R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother Res. 2009;23:347–50. doi: 10.1002/ptr.2629. [DOI] [PubMed] [Google Scholar]
- 108.Sriplang K, Adisakwattana S, Rungsipipat A, Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;109:510–4. doi: 10.1016/j.jep.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 109.Ndiaye M, Diatta W, Sy AN, Dièye AM, Faye B, Bassène E. Antidiabetic properties of aqueous barks extract of Parinari excelsa in alloxan-induced diabetic rats. Fitoterapia. 2008;79:267–70. doi: 10.1016/j.fitote.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Odetola AA, Akinloye O, Egunjobi C, Adekunle WA, Ayoola AO. Possible antidiabetic and antihyperlipidaemic effect of fermented Parkia biglobosa (JACQ) extract in alloxan-induced diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:808–12. doi: 10.1111/j.1440-1681.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 111.Oliveira HC, dos Santos MP, Grigulo R, Lima LL, Martins DT, Lima JC, et al. Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol. 2008;115:515–9. doi: 10.1016/j.jep.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 112.Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia. 2006;77:511–4. doi: 10.1016/j.fitote.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 113.Hannan JM, Ali L, Khaleque J, Akhter M, Flatt PR, Abdel-Wahab YH. Aqueous extracts of husks of Plantago ovata reduce hyperglycaemia in type 1 and type 2 diabetes by inhibition of intestinal glucose absorption. Br J Nutr. 2006;96:131–7. doi: 10.1079/bjn20061819. [DOI] [PubMed] [Google Scholar]
- 114.Badole SL, Bodhankar SL. Investigation of antihyperglycaemic activity of aqueous and petroleum ether extract of stem bark of Pongamia pinnata on serum glucose level in diabetic mice. J Ethnopharmacol. 2009;123:115–20. doi: 10.1016/j.jep.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 115.Gokce G, Haznedaroglu MZ. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol. 2008;115:122–30. doi: 10.1016/j.jep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 116.Zheng J, He J, Ji B, Li Y, Zhang X. Antihyperglycemic activity of Prunella vulgaris L. in streptozotocin-induced diabetic mice. Asia Pac J Clin Nutr. 2007;16(Suppl 1):427–31. [PubMed] [Google Scholar]
- 117.Shen SC, Cheng FC, Wu NJ. Effect of guava (Psidium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother Res. 2008;22:1458–64. doi: 10.1002/ptr.2476. [DOI] [PubMed] [Google Scholar]
- 118.Dhanabal SP, Kokate CK, Ramanathan M, Kumar EP, Suresh B. Hypoglycaemic activity of Pterocarpus marsupium Roxb. Phytother Res. 2006;20:4–8. doi: 10.1002/ptr.1819. [DOI] [PubMed] [Google Scholar]
- 119.Ojewole JA. Analgesic, anti-inflammatory and hypoglycaemic effects of Rhus chirindensis (Baker F.) [Anacardiaceae] stem-bark aqueous extract in mice and rats. J Ethnopharmacol. 2007;113:338–45. doi: 10.1016/j.jep.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 120.Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on alpha-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16:935–41. doi: 10.1016/j.phymed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 121.Lima CF, Azevedo MF, Araujo R, Fernandes-Ferreira M, Pereira-Wilson C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br J Nutr. 2006;96:326–33. doi: 10.1079/bjn20061832. [DOI] [PubMed] [Google Scholar]
