Motor rehabilitation after hemiparetic stroke is essential to soften physical disability (Furlan, 2014). Nevertheless, current interventions are mostly designed for well recovered individuals and often exclude stroke survivors with rather limited motor ability (Sterr and Conforto, 2012). Given that, and further advancing our research agenda in this arena (Sterr et al., 2002; Sterr and Freivogel, 2003, 2004; Sterr, 2004; Sterr et al., 2006; Sterr and Saunders, 2006), we recently tested the efficacy of a 2-week modified constraint-induced (CI) therapy program in chronic stroke individuals with very low-functioning upper limb hemiparesis (Sterr et al., 2014a). We tested the influence of both the intensity of daily motor training (90 vs. 180 minutes) and the restraint of the less affected upper limb (restraint vs. no restraint) on treatment outcomes. Sixty-five individuals were randomly assigned to four experimental conditions (90 minutes of training with or without restraint, and 180 minutes of training with or without restraint). They were assessed at baseline and after the intervention (2 weeks before, immediately before and after, 6, and 12 months after). Across the cohort, motor function improved significantly, and treatment benefits were largely sustained over the 12 months of follow-up. Analysis of the different treatment variants, however, revealed interesting yet unexpected findings, particularly with regards to the relationship between intensity (amount) of daily training and motor outcomes. As suggested by previous work (Sterr et al., 2002), longer sessions of daily training were expected to yield better outcomes than short sessions, a finding in line with the theory that massed practice is essential for neuroplasticity processes driving the functional improvements induced by CI therapy. However, this was not entirely the case. While we found some differences suggesting greater benefit of longer training sessions, the picture was not as clear as one might expect. This pointed to an interaction between training intensity and motor outcomes in low-functioning chronic stroke that appears to be different from that seen in less severe chronic hemiparesis, where the concept of ‘the more the better’ often holds true (Figure 1). We argued that this intensity-outcome relationship is moderated by variables that highly depend on the level of residual recovery. A key candidate for this moderation is fatigue. Fatigue is identified as rather common, yet obscure problem in stroke survivors (Wu et al., 2015). Post-stroke fatigue is multifactorial and seems to result from a complex interaction among biological, psychosocial, and behavioral factors (Wu et al., 2015). Here, we discuss the role of fatigue in motor rehabilitation of low-functioning chronic stroke using the framework recently suggested by Kluger et al. (2013). Although relatively different from, yet not antithetic to other fatigue models (e.g., Wu et al., 2015), we believe their framework provides conceptual and mechanistic support to our hypothesis. According to that framework, neurological, including post-stroke fatigue encompasses two domains: Perception of fatigue and fatigability. Perception of fatigue refers to a subjective sense of effort or exhaustion, whereas fatigability is related to an objective decline in performance. Although these two types of fatigue might be largely interrelated (e.g., an increased sense of effort would usually contribute to impair performance), they might also act independently and still significantly affect the individual's engagement with activities posing high motor and/or cognitive demands. This is because those two types of fatigue are likely to be caused by different, yet potentially interacting factors. For instance, perception of fatigue could be induced by homeostatic (e.g., metabolic stimuli, such as depletion of energy reserves in skeletal muscle and/or brain tissue) and/or psychological (e.g., decreased motivation) mechanisms, while fatigability could occur due to declines in skeletal muscle force production and/or deficits in task-related neural processing (Kluger et al., 2013). Based on that, we propose that low-functioning chronic stroke survivors are highly susceptible to get into a complex fatigued state, which renders motor training ineffective. This state is more likely to be reached by individuals undergoing longer training sessions. Essentially, we elaborate here on the possibility that a combination of general deconditioning and compromised neural processing might greatly increase both perception of fatigue and fatigability in those individuals, which substantially reduces their engagement with motor training and thereby decreases the likelihood for neuroplasticity processes driving behavioral improvements.
Figure 1.
