Abstract
Recent evidence has suggested the neuroprotective effects of physical exercise on cerebral ischemic injury. However, the role of physical exercise in cerebral ischemia-induced hippocampal damage remains controversial. The aim of the present study was to evaluate the effects of pre-ischemia treadmill training on hippocampal CA1 neuronal damage after cerebral ischemia. Male adult rats were randomly divided into control, ischemia and exercise + ischemia groups. In the exercise + ischemia group, rats were subjected to running on a treadmill in a designated time schedule (5 days per week for 4 weeks). Then rats underwent cerebral ischemia induction through occlusion of common carotids followed by reperfusion. At 4 days after cerebral ischemia, rat learning and memory abilities were evaluated using passive avoidance memory test and rat hippocampal neuronal damage was detected using Nissl and TUNEL staining. Pre-ischemic exercise significantly reduced the number of TUNEL-positive cells and necrotic cell death in the hippocampal CA1 region as compared to the ischemia group. Moreover, pre-ischemic exercise significantly prevented ischemia-induced memory dysfunction. Pre-ischemic exercise mighct prevent memory deficits after cerebral ischemia through rescuing hippocampal CA1 neurons from ischemia-induced degeneration.
Keywords: nerve regeneration, physical exercise, cerebral ischemia, hippocampus, apoptosis, Nissl staining, TUNEL, memory, neural regeneration
Introduction
Cerebral ischemia is a pathological phenomenon in which blood supply to the brain is restricted. In this condition, oxygenation and glucose transport to the brain is impaired which can be deleterious. Duration of cerebral ischemia and reperfusion of blood supply to the affected organ are critical factors that should be considered in the treatment of ischemia/reperfusion-induced brain injury (White et al., 1996; Neumar, 2000). Different parts of the brain, especially the hippocampus, are sensitive to ischemia-related structural and functional damage which can be aggravated following reperfusion (White et al., 2000). The hippocampus, as a part of limbic system, plays a pivotal role in the long-term memory formation (Bartsch et al., 2010; Robin et al., 2015). Previous studies indicated that pyramidal neurons in the CA1 region of the hippocampus are among the most susceptible areas of the brain to cerebral ischemia (Mitani et al., 1992).
The underlying mechanisms that lead to ischemia-induced neuronal damage are not fully understood. According to previous studies, several types of cell death, such as autophagy (Adhami et al., 2006), apoptosis and necrosis are involved in the ischemic brain injury (Zheng et al., 2009). It has been established that pyramidal neurons are critically involved in learning and memory functions, and passive avoidance memory impairment after ischemia is related to the degeneration of CA1 neurons (Karasawa et al., 1994).
Duration of ischemia is a prominent contributing factor to the pathogenesis of pyramidal neurons of the CA1 region in the hippocampus (Colbourne et al., 1999). The CA1 pyramidal neurons of rat hippocampus degenerate within 2 to 4 days after transient ischemia (Erfani et al., 2015b). Among the several methods for inducing transient cerebral ischemia in animal models, bilateral common carotid artery occlusion, is considered as one of the most simple and reliable approach. Temporary occlusion of carotid arteries for 5 minutes causes delayed neural cell death in the hippocampal CA1 region (Kirino et al., 1985). Furthermore, this model of ischemia leads to deficits in spatial learning and memory.
In recent years, there has been an increasing interest in the evaluation of the effects of exercise on brain injuries induced by ischemia (Ding et al., 2006). Evidence indicated that exercise might protect cerebral neurons against ischemic brain injury (Zhang et al., 2011). In addition, physical exercise exerts beneficial and protective effects on hippocampal neurons (Prakash et al., 2015). However, the exact role(s) of physical exercise in protecting hippocampal CA1 neurons against cerebral ischemia has/have not been well understood. The aim of this study was to evaluate the effect of exercise preconditioning on apoptosis and necrosis in the hippocampal CA1 neurons and memory impairment following transient global ischemia.