- 122.Deutschländer MS, van de Venter M, Roux S, Louw J, Lall N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J Ethnopharmacol. 2009;124:619–24. doi: 10.1016/j.jep.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 123.Behradmanesh S, Derees F, Rafieian-kopaei M. Effect of Salvia officinalis on diabetic patients. J Ren Inj Prev 2013. 2(2):51–54. doi: 10.12861/jrip.2013.18. DOI: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Kamtchouing P, et al. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol. 2007;110:434–8. doi: 10.1016/j.jep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 125.Mathews JN, Flatt PR, Abdel-Wahab YH. Asparagus adscendens (Shweta musali) stimulates insulin secretion, insulin action and inhibits starch digestion. Br J Nutr. 2006;95:576–81. doi: 10.1079/bjn20051650. [DOI] [PubMed] [Google Scholar]
- 126.Park SH, Lee SG, Kang SK, Chung SH. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch Pharm Res. 2006;29:768–76. doi: 10.1007/BF02974078. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki YA, Tomoda M, Murata Y, Inui H, Sugiura M, Nakano Y. Antidiabetic effect of long-term supplementation with Siraitia grosvenori on the spontaneously diabetic Goto-Kakizaki rat. Br J Nutr. 2007;97:770–5. doi: 10.1017/S0007114507381300. [DOI] [PubMed] [Google Scholar]
- 128.Isah AB, Ibrahim YK, Abdulrahman EM, Ibrahim MA. The hypoglycaemic activity of the aqueous extract of Stachytarpheta angustifolia (Verbanaceae) in normoglycaemic and alloxan-induced diabetic rats. Pak J Biol Sci. 2007;10:137–41. doi: 10.3923/pjbs.2007.137.141. [DOI] [PubMed] [Google Scholar]
- 129.Shinde J, Taldone T, Barletta M, Kunaparaju N, Hu B, Kumar S, et al. Alpha-glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in vitro and in Goto-Kakizaki (GK) rats. Carbohydr Res. 2008;343:1278–81. doi: 10.1016/j.carres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 130.Aguilar-Santamaría L, Ramírez G, Nicasio P, Alegría-Reyes C, Herrera-Arellano A. Herrera-Arellano A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J Ethnopharmacol. 2009;124:284–8. doi: 10.1016/j.jep.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 131.Kamtchouing P, Kahpui SM, Dzeufiet PD, Tédong L, Asongalem EA, Dimo T. Anti-diabetic activity of methanol/methylene chloride stem bark extracts of Terminalia superba and Canarium schweinfurthii on streptozotocin-induced diabetic rats. J Ethnopharmacol. 2006;104:306–9. doi: 10.1016/j.jep.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 132.Miura T, Furuta K, Yasuda A, Iwamoto N, Kato M, Ishihara E, et al. Antidiabetic effect of nitobegiku in KK-Ay diabetic mice. Am J Chin Med. 2002;30:81–6. doi: 10.1142/S0192415X02000090. [DOI] [PubMed] [Google Scholar]
- 133.Sivajothi V, Dey A, Jayakar B, Rajkapoor B. Antihyperglycemic property of Tragia cannabina in streptozotocin-induced diabetic rats. J Med Food. 2007;10:361–5. doi: 10.1089/jmf.2006.030. [DOI] [PubMed] [Google Scholar]
- 134.Schoenfelder T, Cirimbelli TM, Citadini-Zanette V. Acute effect of Trema micrantha (Ulmaceae) on serum glucose levels in normal and diabetic rats. J Ethnopharmacol. 2006;107:456–9. doi: 10.1016/j.jep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 135.Pareek H, Sharma S, Khajja BS, Jain K, Jain GC. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.) BMC Complement Altern Med. 2009:9–48. doi: 10.1186/1472-6882-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fatima SS, Rajasekhar MD, Kumar KV, Kumar MT, Babu KR, Rao CA. Antidiabetic and antihyperlipidemic activity of ethyl acetate: Isopropanol (1:1) fraction of Vernonia anthelmintica seeds in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48:495–501. doi: 10.1016/j.fct.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 137.Zanatta L, de Sousa E, Cazarolli LH, Junior AC, Pizzolatti MG, Szpoganicz B, et al. Effect of crude extract and fractions from Vitex megapotamica leaves on hyperglycemia in alloxan-diabetic rats. J Ethnopharmacol. 2007;109:151–5. doi: 10.1016/j.jep.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 138.Amiri M, Motamedi P, Vakili L, Dehghani N, Kiani F, Taheri Z, et al. Beyond the liver protective efficacy of silymarin; bright renoprotective effect on diabetic kidney disease. J Nephropharmacol. 2014;3:25–6. [PMC free article] [PubMed] [Google Scholar]