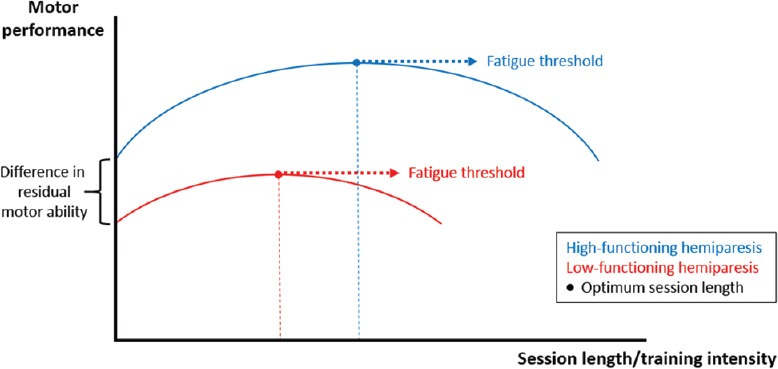
Hypothetical relationship between training intensity and outcomes in chronic hemiparetic stroke.
This figure illustrates the modulation of the optimum session length/training intensity by residual recovery levels. Two assumptions are made. Firstly, as session length increases, performance also increases, until it reaches its peak; increasing session length further, however, results in performance deterioration, which presumably reflects the impact of fatigue. Secondly, in stroke survivors with low-functioning hemiparesis (red line), performance is not only lower in general, but critically, the optimum training intensity is reached earlier than in those with high-functioning hemiparesis (blue line). Optimum session length/training intensity: Mostly determined by both the level of residual recovery and the fatigued status an individual achieves. Of note, the latter is critically influenced by the first. Investigations of dosage effects in motor rehabilitation should, therefore, not only carefully consider the level of residual function, but also take measures of fatigue into consideration.
General deconditioning in low-functioning chronic stroke: The musculoskeletal and cardiorespiratory systems are interdependent. Effective skeletal muscle work requires that muscle fibers have not only relatively good levels of strength, but also adequate supply of oxygen and nutrients. The first is achieved through regular doses of mechanical load normally imposed to the musculoskeletal system during routine tasks, while the latter is implemented by the cardiorespiratory system via the blood stream. On the other hand, to maintain an effective cardiorespiratory system that ensures adequate supply of oxygen and nutrients to working muscle fibers, regular physical activity is mandatory. This is only possible through the contraction of skeletal muscles. After hemiparetic stroke, individuals experience significant cardiorespiratory and musculoskeletal deterioration. Pre-stroke age-related changes and/or comorbid cardiovascular diseases often contribute to a deteriorated cardiorespiratory function already at the sub-acute phase (Kilbreath and Davis, 2005). In parallel, the stroke-induced loss of voluntary motor control results in an important deterioration of musculoskeletal function at the same time. More specifically, the lack of selective control over spinal motor units imposes major limitation on the individual's ability to generate muscle force and coordinate contraction across muscle groups, which critically reduces their capacity to use the paretic body side (Carr and Shepherd, 2011a). Combined to customarily low inpatient and post-discharge physical activity levels, this initial impaired cardiorespiratory and musculoskeletal status greatly increases the likelihood for an inactive lifestyle after hemiparetic stroke (Carr and Shepherd, 2011b). Over time, the sustained immobility of paretic limbs starts maladaptive plastic changes in skeletal muscles that contribute to aggravate even more an already compromised musculoskeletal condition. Some of these changes include: atrophy and shortening of muscle fibers, proliferation of connective (non-contractile) tissue, increased stiffness and fat content, and reduced capillary density and oxidative capacity (Carr and Shepherd, 2011a). Collectively, these changes further subsidise the ongoing physical inactivity process and thereby the aggravation of cardiorespiratory function (Kilbreath and Davis, 2005; Carr and Shepherd, 2011a, b). Thus, chronic stroke individuals often become trapped in a self-perpetuating cycle of general deconditioning, where an early deteriorated cardiorespiratory and musculoskeletal condition fosters physical inactivity and paretic limbs disuse, which in turn contributes to deteriorate cardiorespiratory and musculoskeletal function even further. This is likely to elevate both perception of fatigue and fatigability during motor activities by accelerating depletion of skeletal muscle energy reserves and causing rapid declines in force production, respectively. While the first is likely a compound of an impaired cardiorespiratory system – which may fail to effectively supply contracting muscles with oxygen and nutrients – and a deteriorated musculoskeletal function – in terms of reduced capillary density and oxidative capacity limiting muscle energy production –, the latter mostly results from an impaired neuro-musculoskeletal system, owing primarily to both a reduced ability to activate spinal motor units and skeletal muscle plastic changes. Of note, it is plausible to assume that general deconditioning tends to be more pronounced in low-functioning chronic hemiparesis, as here the likelihood for paretic limbs immobility and an inactive lifestyle is even higher due to greater physical (and often cognitive) limitations.
