Plasticity is a natural property of living organisms that is crucial for adaptation and evolution. Over the last decades, the availability of sophisticated neuroimaging techniques (in particular, functional magnetic resonance imaging (fMRI), and transcranial magnetic stimulation (TMS)), has made it possible to explore in vivo the on-line functioning of brain and its plasticity. However, the impressive visual impact of fMRI and the quick and reliable measures obtained by TMS have tended to divert attention from one of the oldest neuroimaging techniques available in humans: electroencephalography (EEG). Recent advances in EEG signal processing and recording technology have started to reverse this trend with remarkable demonstrations in humans of phenomena previously only recorded invasively in animals such as the electrical correlates of memory consolidation during sleep (for a review see Tononi and Cirelli, 2012). EEG has the advantage over fMRI in that it can explore real-time spontaneous electrical brain activity with an excellent temporal resolution (milliseconds). Coupled with new forms of mathematical signal analysis (signal coherence and perform fractal dimension, small world and entropy analyses), EEG can now be used to investigate functional connectivity and to explore how neural networks are organized and interact (an example in Zappasodi et al., 2014).
A number of approaches have been employed to capture the EEG signatures of neural plasticity. In the awake state these are often masked by the large amount of unrelated activity that occurs at the same time. Experimental strategies to increase the signal: noise ratio include increasing the topographic specificity of tasks required to induce plasticity and increasing spatial resolution of the EEG signal by using a large number of electrodes. Another opportunity is offered by sleep, when spontaneous electric neural patterns are predictable, allowing novel events to be easily characterised. This was the approach used by Tononi and colleagues (2012), who demonstrated that slow delta waves (DW) during non-rapid eye movement (NREM) sleep are associated with memory consolidation. DW are the most prominent EEG feature of human NREM sleep. They originate in cortical neurons and have been proposed as possible effectors of sleep-dependent synaptic plasticity. Experiments in animals have demonstrated that DW are the EEG counterpart of near-synchronous transitions between states of hyper- and hypo-excitability over wide neural networks (Steriade et al., 1993), with the amplitude and slope of DW correlated to the number of neurons oscillating near-synchronously (Vyazovskiy et al., 2009). This synchrony is, in turn, directly related to the number and strength of synaptic connections among neural networks. In humans, high-density EEG studies revealed that there was an increase in parietal cortex DW after learning a visuo-motor task; conversely DW activity was reduced over sensorimotor cortex if the contralateral arm was immobilized during the day. In this case, synaptic depression was confirmed behaviourally by reduced motor performance and physiologically by reduced amplitude sensory evoked responses (Huber et al., 2006). Sensitivity of sleep DW to neural plasticity was also corroborated by demonstrating changes in DW after the induction of long-term potentiation (LTP) and depression-like phenomena in the brain following repetitive TMS.
Brain plasticity is affected in a number of pathological states. In particular, large acute lesions, such as after stroke give rise to a state when the brain devotes much of its energy to induce brain plasticity. In EEG studies, the best experimental model to investigate plasticity is after unilateral monolesional stroke, particularly if restricted to a confined area involving eloquent cortex, i.e., a cortex producing a measurable clinical deficit after its lesion (e.g., middle cerebral artery territory with upper limb sensory/motor deficits). In such cases it is then possible to take into account lesion size, clinical impairment and EEG changes of the affected cortex throughout the subacute and chronic phases of stroke. Additionally, EEG changes specific to the affected cortex can be compared with activity of contralateral non-lesioned hemisphere (even if non-lesioned areas activity can, in turn, be partially influenced from modified transcallosal inputs originating from the lesioned one). Clinical studies on acute stroke patients have demonstrated that DW of the affected hemisphere are a very sensitive indicator of neuronal dysfunction that is related to both the lesion volume and the acute neurological deficit (Assenza et al., 2009). Thus, spread of DW from the affected to unaffected hemisphere is associated with poor prognosis (Finnigan et al., 2008). Indeed, EEG or magnetoencephalography measures of DW activity in acute stroke can provide additional predictive value of clinical recovery at 3 months over and above that given by clinical examination alone (Assenza et al., 2013). Advanced EEG signal analyses (the coherence analysis) elucidated the pathophysiology of DW arising from the unaffected hemisphere. They come from an interhemispheric communication breakdown of electrical signals between the two hemispheres that can interfere with the unaffected hemisphere contribution to plasticity processes sustaining clinical recovery (Graziadio et al., 2012; Assenza et al., 2013). These results confirmed old animal experimental data reporting that EEG DW detection requires an intact cortex (Steriade et al., 1993), as they appear, in stroke models, only when a subcortical lesion occurs and not after a cortical lesion, which sets almost to zero the neural activity.
Although DW during wakefulness in stroke patients is usually regarded as a negative sign, the studies above suggest that they may also have a role in cortical plasticity and stroke recovery. The frequency of oscillatory activity in a neural network is inversely related to the number of synchronously active neurons. Thus the population of neurons that oscillate in the delta rhythm is larger than participates in the alpha rhythm. We postulate that neural plasticity in perilesional areas increases connectivity between neurons, and thus increases the chance that they synchronise their activity at the delta frequency (Figure 1).
Figure 1.