- 139.Wiernsperger N. Metformin as a cellular protector; a synoptic view of modern evidences. J Nephropharmacol. 2015;4:31–36. [PMC free article] [PubMed] [Google Scholar]
- 140.Nasri H, Rafieian-Kopaei M. Medicinal plants and antioxidants: Why they are not always beneficial? Iran J Public Health. 2014;43:255–7. [PMC free article] [PubMed] [Google Scholar]
- 141.Hajian S, Rafieian-Kopaei M, Nasri H. Renoprotective effects of antioxidants against cisplatin nephrotoxicity. J Nephropharmacol. 2014;3:39–42. [PMC free article] [PubMed] [Google Scholar]
- 142.Madihi Y, Merrikhi A, Baradaran A, Rafieian-Kopaei M, Shahinfard N, Ansari R, et al. Impact of sumac on postprandial high-fat oxidative stress. Pak J Med Sci. 2013;29:340–5. [Google Scholar]
- 143.Beladi-Mousavi SS, Bashardoust B, Nasri H, Ahmadi A, Tolou-Ghamari Z, Hajian S, et al. The theme of the world diabetes day 2014; healthy living and diabetes; a nephrology view point. J Nephropharmacol. 2014;3:43–5. [PMC free article] [PubMed] [Google Scholar]
- 144.Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- 145.Rafieian-Kopaei M, Nasri H. The ameliorative effect of Zingiber officinale in diabetic nephropathy. Iran Red Crescent Med J. 2014;16:e11324. doi: 10.5812/ircmj.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nazar CM. Mechanism of hypertension in diabetic nephropathy. J Nephropharmacol. 2014;3:49–55. [PMC free article] [PubMed] [Google Scholar]
- 147.Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28:193–9. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 148.Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J Res Med Sci. 2014;19:358–67. [PMC free article] [PubMed] [Google Scholar]
- 149.Nasri H. The awareness of chronic kidney disease and aging; the focus of world kidney day in 2014. J Nephropharmacol. 2014;3:1–2. [PMC free article] [PubMed] [Google Scholar]
- 150.Tavafi M. Suggestions for attenuation of renal ischemia reperfusion injury based on mechanisms involved in epithelial cells damages. J Nephropharmacol. 2015;4:1–3. [PMC free article] [PubMed] [Google Scholar]
- 151.Madihi Y, Merrikhi A, Baradaran A, Ghobadi S, Shahinfard N, Ansari R, et al. Bioactive components and the effect of hydroalcoholic extract of Vaccinium myrtillus on postprandial atherosclerosis risk factors in rabbits. Pak J Med Sci. 2013;29(1 Suppl):384–9. [Google Scholar]
- 152.Setorki M, Rafieian-Kopaei M, Merikhi A, Heidarian E, Shahinfard N, Ansari R, et al. Suppressive impact of anethum graveolens consumption on biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Int J Prev Med. 2013;4:889–95. [PMC free article] [PubMed] [Google Scholar]
- 153.Shirzad H, Shahrani M, Rafieian-Kopaei M. Comparison of morphine and tramadol effects on phagocytic activity of mice peritoneal phagocytes in vivo. Int Immunopharmacol. 2009;9:968–70. doi: 10.1016/j.intimp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 154.Shirzad H, Taji F, Rafieian-Kopaei M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J Med Food. 2011;14:969–74. doi: 10.1089/jmf.2011.1594. [DOI] [PubMed] [Google Scholar]
- 155.Shirzad H, Kiani M, Shirzad M. Impacts of tomato extract on the mice fibrosarcoma cells. J HerbMed Pharmacol. 2013;2:13–6. [Google Scholar]
- 156.Baradaran A, Rabiei Z, Rafieian M, Shirzad H. A review study on medicinal plants affecting amnesia through cholinergic system. J HerbMed Pharmacol. 2012;1:3–9. [Google Scholar]
