Abstract
Sarcoidosis is a granulomatous disorder mostly could involve intrathoracic structures. The gastric involvement is rare and the symptoms may be non-specific. We herein report a case of a 56-year-old female patient who was admitted due to chest tightness and discomfort. Computed tomography (CT) of the thorax revealed bilaterally nodular lesions in the lower lobes of the lung and pleural effusion on the left side. Positron emission tomography/CT showed lung nodules and gastric involvement with mesenteric lymphadenomegalies with pathological uptake of 18F-fluoro-2-deoxy-d-glucose. Pathological examination of the lung biopsy taken by thoracotomy demonstrated non-caseating granulomas. The gastric biopsies taken by endoscopy also showed non-caseating granulomas consistent with a diagnosis of sarcoidosis.
Keywords: Granulomatous gastritis, pulmonary nodules, sarcoidosis
INTRODUCTION
Sarcoidosis is a multisystemic disease with primary involvement of the thoracic structures, particularly the lungs and the hilar and mediastinal lymph nodes. Although sarcoidosis affects gastrointestinal (GI) tract rarely, the stomach remains the most common site of this involvement. GI involvement occurs in approximately 0.9-1.1% of patients affected.[1,2,3] The stomach is the most commonly involved portion of the GI tract, but sarcoidosis of the esophagus, appendix, colon and rectum has also been described.[1] Upper GI sarcoidosis is often difficult to detect due to the relative lack of symptoms. Gastric involvements of sarcoidosis such as peptic ulceration, inflammation and gastric outlet obstruction had been previously published as case reports.[2,4,5,6,7,8,9]18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) may be helpful in patients with sarcoidosis for determining the intrathoracic and extrathoracic extensity of disease, detecting active disease and accessing the response to treatment.[7,8]
CASE REPORT
This was a case report of a 56-year-old Caucasian woman who presented with the chest tightness and discomfort and inability to take a deep breath to the Adnan Menderes University Hospital, Department of Pulmonary Medicine in Aydin, Turkey. Her symptoms started 2 weeks ago with fever, vomiting, coughing, a small amount of purulent sputum and dyspnea in May 2012. She was given levofloxacin 750 mg oral tablet once a day considering the diagnosis of pulmonary infection. Her chest X-ray and CT of the thorax showed bilateral multiple small pulmonary nodules predominantly in the left lower zone. She referred to our department with findings in question. Her medical history was a 10 pack-year of smoking and had undergone cholecystectomy 20 years ago. She had a family history of breast cancer.
Her vital signs on the admission were all normal. The oxygen saturation was 98% on room air. Lung auscultation revealed crackles predominately at the left lung base. An incision scar was observed with an examination of the abdomen. Laboratory studies demonstrated a hemoglobin level of 11.3 g/dL (normal range for female: 11.7-15.5) with microcytic hypochromic anemia, hematocrit of 37.3%, white blood cell count of 14.01/mm3 (normal range: 4.1-11.2/mm3) with a normal differential cell count. The erythrocyte sedimentation rate was 102 mm/h and C reactive protein level was 81 (on admission)/57.5 (1 week later) mg/L (normal range: 0-5 mg/L).
The level of albumin was 3.3 g/dL (normal range: 3.5-5 g/dL), alkalen phosphatase was 212 U/L (on admission)/158 (1 week later) (normal range: 40-150 U/L), γ-glutamyl transpeptidase was 80 U/L (on admission)/56 (1 week later) (normal range: 9-36 U/L), lactate dehydrogenase was 250 U/L (normal range: 125-243 U/L). Other liver and renal function tests were within the normal ranges. The serum levels of tumor markers include CA 15-3, CA 19-9, alpha-fetoprotein, carcinoembryonic antigen were normal. The level of CA-125 was high as 228.7 U/mL (normal range: 0-35 U/mL).
Chest X-ray and thorax CT showed pleural nodularity associated with pleural effusion in the left side of the thorax. A polygonal subpleural nodule with 6 mm in diameter was identified in the posterior part of the right upper lobe [Figure 1]. CT of the abdomen showed several paraaortic lymphadenomegalies reaching maximally about 1.5 cm in diameter and the gallbladder surgically removed.
Figure 1.
