Abstract
This study investigated whether bone marrow mesenchymal stem cell (BMSC) transplantation protected ischemic cerebral injury by stimulating endogenous erythropoietin. The model of ischemic stroke was established in rats through transient middle cerebral artery occlusion. Twenty-four hours later, 1 × 106 human BMSCs (hBMSCs) were injected into the tail vein. Fourteen days later, we found that hBMSCs promoted the release of endogenous erythropoietin in the ischemic region of rats. Simultaneously, 3 μg/d soluble erythropoietin receptor (sEPOR) was injected into the lateral ventricle, and on the next 13 consecutive days. sEPOR blocked the release of endogenous erythropoietin. The neurogenesis in the subventricular zone was less in the hBMSCs + sEPOR group than in the hBMSCs + heat-denatured sEPOR group. The adhesive-removal test result and the modified Neurological Severity Scores (mNSS) were lower in the hBMSCs + sEPOR group than in the heat-denatured sEPOR group. The adhesive-removal test result and mNSS were similar between the hBMSCs + heat-denatured sEPOR group and the hBMSCs + sEPOR group. These findings confirm that BMSCs contribute to neurogenesis and improve neurological function by promoting the release of endogenous erythropoietin following ischemic stroke.
Keywords: nerve regeneration, stem cells, erythropoietin, ischemic stroke, erythropoietin receptor, cell proliferation, cytokine, BrdU, functional recovery, NSFC grant, neural regeneration
Introduction
The use of bone marrow mesenchymal stem cells (BMSCs) as a therapy for stroke is attractive in that it can provide an exogenous supply of cells capable of neurogenesis, angiogenesis, and endogenous plasticity after ischemic stroke (Chopp and Li, 2002; Chen et al., 2003a, b; Wu et al., 2007; Li et al., 2010; Wakabayashi et al., 2010; Sidoryk-Wegrzynowicz et al., 2011). We demonstrated previously that systemic injection of human BMSCs (hBMSCs) in transient middle cerebral artery occlusion (tMCAo) rats promoted angiogenesis, neurogenesis, and functional recovery (Li et al., 2008). However, the underlying beneficial effects have not been fully investigated. Some functional benefits of these cells arise from their ability to produce brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, vascular endothelial growth factor and hepatocyte growth factor (Chen et al., 2002; Andrews et al., 2008; Bao et al., 2011; Smith and Gavins, 2012). We have shown that this trophic support is related to the degree of neurogenesis (Choi et al., 2010). Erythropoietin (EPO) is a cytokine that plays critical roles in hematopoiesis. Recent studies demonstrated that exogenous EPO acts as a neuroprotectant and regulates neurogenesis (Studer et al., 2000; Shingo et al., 2001; Wang et al., 2004; Choi et al., 2010). A recent study using EPO or EPO receptor (EPOR) transgenic mouse showed that endogenous EPO and EPOR are associated with neurogenesis (Tsai et al., 2006). This study was designed to assess the potential role of BMSCs as trophic supports for EPO to the ischemic brain.
Materials and Methods
Establishment of tMCAo animal model
A total of 36 male Sprague-Dawley rats aged 2 months and weighing 250–300 g were provided by Animal Laboratory of Zhejiang University in China (license No. SCXK (Zhe) 2012-0178). Animals were housed under standard conditions with free access to food and water. The use of animals in this study was approved by the Local Animal Care and Use Committee of Sir Run Run Shaw Hospital in China, and all procedures were carried out in accordance with institutional guidelines. Precautions were taken to minimize suffering and limit the number of animals used in each experiment. All rats were anesthetized with 4% isoflurane and maintained with 1.5% isoflurane in 70% N2O and 30% O2 using a face mask. Rectal temperature was maintained at 37.0–37.5°C with heating pads. Briefly, tMCAo was induced using a protocol of intraluminal vascular occlusion as previously described by our laboratory (Chen et al., 1992). To block the origin of the MCAo, a 4-0 surgical monofilament nylon suture with a rounded tip was moved from the left common carotid artery into the lumen of the internal carotid artery. At 2 hours after MCAo, reperfusion was performed by withdrawing the suture to the tip of the common carotid artery. Sham animals underwent an identical surgical procedure without the occlusion.