- 157.Rabiei Z, Hojjati M, Rafieian-Kopaei M, Alibabaei Z. Effect of Cyperus rotundus tubers ethanolic extract on learning and memory in animal model of Alzheimer. Biomed Aging Pathol. 2013;3:185–91. [Google Scholar]
- 158.Rafieian-Kopaei M, Shahinfard N, Rouhi-Boroujeni H, Gharipour M, Darvishzadeh-Boroujeni P. Effects of Ferulago angulata extract on serum lipids and lipid peroxidation. Evidence-Based Complementary and Alternative Medicine. 2014 doi: 10.1155/2014/680856. (2014) Article ID 680856, 4 pages http://dx.doi.org/10.1155/2014/680856 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rabiei Z, Rafieian-Kopaei M, Mokhtari S, Alibabaei Z, Shahrani M. The effect of pretreatment with different doses of Lavandula officinalis ethanolic extract on memory, learning and nociception. Biomed Aging Pathol. 2014;4:71–6. [Google Scholar]
- 160.Bahmani M, Rafieian M, Baradaran A, Rafieian S, Rafieian-Kopaei M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J Nephropathol. 2014;3:81–5. doi: 10.12860/jnp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Delfan B, Bahmani M, Rafieian-Kopaei M, Delfan M, Saki K. A review study on ethnobotanical study of medicinal plants used in relief of toothache in Lorestan Province, Iran. Asian Pac J Trop Dis. 2014;4(Suppl 2):879–84. [Google Scholar]
- 162.Saki K, Bahmani M, Rafieian-Kopaei M, Hassanzadazar H, Dehghan K, Bahmani F, et al. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac J Trop Dis. 2014;4(Suppl 2):895–901. [Google Scholar]
- 163.Chandra A, Biersmith M, Tolouian R. Obesity and kidney protection. J Nephropathol. 2014;3:91–7. doi: 10.12860/jnp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nasri H, Rafieian-Kopaei M. Oxidative stress and aging prevention. Int J Prev Med. 2013;4:1101–2. [PMC free article] [PubMed] [Google Scholar]
- 165.Bagheri N, Rahimian GH, Salimzadeh L, Azadegan F, Rafieian-Kopaei M, Taghikhani A, et al. Association of the virulence factors of Helicobacter pylori and gastric mucosal interleukin-17/23 mRNA expression in dyspeptic patients. EXCLI J. 2013;12:5–14. [PMC free article] [PubMed] [Google Scholar]
- 166.Tamadon MR, Zahmatkesh M, Beladi Mousavi SS. Administration of antioxidants in chronic kidney disease. J Nephropharmacol. 2015;4:9–11. [PMC free article] [PubMed] [Google Scholar]
- 167.Bagheri N, Taghikhani A, Rahimian G, Salimzadeh L, Azadegan Dehkordi F, Zandi F, et al. Association between virulence factors of Helicobacter pylori and gastric mucosal interleukin-18 mRNA expression in dyspeptic patients. Microb Pathog. 2013;65:7–13. doi: 10.1016/j.micpath.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 168.Tavakoli M. Kidney protective effects of melatonin. J Nephropharmacol. 2014;3:7–8. [PMC free article] [PubMed] [Google Scholar]
- 169.Amirmohammadi M, Khajoenia S, Bahmani M, Rafieian-Kopaei M, Eftekhari Z, Qorbani M. In vivo evaluation of antiparasitic effects of Artemisia abrotanum and Salvia officinalis extracts on Syphacia obvelata, Aspiculoris tetrapetra and Hymenolepis nana parasites. Asian Pac J Trop Dis. 2014;4(Suppl 1):S250–4. [Google Scholar]
- 170.Motamedi P, Dehghani N, Kiani F, Taheri Z, Torkamaneh S, Nasri H. New concepts in diabetic kidney disease. J Nephropharmacol. 2015;4:47–8. [PMC free article] [PubMed] [Google Scholar]
- 171.Rahimian G, Sanei MH, Shirzad H, Azadegan-Dehkordi F, Taghikhani A, Salimzadeh L, et al. Virulence factors of Helicobacter pylori vacA increase markedly gastric mucosal TGF-ß1 mRNA expression in gastritis patients. Microb Pathog. 2014;67-68:1–7. doi: 10.1016/j.micpath.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 172.Baradaran A, Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Antioxidant activity and preventive effect of aqueous leaf extract of Aloe vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter. 2014;165:7–11. doi: 10.7471/CT.2014.1653. [DOI] [PubMed] [Google Scholar]