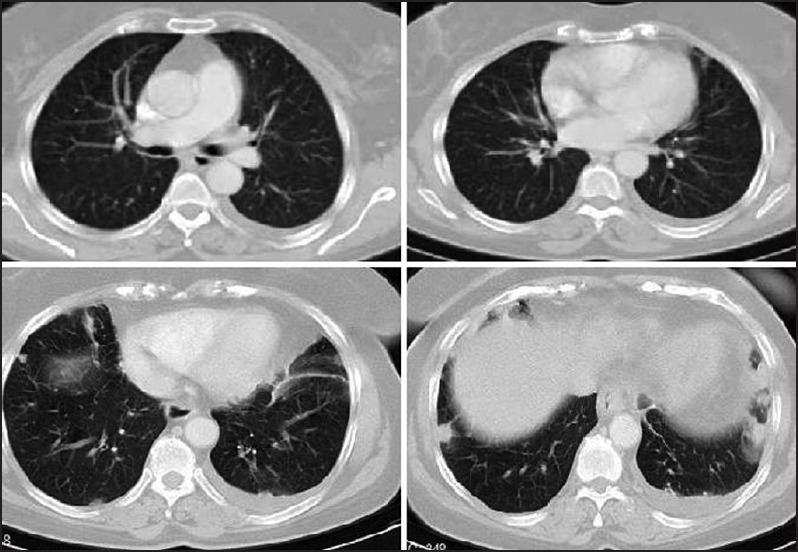
Thorax computed tomography from different sections of the thorax. Minimal amount of pleural effusion associated with collapsed lung with air bronchogram in the left hemithorax and bilateral discrete nodules in different sizes were seen
Because the pneumonia was diagnosed, it has been started sulbactam-ampicillin intravenously four times a day for 1 gram and peroral 500 mg clarithromycin per 2 times a day. Three days after treatment begins, the culture of sputum yields Klebsiella pneumonia that is sensitive to cefazoline and levofloxacine. Treatment was continued without change and completed to ten days.
Oesophagogastroduodenoscopy was performed due to gastric symptoms and it was revealed a mucosal hyperemia with edema of the gastric antrum and some erosive lesions [Figure 2]. The antral erythematous gastritis and erythematous pangastritis were diagnosed. The specimen was negative for Helicobacter pylori. Mucosal biopsies from the lesions of the gastric antrum showed non-caseating granulomas composed of epithelial and multinucleated giant cells [Figure 3]. Nor microorganism was detected with Periodic Acid-Schiff (PAS) ve Ziehl-Neelsen staining nor foreign or phagocytic particles.
Figure 2.
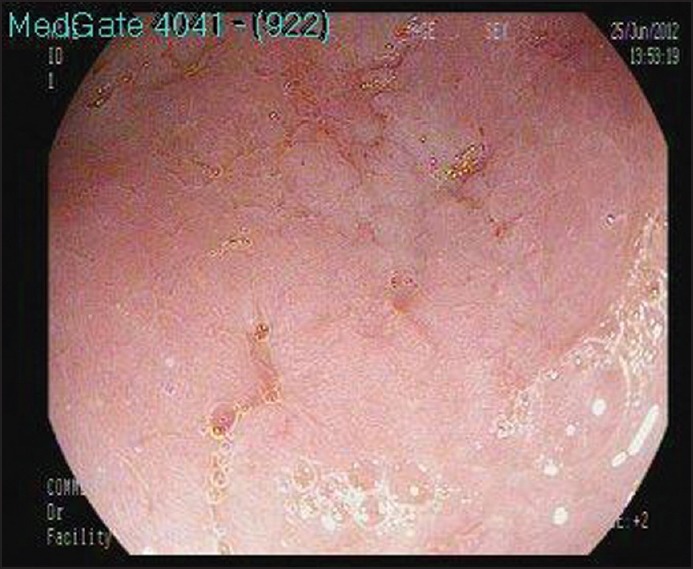
Gastric endoscopy. Oesophagogastroduodenoscopy revealed mucosal hyperemia with edema of the gastric antrum with some erosive lesions
Figure 3.