Compromised neural processing in low-functioning chronic stroke: The adult brain can be thought of as having both primary, specialized, and secondary, less specialized circuits/networks. Primary circuits are critical for the generation of behavior and therefore are normally recruited. Secondary circuits, however, are not essential for behavior expression, but because they have some capacity to contribute to it, they may be recruited under special circumstances, such as when primary circuits can no longer afford behavioral/task demands, which can happen after stroke (Kleim and Schwerin, 2010; Ward, 2011). In that case, enlisting secondary networks often allows for the brain to compensate for damage and preserve behavior integrity to varying degrees (Kleim and Schwerin, 2010; Ward, 2011). The extension of the recruitment of secondary brain networks largely depends on the remaining availability of primary circuits – i.e., the less the sparing of the latter due to more severe damage, the more extensive the recruitment of the first (Ward, 2011). Besides, the functional relevance of shifting activity to secondary circuits in that context relies essentially on how well these circuits can characterize the relevant behavior, which is primarily dictated by their pattern of neuronal connections (Ward, 2011). Because secondary brain networks usually share only part of the highly specific connections displayed by their primary counterparts, as reliance on these networks increases, the ability to maintain behavior integrity is progressively lowered (Kleim and Schwerin, 2010; Ward, 2011). After hemiparetic stroke, primary brain circuits normally controlling skillful motor behaviors are disrupted to different degrees (Frey et al., 2011). The resulting compromised neural processing state manifests itself not only at the motor execution level (see previous paragraph), but also at the more cognitive level. For example, studies by our group have shown deficits in up-stream motor processes, such as motor preparation (Dean et al., 2012). Therefore, it is not only the movement execution-related mental effort that is increased during motor tasks in order to preserve behavior integrity, but critically, also the mental effort associated with the processing of other movement-related information. In chronic stroke, this translates into a pattern of widespread brain activation, which is characterized by enhanced activity in potentially spared primary networks and recruitment of many secondary circuits (Ward, 2011). This has come mostly from studies utilizing functional magnetic resonance imaging to investigate changes in brain activity after stroke. Because of the theoretical/methodological assumptions underlying interpretations of this type of data (Ward, 2009), it seems reasonable to conclude that the pattern of increased brain activation described above reflects a condition of elevated neuronal metabolism in chronic hemiparetic stroke, which favors rapid depletion of brain energy reserves. This, in turn, might elevate perception of fatigue during motor tasks. Besides, an imposed reliance on secondary, less specialised motor control networks is very likely to also increase fatigability in chronic stroke individuals owing to deficits in motor task-related neural processing. Importantly, compromised neural processing is likely to be more aggravated in low-functioning chronic hemiparesis, as here (1) individuals often recruit more secondary circuits and therefore have stronger brain activation (Ward, 2011), causing higher neurometabolic demands and hence more elevated perception of fatigue, and (2) the reliance on a less specialized brain system is stronger (Ward, 2011) – which reflects itself in a low-functioning status –, making task performance even more challenging and thus causing greater fatigability.