Materials and Methods
Animals and surgical procedures
Twenty-one adult male Wistar rats, weighting 260–300 g, were obtained from Pasteur Institute of Iran. For acclimatization to new condition, rats were kept on standard conditions (22–24°C, 45–50% humidity, 12-hour light/dark cycle) 2 weeks prior to commencement of study. All animal experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All efforts were made to minimize animal discomfort and reduce the number of animals used.
Twenty-one rats were randomly and equally divided into three groups with 7 rats per group: sham (control) group, ischemia group, and exercise + ischemia group (ischemia with 4 weeks of exercise preconditioning). Transient cerebral ischemia was induced in rats according to the procedure which has been described by Sharifi et al. (2011). In brief, rats were anesthetized with a mixture of ketamine/xylazine (40 mg/kg, i.p.) and surgical dissection was performed to expose the common carotid arteries and then the vagus nerve was carefully separated from these arteries. Common carotid arteries were occluded for approximately 20 minutes using Yashargil Aneurism microclips. In the next step, the microclips were removed and the arteries were monitored for observing the blood flow. During the operation, the rectal temperature of rats were monitored and maintained in an appropriate range (36.5 ± 0.5°C). At the end of surgery, rats were placed back into the standard cages and fed ad libitum. The rats which were treated by surgical operations were kept in separate cages for 4 days (96 hours). For sham operation, the carotid arteries were exposed by surgical dissection except that the occlusion of these arteries was not performed.
Exercise training protocol
With some modifications, the protocol for exercise training was performed according to the method described by Chen et al. (2007). In brief, the rats were trained to run on a treadmill (4-Lane animal treadmill; IITC Life Science Inc., USA) 5 days a week for 4 weeks. In order to acclimatize for running on the treadmill, rats were subjected to running for a period of 10–15 minutes at 5–7 m/min without slope on the running area. The acclimatization procedure was executed for 2 days before commencement of experiment. Initially, the rats were stimulated by electrical shocks (1.0 mA) to run forward. After adaptive running, the rats were trained based on the aforementioned time schedule (5 days/week for 4 weeks). The formal treadmill training was started at 18 m/min for 35 minutes with 0° slope in the first week and then the time interval and the slope of running area were progressively increased in the remaining weeks of training. Rats were subjected to exercise for 40 minutes at 18 m/min with 5° slope, for 45 minutes at 18 m/min with 10° slope and for 50 minutes at 18 m/min with 15° slope after 2, 3 and 4 weeks of training, respectively. The rats in the sham and ischemia groups (sedentary rats) were located on an immotile treadmill every day, and were stimulated by electrical shock in a similar manner used for the exercise group. As treadmill training may cause some unpredictable stress in rats, rats were weighted regularly during the experiment (the body weight of rats during the experiment was 260–300 g).
Passive avoidance memory test
In strict accordance with the procedures described previously (Harooni et al., 2009; Erfani et al., 2015b), passive avoidance memory test was performed in all included rats at 4 days after induction of cerebral ischemia. The apparatus used for this test was the two-way shuttle box (Borj Sanaat Co., Tehran, Iran), which was comprised of two distinct parts. One part was an illumination compartment with identical dimensions (30 × 20 × 20 cm3) which was made of transparent plastic and the other part was a dark compartment (30 × 20 × 20 cm3), the walls and ceiling of which were made of dark opaque plastic. The two chambers were separated from each other by an arced angular opening (8 × 8 cm2) which can be closed by an opaque guillotine door. The grid floor of the box was made of stainless steel rods (2 mm diameter), spaced 1 cm apart. The floor of the dark compartment could be connected with an electrical power supply to stimulate electrical shock.