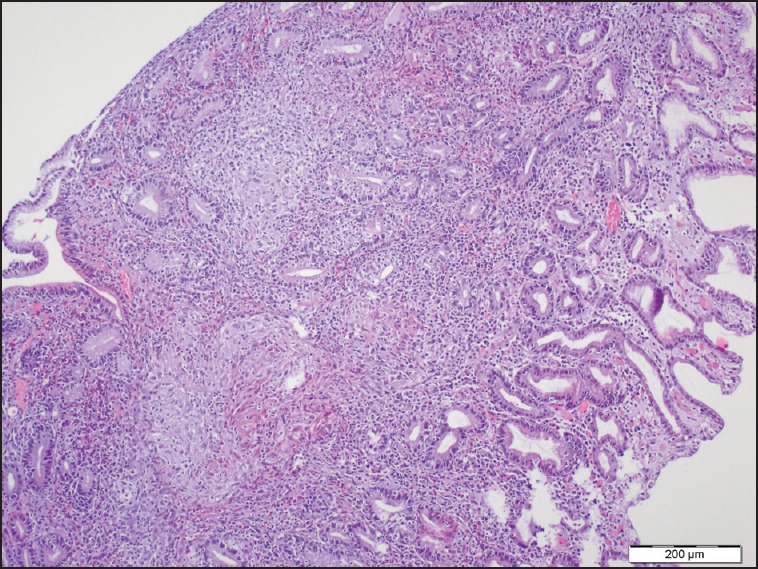
Pathology of the gastric antral biopsy (H and E, ×100) showed non-caseating granulomas composed of epithelial and multinucleated giant cells extend under the epithelium from gastric biopsy
Due to suspicion of malignancy for pulmonary nodules, we performed 18F-FDG PET/CT that it is revealed multiple nodules of different size bilaterally in the lower lobes. The nodules showed pathological uptake of 18F-fluoro-2-deoxy-d-glucose (18F-FDG) (The SUV of the larger nodule which is 1.5 cm diameter was 5.5). Multiple mesenteric lymphadenomegaly which are different sizes from the coeliac area, peripancreatic area to the iliac bifurcation were detected with pathological uptake of 18F-FDG (maximum SUVs were 6.6). Figure 4 showed 18F-FDG PET/CT sections which demonstrate pathological uptake of 18F-FDG of the stomach and mesenteric lymphadenopathies.
Figure 4.
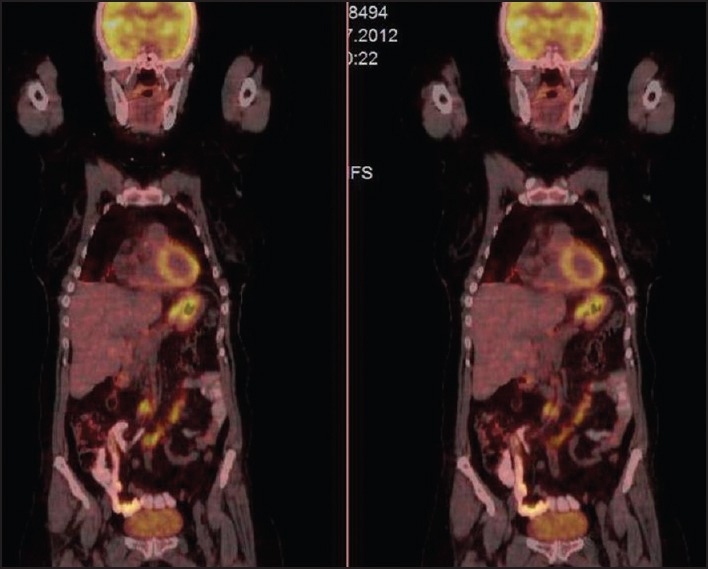
The image of positron emission tomography/computed tomography with involvement of pathological uptake of 18F-fluoro-2-deoxy-d-glucose of the stomach and mesenteric lymphadenopathies
Thoracotomy was performed due to increase in the probability of malignancy of the lung nodules. Furthermore, bilateral pleural irregularities which had pathological uptake of 18FDG suggested empyema, though it is aimed to clarify. Partial pleural decortication for pleural thickening and wedge resection for lung nodules were performed. The pathological examination of the wedge resection material of the lung revealed non-caseating granulomas including giant cells, accompanying inflammatory cells, including monocytes, macrophages and lymphocytes. Moreover, chronic active inflammation from decortication material of the pleura was found. No specific microorganism was detected with PAS ve Ziehl-Neelsen staining. Tuberculin skin test was 8 mm in diameter and she had 2 scar of Bacille Calmette Guerin vaccine. Sarcoidosis considered in the differential diagnosis and the level of angiotensin-converting enzyme (ACE) was measured. The ACE level was within the normal limits as 48.60 (8-52) U/L.