hBMSCs culture
BMSCs were obtained according to the World Medical Association outlined in the Declaration of Helsinki. All participants provided written informed consent. hBMSCs were obtained from 20-mL aspirates from the iliac crest of normal human donors (Bang et al., 2005) according to a protocol approved by the Scientific-Ethical Review Board of Sir Run Run Shaw Hospital in China. Briefly, cells were incubated in 150-cm2 rectangular canted neck cell culture flasks (Corning Incorporated Life Sciences, Tewksbury, MA, USA) at 37°C in 5% CO2 for 1 day; the non-adherent cells were removed by changing the medium. The medium was changed every 2–3 days until the adherent cells were 80% confluent (usually 10–14 days). After the cells were passaged twice, they were harvested with 0.05% trypsin and 0.53-mM ethylenediamine tetraacetic acid (Gibco, Carlsbad, CA, USA) for 5 minutes at 37°C, replated in a flask, and cultured for an additional 3–5 days. The expression levels of surface markers CD105 and CD73 in hBMSCs were evaluated using flow cytometry (n = 6; FACScan; Becton Dickinson, Rutherford, NJ, USA), and hBMSCs exhibited high expression levels (96–99%) of these stem cell markers (data not shown).
Experimental groups
The rats were randomly allocated to the two parts of the study. The first part was designed to assess whether hBMSCs therapy influenced EPO levels using enzyme-linked immunosorbent assay (ELISA). This was divided into sham, tMCAo, and hBMSCs groups (n = 6 for each group). The second part of this study assessed whether blocking EPO influenced hBMSCs-induced 5-bromo-2-deoxyuridine (BrdU)-positive cell proliferation and recovery of neurological functional, using BrdU staining and a behavior test. There were a heat-denatured sEPOR group, an hBMSCs + heat-denatured sEPOR group, and an hBMSCs + sEPOR group (n = 6 for each group).
hBMSCs injection
hBMSCs (1 × 106) were injected into the tail vein of each rat at 24 hours after tMCAo in the hBMSCs, hBMSCs + heat-denatured sEPOR and hBMSCs + sEPOR groups and rats were not immunosuppressed after the hBMSCs transplantation. PBS (1-mL) was injected into the tail vein of the rats in the tMCAo group and heat-denatured sEPOR only group.
Tissue preparation and immunohistochemical assay
Rats in the second part of the study received daily intraperitoneal injections of 50 mg/kg BrdU at 24 hours after tMCAo for 13 consecutive days. The rats were sacrificed at 14 days after tMCAo by an overdose of anesthesia. The rat brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde solution. The brains were removed quickly and kept in 4% paraformaldehyde solution overnight at 4°C, embedded in 30% sucrose solution until they sank. Preliminary immunohistochemical assay was performed using 30-μm-thick coronal sections. Sections (60-μm-thick) were collected from every sixth section between bregma levels +1.0 and –1.0 mm (six sections per brain) to quantify BrdU labeling with unbiased stereological analysis. We counted BrdU-positive cells to evaluate the degree of proliferation as described by Li et al. (2008). To do so, DNA was first denatured by incubating sections in 2-N HCl at 37°C for 1 hour. The sections were then rinsed with Tris buffer and treated with 0.3% H2O2 to block endogenous peroxidase. The sections were blocked with 10% normal horse serum and 0.1% Triton X-100 in PBS for 30 minutes at room temperature, incubated with a mouse anti-BrdU monoclonal antibody (1:200; Roche Diagnostics, Penzberg, Germany) overnight at 4°C, with horse anti-mouse IgG (1:500; Vector Laboratories, Burlingame, CA, USA) for 1 hour at room temperature and with an avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories) for 1 hour at room temperature before developing with 3,3′-diaminobenzidine (Dako Cytomation, Glostrup, Denmark). After rinsing in 0.1 M PBS, sections were mounted on gelatin-coated slides and analyzed under a bright-field microscope (E600; Nikon, Tokyo, Japan) to determine the number of BrdU-labeled cells in the subventricular zone of the ipsilateral hemisphere (six sections per brain). The ischemic zone was defined as in a