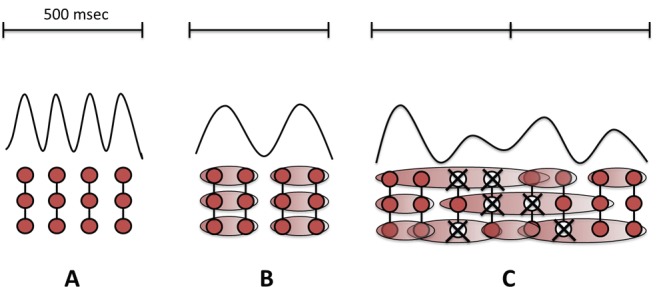
Delta waves and brain plasticity.
In physiological conditions with preserved interneuronal connections, background electroencephalography (EEG) activity oscillates in alpha frequency (8–12 Hz) band (A). When cortical plasticity is locally stimulated (as in Assenza et al., 2014) new connections are created and larger groups of neurons start oscillating simultaneously generating delta waves (B). When a lesion hit the brain, spared neurons on the boundaries of the lesion try to connect each other according to novel networks in the attempt to overcome the occurred neuronal deficit and start oscillating in delta frequency band.
In order to test this possible relationship between DW and plasticity we used intermittent theta burst (iTBS) TMS to manipulate levels of cortical plasticity in healthy volunteers (Assenza et al., 2015). This method has been shown to transiently increase cortical excitability for up to 30 minutes after administration (Di Pino et al., 2014). We could therefore test whether increased plasticity in the healthy brain led to a period of increased DW activity in the EEG. As predicted we found that iTBS (but not sham iTBS) increased the power of DW, thus confirming in wakefulness the concept from Tononi's experiments during sleep showing an association between synaptic consolidation and DW. Thus, DW in lesional and controlateral areas of stroke patients may be not merely a marker of network dysfunction, but more a sign of neuronal rearrangement accompanying acute and chronic phases of recovery. Admittedly, the data so far only show correlations between plasticity and DW activity and further complementary experiments are planned to try to answer this question. In particular, present data do not permit to establish the exact relationship between DW and plasticity (because of the lack of a correlation between motor evoked potential amplitude and DW power and of a temporal segregation of DW and motor evoked potential increase), but clearly reveal their physiological association. Animal studies provided further data supporting an active role of DW in the awake state. In stroke rats, Carmichael and Chesselet (2002) demonstrated that DW in the controlesional hemisphere might function as an attraction guide for interhemispheric fibers sprouting.
We note that several studies have changes in alpha and beta band activity in association with learning and plastic phenomena, but the effects are smaller and less consistent than those reported above. However, the implication is that plasticity phenomena may manifest other changes in addition to those in the delta band EEG. Nevertheless, we propose that data from delta band activity has huge potential which should be explored in more detail to confirm its involvement in processes related to neural plasticity. Our hypothesis is that DWs are a non-invasive and easily-recordable correlate of neural plasticity that open new and exciting scenarios in neurological rehabilitation as well as in a wide context of learning programs. It could also provide an objective measure of obtained learning.
In conclusion, EEG is a reliable technique for exploring functional activity of the brain and is sensitive to changes in neural plasticity processes related to LTP-like phenomena and clinical recovery after brain lesions. Its use in this field of neuroscience should be encouraged, in particular in searching traces of plasticity processes to provide a biological marker to lead improvements of brain functioning.
References
- Assenza G, Zappasodi F, Squitti R, Altamura C, Ventriglia M, Ercolani M, Quattrocchi CC, Lupoi D, Passarelli F, Vernieri F, Rossini PM, Tecchio F. Neuronal functionality assessed by magnetoencephalography is related to oxidative stress system in acute ischemic stroke. Neuroimage. 2009;44:1267–1273. doi: 10.1016/j.neuroimage.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Assenza G, Zappasodi F, Pasqualetti P, Vernieri F, Tecchio F. Prognostic potential of contralesional eeg power increase is mediated by interhemispheric disconnection in acute stroke. Restor Neurol Neurosci. 2013;31:177–188. doi: 10.3233/RNN-120244. [DOI] [PubMed] [Google Scholar]
- Assenza G, Pellegrino G, Tombini M, Di Pino G, Di Lazzaro V. Wakefulness delta waves increase after cortical plasticity induction. Clin Neurophysiol. 2015;126:1221–1227. doi: 10.1016/j.clinph.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Finnigan SP, Rose SE, Chalk JB. Contralateral hemisphere delta EEG in acute stroke precedes worsening of symptoms and death. Clin Neurophysiol. 2008;119:1690–1694. doi: 10.1016/j.clinph.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Graziadio S, Tomasevic L, Assenza G, Tecchio F, Eyre JA. The myth of the ‘unaffected’ side after unilateral stroke: is reorganisation of the non-infarcted corticospinal system to reestablish balance the price for recovery. Exp Neurol. 2012;238:168–175. doi: 10.1016/j.expneurol.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast 2012. 2012 doi: 10.1155/2012/415250. 415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappasodi F, Olejarczyk E, Marzetti L, Assenza G, Pizzella V, Tecchio F. Fractal dimension of EEG activity senses neuronal impairment in acute stroke. PLoS One. 2014;9:e100199. doi: 10.1371/journal.pone.0100199. [DOI] [PMC free article] [PubMed] [Google Scholar]