The experiment consisted of three chronologically distinct steps. The adaptation session was the first step in which rats were placed in the transparent chamber for 1 hour. This step was performed in order to acclimatize rats to the new habitat. In the second step, namely training session, rats were allowed to move freely in the box, but when they entered into the dark chamber the opening was closed and immediately an electrical shock was induced (0.5 mA, 3 seconds). The training session took for 24 hours and then the last session which was called “memory retention” was performed. In the third step, the rats were placed back into the transparent chamber and the guillotine door remained open. Step-through latency (STL) and total time spent in dark chamber during the memory retention session were recorded. In this session, no electrical shock was delivered and the experiment lasted for 300 seconds in which the retention latency of rats to enter the dark chamber was recorded.
Tissue preparation
Four days (96 hours) after induction of cerebral ischemia, rats were anesthetized and perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde dissolved in 0.1M phosphate buffer (pH 7.4) (Aboutaleb et al., 2014). The brains were carefully removed from the skulls, fixed in the same fixative overnight and then embedded in paraffin. Coronal sections were prepared from paraffin-embedded brain tissues using a standard microtome which was adjusted to cut the blocks at 7 μm thicknesses. The sections between 3.3 and 4.2 mm posterior to bregma according to the Paxinos atlas were used for Nissl and TUNEL staining (Paxinos and Watson, 2007).
Nissl staining
Nissl staining is commonly used for identifying the basic neuronal structure from necrotic neurons in brain (Erfani et al., 2015a). For Nissl staining, 7 μm-thick sections (three sections per rat) were mounted directly onto glass slides which were pre-coated by gelatin. The slides were remained to dry and stained with 0.1% cresyl violet (Sigma-Aldrich, St. Louis, MO, USA), dehydrated, and finally cover-slipped with Entellan. Three photomicrographs were prepared from each rat and the numbers of necrotic cells were blindly counted by a light microscope (Olympus AX-70) at 400× magnification. The samples were investigated by an observer. Analyses of images were executed by image tool software (OLYSIABio Report Soft Imaging System GmbH, Version 3.2 (Build 670), Boston, MA, USA). The number of cells was counted along a transect (400 μm long, 0.160 mm2) in the CA1 region of the right hippocampus. Only sparsely arranged cells with fuzzy shape and had no evident nuclei and nucleoli were considered as necrotic cells and included.
TUNEL staining
The occurrence of apoptosis in hippocampal CA1 cells was assessed using TUNEL assay (Khaksari et al., 2012; Gheibi et al., 2014). TUNEL staining was performed using a commercial kit (In Situ Cell Death Detection Kit; Roche, Mannheim, Germany) according to the instruction provided by manufacturer. In brief, the sections (three sections per rat) were incubated in xylol for deparaffinization, dehydrated in a series of alcohols, rinsed in PBS, and treated by 10 mM proteinase K for 30 minutes at room temperature to permeabilize the sections. Subsequently, to block endogenous peroxidase activity which could lead to false positive results, the sections were washed and incubated with 3% H2O2 in methanol for 10 minutes in a dark place. After addition of TUNEL reaction mixture, the sections were incubated for 60 minutes at 37°C in a chamber with moderate humidity, rinsed with PBS and visualized using converter-POD for 30 minutes at 37°C in a dark place with appropriate humidity. Then, the sections were washed with PBS and incubated with 50–100 μL 0.05 % 3,3′-diaminobenzidine (DAB) as a chromogen for 10 minutes. After PBS washes, the sections were mounted by acover slip. Under the light microscope, the number of TUNEL-positive cells was counted along a transect (400 μm long, 0.160 mm2) in the CA1 area of the right hippocampus. All counting procedures were performed blindly.
Statistical analysis
Data analysis was performed using 16.0 SPSS software (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to determine if the distribution of data is normal. In order to compare the mean differences among the groups, one-way analysis of variance (ANOVA) was used. When a significant difference was observed, the mean difference between each two groups was assessed using Dunnett's T3 or Scheffe's post hoc test. The Scheffe's post hoc test was used when the homogeneity of variance was established; otherwise, the Dunnett's T3 post hoc test was applied. The results are shown as the mean ± SD and a level of P ≤ 0.05 was considered statistically significant.