- 173.Rafieian-Kopaei M, Motamedi P, Vakili L, Dehghani N, Kiani F, Taheri Z, et al. Green tea and type 2 diabetes mellitus. J Nephropharmacol. 2014;3:21–3. [PMC free article] [PubMed] [Google Scholar]
- 174.Nasri H. Consequences of hypomagnesemia in type 2 diabetes mellitus patients. J Renal Inj Prev. 2014;3:99–100. doi: 10.12861/jrip.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rafieian-Kopaei M, Nasri H. Re: Erythropoietin ameliorates oxidative stress and tissue injury following renal ischemia/reperfusion in rat kidney and lung. Med Princ Pract. 2014:23–95. doi: 10.1159/000350842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baradaran A, Nasri H, Rafieian-Kopaei M. Comment on: Anti-oxidative stress activity of Stachys lavandulifolia aqueous extract in humans. Cell J. 2013;15:272–3. [PMC free article] [PubMed] [Google Scholar]
- 177.Baradaran A, Madihi Y, Merrikhi A, Rafieian-Kopaei M, Nematbakhsh M, Asgari A, et al. Nephrotoxicity of hydroalcoholic extract of Teucrium polium in Wistar rats. Pak J Med Sci. 2013;29(1 Suppl):329–33. [Google Scholar]
- 178.Amini FG, Rafieian-Kopaei M, Nematbakhsh M, Baradaran A, Nasri H. Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. J Res Med Sci. 2012;17:621–5. [PMC free article] [PubMed] [Google Scholar]
- 179.Ghaderian SB, Beladi-Mousavi SS. The role of diabetes mellitus and hypertension in chronic kidney disease. J Renal Inj Prev. 2014;3:109–10. doi: 10.12861/jrip.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Taheri Z, Ghafari M, Hajivandi A, Amiri M. Vitamin D deficiency in children and adolescents; an international challenge. J Parathyr Dis. 2014;2:27–31. [Google Scholar]
- 181.Bahmani M, Zargaran A, Rafieian-Kopaei M. Identification of medicinal plants of Urmia for treatment of gastrointestinal disorders. Rev Bras Farmacognosia. 2014;24:468–80. [Google Scholar]
- 182.Taghikhani A, Afrough H, Ansari-Samani R, Shahinfard N, Rafieian-Kopaei M. Assessing the toxic effects of hydroalcoholic extract of Stachys lavandulifolia Vahl on rat's liver. Bratisl Lek Listy. 2014;115:121–4. doi: 10.4149/bll_2014_026. [DOI] [PubMed] [Google Scholar]
- 183.Heidarian E, Rafieian-Kopaei M. Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm Biol. 2013;51:1104–9. doi: 10.3109/13880209.2013.777931. [DOI] [PubMed] [Google Scholar]
- 184.Rafieian-Kopaei M, Hosseini M, Shirzad H. Comment on: Effect of pomegranate flower extract oncis platin-induced nephrotoxicity in rats. J Nephropathol. 2014;3:121–3. doi: 10.12860/jnp.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beladi-Mousavi SS, Bashardoust B, Nasri H, Ahmadi A, Hajian S, Torkamaneh S. Association of serum magnesium with Vitamin D level in normal and renal disease patients. J Parathyr Dis. 2014;2:45–6. [Google Scholar]
- 186.Asadi SY, Parsaei P, Karimi M, Ezzati S, Zamiri A, Mohammadizadeh F, et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg. 2013;11:332–7. doi: 10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 187.Rahimi Z. Parathyroid hormone, glucose metabolism and diabetes mellitus. J Parathyr Dis. 2014;2:55–6. [Google Scholar]
- 188.Gohari AR, Saeidnia S. The role of herbal medicines in treatment of urinary tract diseases. J Nephropharmacol. 2014;3:13–4. [PMC free article] [PubMed] [Google Scholar]
- 189.Nazar CM. Diabetic nephropathy; principles of diagnosis and treatment of diabetic kidney disease. J Nephropharmacol. 2014;3:15–20. [PMC free article] [PubMed] [Google Scholar]
- 190.Rafieian-Kopaei M, Baradaran A. On the occasion of world diabetes day 2105; act today to change tomorrow. J Renal Endocrinol. 2015;1:e02. [Google Scholar]