previous report (Li et al., 2008). An unbiased stereological estimate of the total number of BrdU-immunoreactive cells in the subventricular zone was made using the optical fractionator technique (Plane et al., 2004). This sampling technique is not affected by tissue volume changes and does not require reference volume determinations (West et al., 1991). Sampling was performed with the Computer-Assisted Stereological Toolbox system (version 2.3.1.5; Olympus Denmark A/S, Ballerup, Denmark) under an Olympus BX51 microscope. The subventricular zone was delineated using a 1.25× objective, which generated counting areas of 84.6 × 84.6 μm2. A counting frame (3,580 μm2) was placed randomly on the first counting area and moved through all counting areas systemically until the entire delineated area was sampled. The sampling frequency was chosen so that 100–200 BrdU-positive cells were counted in each specimen in the ipsilateral hemisphere. Actual counting was performed using a 100× oil objective lens. Guard volumes (4 μm from the top and 4–6 μm from the bottom of the section) were excluded from both surfaces to avoid the problem of lost caps, and only profiles that came into focus within the counting volume (with a depth of 20 μm) were counted. The total number of BrdU-positive cells was estimated using the optical fractionator formula (West et al., 1991).
ELISA
All animals were sacrificed at 14 days after tMCAo or sham surgery. Quantitative immunoassay was used for EPO levels, according to the manufacturer's protocol (R&D system, Shanghai, China). Tissue from the ischemic hemisphere was homogenized and lysed with 25 mM Tris, 1% Triton X-100, 0.5 mM ethylenediamine tetraacetic acid, 150 mM NaCl, and a protease inhibitor, cocktain (Calbiochem, Darmstadt, Germany). Total protein was quantified by the Bradford assay (Bradford, 1976) as previously reported (Li et al., 2008). Each sample was analyzed in triplicate.
Blocking EPO by infusion of sEPOR
A cannula, connected to an osmotic minipump (Alzet; model 1007D, 100 μL, flow rate 0.5 μL/h; Cupertino, CA, USA), was implanted into the left lateral ventricle, i.e., ipsilateral side to the stroke, to give better delivery of agents to the peri-infarct tissue. Recombinant sEPOR was infused into the left lateral ventricle as described previously (Sakanaka et al., 1998; Prass et al., 2003; Malhotra et al., 2006). The pump and cannula were primed overnight in sterile saline at 37°C to avoid any delay in pump delivery of the agent after implantation. sEPOR (R&D systems) was infused at 24 hours after tMCAo in the left lateral vehicle (PBS, pH 7.0–7.4, and 0.1% bovine serum albumin) for 13 consecutive days, in accordance with a previous method (Malhotra et al., 2006). Heat-denatured sEPOR was heated at 56°C by heating in water bath for 30 minutes. A dose of sEPOR/heat-denatured sEPOR 3 μg/day was selected because our preliminary ELISA results showed that the levels of EPO in stroke rats treated with hBMSCs were 2–3 times higher than those in rats that received PBS, and in previous reports 1 μg/day was used in a rat model of transient ischemic attack (Sakanaka et al., 1998; Prass et al., 2003; Malhotra et al., 2006).
Behavioral test
In all rats, a battery of behavioral tests was performed before tMCAo and at 1, 7 and 14 days after tMCAo. For the adhesive-removal somatosensory test (Chen et al., 2001, 2004; Li et al., 2002), two small pieces of adhesive-backed paper dots (of equal size, 113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The time taken to remove each stimulus from forelimbs was recorded in five trials per day. Before surgery, rats were trained for 3 days. If the rats were able to remove the dots within 10 seconds, they were chosen for surgery. The time taken to remove the dot was recorded. Using the modified Neurological Severity Score (mNSS), neurological function was graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18) (Markgraf et al., 1992). The mNSS is a composite of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), reflex, and balance tests. The behavioral test was conducted in triplicate on each rat.