Results
Exercise preconditioning improved ischemia-induced memory deficits
Preliminary data obtained from the passive avoidance memory test showed that the average time for rats to cross from the transparent (illuminated) chamber to the dark one, was about 30 seconds (data not shown). In the retention session, just after a 24-hour training session, the latency to cross into the dark chamber was evaluated and the results showed a statistically significant difference among groups. As shown in Figure 1, rats in the sham group showed a significant increase in the average time for entering into the dark compartment. This latency after electrical stimulation training could be due to acquired memory of the aversive stimulation. Moreover, a significant reduction in response latency was observed in the ischemia group in comparison with sham group (P < 0.05); this response latency was significantly increased in the exercise group compared to the ischemia group (P < 0.05) (Figure 1A). Figure 1B shows the effect of exercise preconditioning on total time which rats in different groups spent in the dark chamber during the retention step of the passive avoidance memory test. Further analysis of this experiment also revealed that the time spent in the dark compartment increased in the ischemia group compared with the sham group (P < 0.05), this response was significantly decreased in the exercise group compared to the ischemia group (P < 0.05) (Figure 1).
Figure 1.
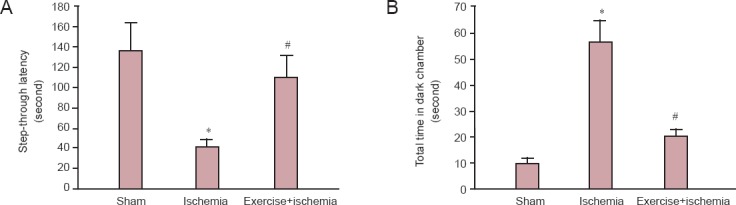
The effects of pre-ischemic exercise on memory function of rats with cerebral ischemia (passive avoidance memory test).
(A) Step-through latency. (B) Total time that the rats spent in the dark chamber during the retention session (n = 7 rats per group, 3 sections per rat). The results are shown as the mean ± SD. *P < 0.05, vs. sham group. #P < 0.05, vs. ischemia group (analysis of variance with Dunnett's T3 post-hoc test).
Exercise preconditioning modulated ischemia/reperfusion-induced neuronal injury in the hippocampal CA1 region
Nissl staining results showed that in the hippocampal CA1 area of ischemic rats, the cells were sparsely distributed and the number of cells with eumorphism was significantly reduced (Figure 2A–D). Based on the results, transient cerebral ischemia led to significantly increased necrotic death in the hippocampal CA1 region in the ischemia group (10.56 ± 7.099) as compared with the sham group (P < 0.001) which failed to show any sign of cell death in the hippocampal CA1 region. The rate of necrotic cell death was significantly decreased in the rats that received exercise preconditioning compared to the ischemia rats that did not receive (P < 0.001; Figure 2E).
Figure 2.
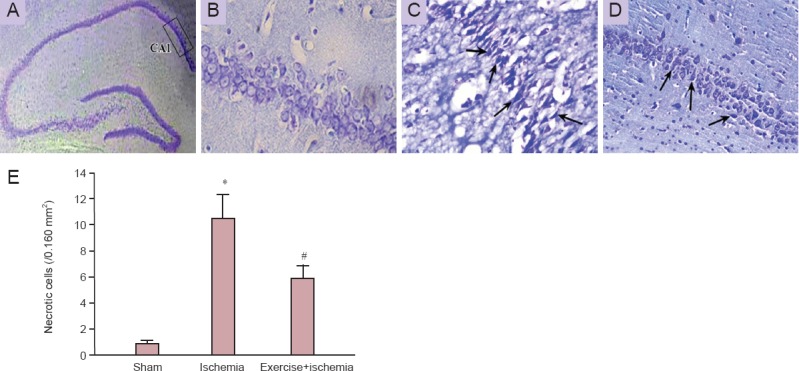
Nissl staining of hippocampal CA1 region after induction of transient global cerebral ischemia.