In our CI therapy study, because we did not measure fatigue, the interpretation outlined in this article can only be speculative. However, given the prevailing characteristics of our sample, the aspects of the intervention that was delivered, and the mechanisms underpinning the processes of fatigue described before, we nonetheless feel that this is a relevant interpretation. The very low-functioning motor status and long chronicities prevailing in our cohort are rather suggestive of pronounced cardiorespiratory and musculoskeletal deterioration, and hence important general deconditioning. When combined to a physically demanding motor training regimen such as CI therapy, this may well have exacerbated perception of fatigue and fatigability in our sample. Moreover, although we did not obtain specific information about stroke lesion size and location from our cohort, investigations on the association between motor status and the integrity of movement-related primary brain circuits (Sterr et al., 2010, 2014b) show that poor motor function at the chronic phase highly depends on the overlap of the stroke with those circuits, which in turn is very likely to cause overreliance on secondary neural substrates for movement control, and thereby rather compromised neural processing. Given the high cognitive/motor processing demands of our intervention, this may well have contributed to further aggravate perception of fatigue and fatigability in our cohort. Accordingly, a recent cross-sectional study with chronic stroke survivors revealed an inverse correlation between levels of fatigue and the excitability of movement-related primary brain networks (Kuppuswamy et al., 2015). Nevertheless, it is difficult to determine whether the poor motor status of our sample indeed reflected a severe neurological damage or, instead, the manifestation of maladaptive musculoskeletal changes that may have evolved over time as a result of inactivity/immobility. A more sensible appreciation of this point assumes that these two phenomena interact and jointly contribute to the fatigued status, as well as the residual recovery an individual achieves. Clearly, further research is needed to properly investigate the proposed mechanisms and their interaction.
Fatigue, motor training, and neuroplasticity in low-functioning chronic stroke: Motor training contributes to restore motor behaviors lost due to hemiparetic stroke by providing the brain with neural signals that drive functionally relevant neuroplastic changes within spared primary/secondary motor control networks (Nudo et al., 1996). However, to do that, trained motor tasks need to be not only actively and repetitively practiced, but also challenging enough to stimulate individuals to go beyond the current state of their motor capacity and thereby achieve the adaptive brain reorganization driving behavioral improvements (Nudo, 2003). The CI therapy intervention is grounded on this principle (Morris and Taub, 2006). In our study, the individuals receiving longer daily sessions indeed spent more time in active, repetitive motor practice than those receiving shorter sessions, but treatment outcomes did not coherently reflect this. One could henceforth conclude that 90 minutes of daily training is enough. But we do not take this position. Rather, we argue that those in the group receiving longer daily sessions are more likely to reach an exacerbated fatigued status – characterized by both elevated perception of fatigue and fatigability –, which adversely directs motor training towards the repetition of movements that are more accommodated to their motor impairments, and hence reduces the neural activation required for the relevant neuroplastic changes mediating motor improvements (Nudo, 2003). Furthermore, an increased fatigued status is also likely to negatively impact on motivation and compliance, which in our study could have not only contributed to aggravate an already existing condition of elevated perception of fatigue in the individuals exposed to the more intensive CI therapy protocol (see mechanisms of fatigue), but also directly affected their ability to engage with the training as well as their commitment to it. Thus, the fact that those individuals may have spent their extra training time practicing tasks while they were physically and/or mentally too fatigued to do so effectively, might explain the limited added value of the more intensive 30 hour training regimen.
Conclusion: We believe the results from our study, when interpreted under the perspective presented in this article, harbor important implications for post-stroke motor rehabilitation research. Two of the many challenges in this field have been to define the optimal intensity of motor training-based interventions (Cooke et al., 2010) and to account for potential individual differences in motor outcomes after such interventions. Taking critical modulators such as fatigue into consideration is very important here. This is because not only it might explain individual differences to some extent, but also it will contribute to prevent misconceptions around the intensity-outcome relationship of those interventions. Because fatigue is very likely to be more pronounced in low-functioning chronic stroke, studies with this group have an even stronger mandate to take it into consideration when both, seeking for optimal training intensity-related parameters as well as interpreting motor outcome measures.
This work was supported by the MRC, UK (G0200128, awarded to AS) and the CAPES Foundation, Ministry of Education, Brazil (BEX 0996/14-9, awarded to LF).