The evaluation of patient by the gastroenterologist was concluded that the clinical signs, endoscopic and pathologic findings of the patients are not compatible with Crohn's disease. Clinical assessments and 18F-FDG PET/CT examination showed no other organ involvement. Spirometry was normal. It is considered the case is limited sarcoidosis with pulmonary and gastric involvement. Pantoprazole of 40 mg per day, metoclopramide hydrocloride of 10 mg tablet three times per day, sucralfate 2 gr two times per day were recommended for gastric symptoms.
The patient experienced no further symptoms and remained well on proton pump inhibitor (PPI) (pantoprazole) therapy. She experienced gastric symptoms occasionally within 1-year follow-up. Endoscopy was performed again in order to check the findings and no abnormality was found. The biopsy from the gastric antrum revealed non-caseating granulomatous inflammation consistent with sarcoidosis.
DISCUSSION
In this article, we presented a case with gastric sarcoidosis concomitant spread to mesenteric lymph node diagnosed by 18F-FDG PET/CT. It was surprising to see no evidence of any systemic disease except atypical pulmonary sarcoidosis characterized by bilateral discrete nodules with no spread to mediastinal and hilar lymph nodes. Clinically, recognizable GI disease occurs up to 1% of patients with sarcoidosis, although the incidence of subclinical involvement may be much higher.
GI sarcoidosis may present in the context of generalized disease or as an isolated finding.[2,10] Clinical presentation of gastric involvement varies depending on the intensity of granulomatous inflammation and anatomic location of gastric involvement.[11] A great number of gastric sarcoidosis are clinically silent and may therefore be overlooked during upper endoscopy in the absence of abnormal gastric mucosa.[11] When symptomatic, abdominal pain, nausea, emesis and weight loss, hematemesis and melena are the primary symptoms. It may mimic gastric ulcers or gastric cancers as well as Crohn disease.[4,5,11]
Granulomas in gastric biopsy specimens are extremely rare and in developed countries, more than half are associated with tuberculosis, Crohn's disease, sarcoidosis, Wegener's granulomatosis, histoplasmosis and isolated granulomatous gastritis.[2,4,12] Granulomatous disease has also been reported in association with vasculitis, lymphoma, carcinoma, Whipple's disease, Langerhans cell histiocytosis and recently H. pylori infection.[8] There was no symptoms and clinical findings in relation to mentioned differential diagnosis in our case, nor at the diagnosis nor 18 months follow-up. The evaluations for infections were not revealed any pathology.
The presence and severity of symptoms is important in treatment approach. When GI sarcoidosis is asymptomatic, it may be monitored. PPI may be effective in treating epigastralgia, but oral glucocorticoids may be necessary for some symptomatic patients. In some case studies, other immunosuppressive and surgical treatment options were also suggested. The optimal method for treating GI sarcoidosis therefore remains to be determined.[10]
The disease extent and severity are judged from the number of involved organs and the density of granuloma within the organs. Increased 18F-fluoro-2-deoxy-d-glucose accumulation is seen in the setting of granulomatous processes such as sarcoidosis or mycobacterial infection by 18F-FDG PET/CT. Inflammatory cells such as neutrophils and activated macrophages at the site of inflammation or infection are responsible for the accumulation of 18F-FDG.[7] In a patient with proven diagnosis of sarcoidosis, the extent of involvement and quantification of the disease activity can be more accurately assessed by 18F-FDG PET/CT than with Gallium-67 scan. Gallium-67 scan requires more than one visit for assessment and not available everywhere. With the advent of 18F-FDG PET/CT, involvement of the occult manifestations of sarcoidosis such as sinonasal and pharyngo-laryngeal localizations, mesenteric lymph nodes, small lung nodules, gastric and cardiac involvement has been recognized.[7,8,13] 18F-FDG PET/CT is also useful to follow the efficacy of treatment in patients with sarcoidosis, particularly in its atypical, complex and multisystem forms.[8]
CONCLUSION
Upper GI sarcoidosis is often difficult to detect due to the relative lack of symptoms, but, as in our case, especially when concomitant with lung involvement, the diagnosis is easier. 18FDG PET/CT was very helpful for detecting extrathoracic involvement of sarcoidosis in this case and it was useful in showing biopsy taken place.