(A) The CA1 region of the hippocampus; (B) sham group; (C) ischemia group; (D) exercise + ischemia group. Damaged cells were sparsely arranged and their shapes were fuzzy (arrows indicate the necrotic cells; B–D are higher magnification (400×) of the boxed area in A). (E) Effects of pre-ischemic exercise on ischemia-induced necrosis in the hippocampal CA1 neurons (n = 7 rats per group, 3 sections per rat). The results are shown as the mean ± SD. *P < 0.001, vs. sham group; #P < 0.001, vs. ischemia group (analysis of variance with Scheffe's post-hoc test).
TUNEL staining results showed that the number of TUNEL-positive cells in the CA1 region of the rat hippocampus in the ischemia group was significantly increased compared with that in the sham group (P < 0.001). The number of TUNEL-positive cells was significantly decreased in the ischemia rats that received exercise preconditioning than in the ischemia rats that did not receive (P < 0.05) (Figure 3).
Figure 3.
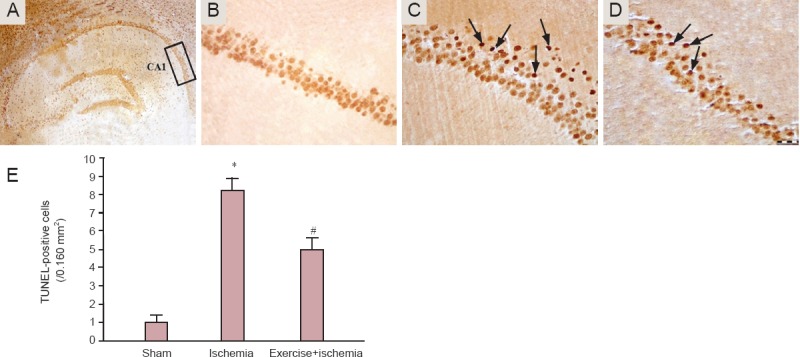
TUNEL staining of hippocampal CA1 region after induction of transient global cerebral ischemia.
(A) The CA1 region of the hippocampus; (B) sham group; (C) ischemia group; (D) exercise + ischemia group (arrows indicate TUNEL-positive cells; B, C and D are higher magnification (400×) of the boxed area in A). (E) Effect of pre-ischemic exercise on the number of TUNEL-positive cells in the hippocampal CA1 region after cerebral ischemia (n = 7 rats per group, 3 sections per rat). The results are shown as the mean ± SD. *P < 0.001, vs. sham group; #P < 0.05, vs. ischemia group; analysis of variance with Scheffe's post-hoc test).
Discussion
Results from this study revealed that ischemic brain injury accelerated cellular apoptosis and necrosis in the hippocampal CA1 pyramidal cells, leading to memory deficits. However, exercise preconditioning significantly reduced the rate of apoptotic and necrotic cell death in the hippocampal CA1 neurons of rats with cerebral ischemia. In addition, exercise preconditioning ameliorated ischemia-induced memory deficits.
One possible mechanism for the ischemia-induced neural cell death is to deregulate the release of the glutamate and excitotoxicity (Jia et al., 2010; Moskowitz et al., 2010). It has been shown that uncontrolled release of glutamate, as a neurotransmitter, and over-activation of its respective receptors might aggravate neuronal damages induced by cerebral ischemia, especially in hippocampal neurons.