References
- Carr JH, Shepherd RB. Upper motor neuron lesions. In: Carr JH, Shepherd RB, editors. Neurological rehabilitation: optimizing motor performance. New York: Churchill Livingstone Elsevier; 2011a. pp. 193–215. [Google Scholar]
- Carr JH, Shepherd RB. Enhancing physical activity and brain reorganization after stroke. Neurol Res Int. 2011b;2011:1–7. doi: 10.1155/2011/515938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: a systematic review and meta-analysis. BMC Med. 2010;8:1–13. doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean PJ, Seiss E, Sterr A. Motor planning in chronic upper-limb hemiparesis: evidence from movement-related potentials. PLoS One. 2012;7:1–15. doi: 10.1371/journal.pone.0044558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Fogassi L, Grafton S, Picard N, Rothwell JC, Schweighofer N, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: computation, anatomy and physiology (CAP) model. Neurorehabil Neural Repair. 2011;25:6S–20S. doi: 10.1177/1545968311410940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan L. Potential barriers and promising opportunities for stroke rehabilitation in Brazil. Int J Stroke. 2014;9:144. doi: 10.1111/ijs.12338. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Davis GM. Cardiorespiratory fitness after stroke. In: Refshauge K, Ada L, Ellis E, editors. Science-based rehabilitation: theories into practice. China: Elsevier Science; 2005. pp. 131–158. [Google Scholar]
- Kleim JA, Schwerin S. Motor map plasticity: a neural substrate for improving motor function after stroke. In: Cramer SC, Nudo RJ, editors. Brain repair after stroke. New York: Cambridge UP; 2010. pp. 1–10. [Google Scholar]
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy A, Clark EV, Turner IF, Rothwell JC, Ward NS. Post-stroke fatigue: a deficit in corticomotor excitability? Brain. 2015;138:136–148. doi: 10.1093/brain/awu306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42:257–268. [PubMed] [Google Scholar]
- Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14:S57–76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, Si Fuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Sterr A. Training-based interventions in motor rehabilitation after stroke: theoretical and clinical considerations. Behav Neurol. 2004;15:55–63. doi: 10.1155/2004/703746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Conforto AB. Plasticity of adult sensorimotor system in severe brain infarcts: challenges and opportunities. Neural Plast. 2012;2012:1–10. doi: 10.1155/2012/970136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Freivogel S. Motor-improvement following intensive training in low-functioning chronic hemiparesis. Neurology. 2003;61:842–844. doi: 10.1212/wnl.61.6.842. [DOI] [PubMed] [Google Scholar]
- Sterr A, Freivogel S. Intensive training in chronic upper limb hemiparesis does not increase spasticity or synergies. Neurology. 2004;63:2176–2177. doi: 10.1212/01.wnl.0000145605.20476.07. [DOI] [PubMed] [Google Scholar]
- Sterr A, Saunders A. CI therapy distribution: theory, evidence and practice. NeuroRehabilitation. 2006;21:97–105. [PubMed] [Google Scholar]
- Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–1377. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- Sterr A, Szameitat A, Shen S, Freivogel S. Application of the CIT concept in the clinical environment: hurdles, practicalities, and clinical benefits. Cogn Behav Neurol. 2006;19:48–54. doi: 10.1097/00146965-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24:413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- Sterr A, O’Neill D, Dean PJ, Herron KA. CI therapy is beneficial to patients with chronic low-functioning hemiparesis after stroke. Front Neurol. 2014a;5:1–10. doi: 10.3389/fneur.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Dean PJ, Szameitat AJ, Conforto AB, Shen S. Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair. 2014b;28:335–343. doi: 10.1177/1545968313510972. [DOI] [PubMed] [Google Scholar]
- Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol. 2011;589:5625–5632. doi: 10.1113/jphysiol.2011.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. fMRI in cerebrovascular disorders. In: fMRI techniques and protocols. In: Filipi M, editor. Dordrecht: Humana Press; 2009. pp. 597–613. [Google Scholar]
- Wu S, Mead G, Macleod M, Chalder T. Model of understanding fatigue after stroke. Stroke. 2015;46:893–898. doi: 10.1161/STROKEAHA.114.006647. [DOI] [PubMed] [Google Scholar]