AUTHOR'S CONTRIBUTIONS
EC acquisition, analysis, or interpretation of data for the work, evaluating the case, contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript. SŞ acquisition, analysis, or interpretation of data for the work, evaluating the case, contributed in the conception of the work, approval of the final version of the manuscript. AC acquisition, analysis, or interpretation of data for the work, evaluating the case, contributed in the conception of the work, approval of the final version of the manuscript. İM acquisition, analysis, or interpretation of data for the work, evaluating the case, contributed in the conception of the work, approval of the final version of the manuscript. ND acquisition, analysis, or interpretation of data for the work, evaluating and monitoring the case, approval of the final version of the manuscript, agreed for all aspects of the work. OÇ final approval of the version to be published, agreed for all aspects of the work.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Sharma A, Kadakia J, Sharma O. Gastrointestinal sarcoidosis. Semin Respir Crit Care Med. 1992:6–442. [Google Scholar]
- 2.Friedman M, Ali MA, Borum ML. Gastric sarcoidosis: A case report and review of the literature. South Med J. 2007;100:301–3. doi: 10.1097/SMJ.0b013e318030ed94. [DOI] [PubMed] [Google Scholar]
- 3.Okumus G, Musellim B, Cetinkaya E, Turker H, Uzaslan E, Yenturk E, et al. Extrapulmonary involvement in patients with sarcoidosis in Turkey. Respirology. 2011;16:446–50. doi: 10.1111/j.1440-1843.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 4.Afshar K, BoydKing A, Sharma OP, Shigemitsu H. Gastric sarcoidosis and review of the literature. J Natl Med Assoc. 2010;102:419–22. doi: 10.1016/s0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 5.Leeds JS, McAlindon ME, Lorenz E, Dube AK, Sanders DS. Gastric sarcoidosis mimicking irritable bowel syndrome - Cause not association? World J Gastroenterol. 2006;12:4754–6. doi: 10.3748/wjg.v12.i29.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fireman Z, Sternberg A, Yarchovsky Y, Abu-Much S, Coscas D, Topilsky M, et al. Multiple antral ulcers in gastric sarcoid. J Clin Gastroenterol. 1997;24:97–9. doi: 10.1097/00004836-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Saboury B, Werner T, Alavi A. Clinical utility of FDG-PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol. 2011;13:1051–60. doi: 10.1007/s11307-010-0459-x. [DOI] [PubMed] [Google Scholar]
- 8.Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: Review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;35:1537–43. doi: 10.1007/s00259-008-0770-9. [DOI] [PubMed] [Google Scholar]
- 9.Akinyemi E, Rohewal U, Tangorra M, Abdullah M. Gastric sarcoidosis. J Natl Med Assoc. 2006;98:948–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Inomata M, Ikushima S, Awano N, Kondoh K, Satake K, Masuo M, et al. Upper gastrointestinal sarcoidosis: Report of three cases. Intern Med. 2012;51:1689–94. doi: 10.2169/internalmedicine.51.7367. [DOI] [PubMed] [Google Scholar]
- 11.Vahid B, Spodik M, Braun KN, Ghazi LJ, Esmaili A. Sarcoidosis of gastrointestinal tract: A rare disease. Dig Dis Sci. 2007;52:3316–20. doi: 10.1007/s10620-006-9448-y. [DOI] [PubMed] [Google Scholar]
- 12.Ectors NL, Dixon MF, Geboes KJ, Rutgeerts PJ, Desmet VJ, Vantrappen GR. Granulomatous gastritis: A morphological and diagnostic approach. Histopathology. 1993;23:55–61. doi: 10.1111/j.1365-2559.1993.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 13.Hashefi M, Curiel R. Future and upcoming non-neoplastic applications of PET/CT imaging. Ann N Y Acad Sci. 2011;1228:167–74. doi: 10.1111/j.1749-6632.2011.06082.x. [DOI] [PubMed] [Google Scholar]