A very recent study suggests that exercise preconditioning might alleviate the over-activation of glutamate and its receptor and consequently modulates ischemia-induced brain damages (Wang et al., 2015). On the other hand, Jia et al. (2010) showed that treadmill training can protect striatal neurons from ischemic injury via preventing N-methyl-D-aspartate (NMDA)-induced cytotoxicity. Accordingly, NMDA receptors are possibly involved in the neuroprotective effects of exercise preconditioning. The polarity of mitochondrial membrane is strictly dependent on calcium ion. Interestingly, NMDA receptor increases the concentration of intracellular calcium ([Ca2+]i) and so is involved in regulation of mitochondrial membrane depolarization and permeability (Kamat et al., 2015). As it is clear, calcium ion dysregulation in the mitochondria is a critical step for initiating the apoptosis (Simpkin et al., 2007).
The other possible mechanism for neuroprotective ability of exercise preconditioning could be its capacity in blocking free radical formation. Reactive oxygen species (ROS)-induced oxidative stress plays a vital role in the ischemic cascade (Chan, 2001). Physical exercise is known to improve anti-oxidant capacity and increase CuZn-SOD protein expression in mice (Hoffman-Goetz and Spagnuolo, 2007).
Inflammation plays a key role in the pathogenesis of cerebral ischemia. Tumor necrosis factor (TNF)-α, as a pro-inflammatory cytokine, is over-expressed under certain conditions such as stroke and brain injury (Sairanen et al., 2001). However, interestingly, in the case of cerebral ischemia, TNF-α has a neuroprotective effect and is involved in the brain tissue repair (Wang et al., 2001). Moreover, the role of TNF-α as a potential neuroprotective factor in those subjected to exercise preconditioning, is an important issue that should not be ruled out.
Previous studies suggested that chronic physical exercise leads to an increase in the concentration of TNF-α which in turn causes down regulation of its respective receptor during cerebral ischemia (Reyes et al., 2006). The exact mechanism(s) underlying the beneficial effects of TNF-α is/are not well understood and therefore further studies are required.
It has been suggested that heat shock protein (HSP)-70 exerts it effects through over-expression of anti-apoptotic (pro-survival) proteins such as Bcl-2 and Bcl-xL (Liebelt et al., 2010). Accordingly, it is plausible to hypothesize that HSP-70 might be involved in the protection of nerve cells during the course of cerebral ischemia (Giffard and Yenari, 2004). A recent study demonstrated that pre-ischemic treadmill training may reduce neuronal apoptosis and brain infarction volume through the up-regulation of HSP-70 (Liebelt et al., 2010).
Memory deficit is another adverse outcome of ischemic injury which may be due to the hippocampal damage (Squire and Zola, 1996). Results from the present study indicated that treadmill exercise preconditioning for 4 weeks significantly ameliorated ischemia-induced memory deficits in ischemic rats by preventing the two main types of cell death (apoptosis and necrosis) in the CA1 region of the hippocampus.
Neurotrophic factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are a part of very diverse and complicated factors implicated in memory functions of the hippocampus. The production of such growth factors is affected by several factors including physical exercise (Russo-Neustadt et al., 2000). The neuroprotective effects of physical exercise are in part, conveyed through the up-regulation of neurotrophic factors such as BDNF and NGF. Neural regeneration and networking are examples of the several effects which are attributed to the action of neurotrophins (Cohen-Cory et al., 2010). BDNF and NGF gene expression levels are increased as a result of several weeks of regulated physical exercise (Ding et al., 2004). Over-expression of neurotrophic factors as a result of chronic physical exercise can improve the capacity of nerve cells against cerebral ischemia. Preclinical studies in rats showed that physical exercise can lead to an increase in the production of BDNF and NGF in a cerebral ischemia model, and consequently potentiate the response of the rats against cerebral ischemia-induced brain injury (Ang et al., 2003).
In conclusion, pre-ischemic exercise reduces hippocampal injuries after cerebral ischemia through preventing neuronal necrosis and apoptosis. In addition, exercise preconditioning ameliorated ischemia-induced memory dysfunction. The present study suggests that pre-treatment with exercise might be a beneficial strategy for reducing brain complications caused by ischemic brain injury.
Footnotes
Funding: This study was supported by a grant (under the contract number 91052159) sponsored by the Iran National Science Foundation (INSF).
Conflicts of interest: None declared.
Copyedited by Mehrijerdi FZ, Ahmed A, Bauchet L, Li CH, Song LP, Zhao M
References
- Aboutaleb N, Kalalianmoghaddam H, Eftekhari S, Shahbazi A, Abbaspour H, Khaksari M. Apelin-13 inhibits apoptosis of cortical neurons following brain ischemic reperfusion injury in a transient model of focal cerebral ischemia. Int J Pept Res Ther. 2014;20:127–132. [Google Scholar]
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Wong P, Moochhala S, Ng Y. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118:335–345. doi: 10.1016/s0306-4522(02)00989-2. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Schönfeld R, Müller F, Alfke K, Leplow B, Aldenhoff J, Deuschl G, Koch J. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chen SH, Chou W, Lo YM, Hung CH, Lin MT. Exercise pretraining protects against heatstroke-induced cerebral ischemia in rats. Br J Sports Med. 2007;41:597–602. doi: 10.1136/bjsm.2006.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain‐derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Li H, Buchan AM. Continuing postischemic neuronal death in CA1 influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke. 1999;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- Ding YH, Mrizek M, Lai Q, Wu Y, Reyes R, Li J, Davis WW, Ding Y. Exercise preconditioning reduces brain damage and inhibits TNF-α receptor expression after hypoxia/reoxygenation: an in vivo and in vitro study. Curr Neurovasc Res. 2006;3:263–271. doi: 10.2174/156720206778792911. [DOI] [PubMed] [Google Scholar]
- Erfani S, Aboutaleb N, Oryan S, Shamsaei N, Khaksari M, Kalalian-Moghaddam H, Nikbakht F. Visfatin inhibits apoptosis and necrosis of hippocampus CA3 cells following transient global ischemia/reperfusion in rats. Int J Pept Res Ther. 2015a;21:223–228. [Google Scholar]
- Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, Jamali-Raeufy N, Gorjipour F. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides. 2015b;49:63–68. doi: 10.1016/j.npep.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, Mehrjerdi FZ, Gheibi A. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. J Mol Neurosci. 2014;54:264–70. doi: 10.1007/s12031-014-0284-9. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Yenari MA. Many mechanisms for Hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Harooni HE, Naghdi N, Sepehri H, Rohani AH. The role of hippocampal nitric oxide (NO) on learning and immediate, short-and long-term memory retrieval in inhibitory avoidance task in male adult rats. Behav Brain Res. 2009;201:166–172. doi: 10.1016/j.bbr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Hoffman-Goetz L, Spagnuolo P. Effect of repeated exercise stress on caspase 3 Bcl-2 HSP 70 and CuZn-SOD protein expression in mouse intestinal lymphocytes. J Neuroimmunol. 2007;187:94–101. doi: 10.1016/j.jneuroim.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Jia J, Hu YS, Wu Y, Yu HX, Liu G, Zhu DN, Xia CM, Cao ZJ, Zhang X, Guo QC. Treadmill pre-training suppresses the release of glutamate resulting from cerebral ischemia in rats. Exp Brain Res. 2010;204:173–179. doi: 10.1007/s00221-010-2320-5. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Tyagi SC, Tyagi N. Hydrogen sulfide epigenetically attenuates homocysteine‐induced mitochondrial toxicity mediated through NMDA receptor in mouse brain endothelial (bEnd3) cells. J Cell Physiol. 2015;230:378–394. doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa Y, Araki H, Otomo S. Changes in locomotor activity and passive avoidance task performance induced by cerebral ischemia in Mongolian gerbils. Stroke. 1994;25:645–650. doi: 10.1161/01.str.25.3.645. [DOI] [PubMed] [Google Scholar]
- Khaksari M, Aboutaleb N, Nasirinezhad F, Vakili A, Madjd Z. Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J Mol Neurosci. 2012;48:201–208. doi: 10.1007/s12031-012-9808-3. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tamura A, Sano K. Selective vulnerability of the hippocampus to ischemia-reversible and irreversible types of ischemic cell damage. Prog Brain Res. 1985;63:39–58. doi: 10.1016/S0079-6123(08)61974-3. [DOI] [PubMed] [Google Scholar]
- Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Mitani A, Andou Y, Kataoka K. Selective vulnerability of hippocampal CA1 neurons cannot be explained in terms of an increase in glutamate concentration during ischemia in the gerbil: brain microdialysis study. Neuroscience. 1992;48:307–313. doi: 10.1016/0306-4522(92)90492-k. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 6th ed. Amsterdam: Elsevier Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- Reyes R, Jr, Wu Y, Lai Q, Mrizek M, Berger J, Jimenez DF, Barone CM, Ding Y. Early inflammatory response in rat brain after peripheral thermal injury. Neurosci Lett. 2006;407:11–15. doi: 10.1016/j.neulet.2006.07.071. [DOI] [PubMed] [Google Scholar]
- Robin J, Hirshhorn M, Rosenbaum RS, Winocur G, Moscovitch M, Grady CL. Functional connectivity of hippocampal and prefrontal networks during episodic and spatial memory based on real‐world environments. Hippocampus. 2015;25:81–93. doi: 10.1002/hipo.22352. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard R, Huang Y, Cotman C. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Lindsberg P, Brenner M, Carpen O, Sirén AL. Differential cellular expression of tumor necrosis factor-α and type I tumor necrosis factor receptor after transient global forebrain ischemia. J Neurol Sci. 2001;186:87–99. doi: 10.1016/s0022-510x(01)00508-1. [DOI] [PubMed] [Google Scholar]
- Sharifi ZN, Abolhassani F, Zarrindast MR, Movassaghi S, Rahimian N, Hassanzadeh G. Effects of FK506 on hippocampal CA1 cells following transient global ischemia/reperfusion in Wistar rat. Stroke Res Treat. 2011;2012:1–8. doi: 10.1155/2012/809417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemiareperfusion injury. Basic Res Cardiol. 2007;102:518–528. doi: 10.1007/s00395-007-0671-2. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Ischemic brain damage and memory impairment: a commentary. Hippocampus. 1996;6:546–552. doi: 10.1002/(SICI)1098-1063(1996)6:5<546::AID-HIPO7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang RY, Yang YR, Yu SM. Protective effects of treadmill training on infarction in rats. Brain Res. 2001;922:140–143. doi: 10.1016/s0006-8993(01)03154-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Feng R, Li WB, Ren SQ, Zhang F. Exercise pre-conditioning alleviates brain damage via excitatory amino acid transporter 2 and extracellular signal-regulated kinase 1/2 following ischemic stroke in rats. Mol Med Rep. 2015;11:1523–1527. doi: 10.3892/mmr.2014.2834. [DOI] [PubMed] [Google Scholar]
- White BC, Grossman LI, O’neil BJ, Degracia DJ, Neumar RW, Rafols JA, Krause GS. Global brain ischemia and reperfusion. Ann Emerg Med. 1996;27:588–594. doi: 10.1016/s0196-0644(96)70161-0. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, Degracia DJ, O’neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang Q, Pu H, Wu Y, Bai Y, Vosler P, Chen J, Shi H, Gao Y, Hu Y. Very early-initiated physical rehabilitation protects against ischemic brain injury. Front Biosci. 2011;4:2476–2489. doi: 10.2741/e559. [DOI] [PubMed] [Google Scholar]
- Zheng YQ, Liu JX, Li X. Z, Xu L, Xu YG (2009) RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol Sin. 30:919–927. doi: 10.1038/aps.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]