Statistical analysis
All data are expressed as the mean ± SD. Statistical differences between groups were evaluated using one-way analysis of variance (SPSS 13.0, SPSS, Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
Results
hBMSCs promoted the release of endogenous EPO from ischemic brain tissues
ELISA results demonstrated that at 14 days after surgery, the endogenous EPO levels from ischemic tissues were higher in the tMCAo group than in the sham group (P < 0.05). Endogenous EPO levels were significantly higher in the hBMSCs group (more than twice) than in the tMCAo group and sham group (P < 0.01; Figure 1).
Figure 1.
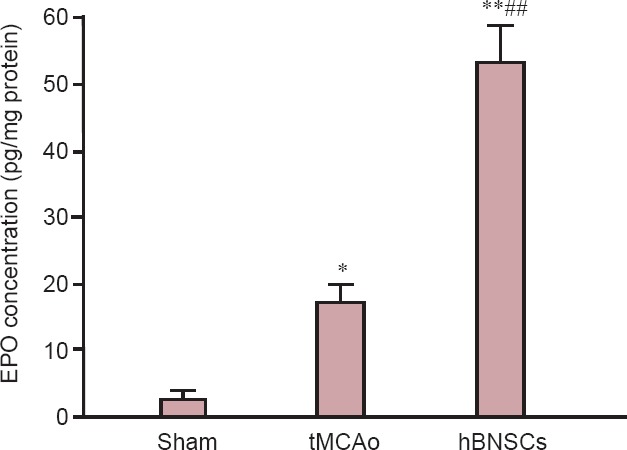
Effect of hBMSCs injection on EPO levels in brain tissue of ischemic stroke rats (enzyme linked immunosorbent assay).
Data are expressed as the mean ± SD (six rats in each group). Each sample was analyzed in triplicate. *P < 0.05, **P < 0.01, vs. sham group; ##P < 0.01, vs. tMCAo group (one-way analysis of variance). EPO: Erythropoietin; tMCAo: transient middle cerebral artery occlusion; hBMSCs: human bone marrow mesenchymal stem cells.
Blocking EPO affected hBMSCs effects on the neurological function in ischemic stroke rats
The adhesive-removal test times and mNSS score gradually decreased in the heat-denatured sEPOR, hBMSCs + heat-denatured sEPOR and hBMSCs + sEPOR groups with time. Moreover, the adhesive-removal test times and mNSS score were lower in the hBMSCs + heat-denatured sEPOR group than in the heat-denatured sEPOR-only group (P < 0.05). The adhesive-removal test time and mNSS score were similar between hBMSCs + heat-denatured sEPOR and hBMSCs + sEPOR groups (P > 0.05; Figure 2).
Figure 2.
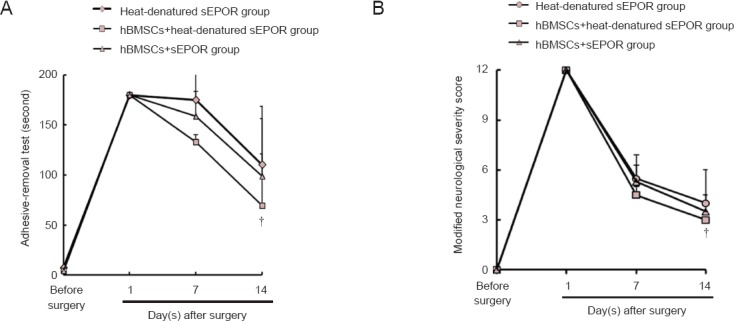
Effects of hBMSCs on neurological function in rats with ischemic stroke after blocking EPO.
Long time taken to remove each stimulus from forelimbs in the adhesive-removal test and high modified neurological severity score represent severe nerve injury. Data are expressed as the mean ± SD (six rats in each group). Behavioral test was conducted in triplicate in each rat. †P < 0.05, vs. heat-denatured sEPOR group (one-way analysis of variance). tMCAo: Transient middle cerebral artery occlusion; sEPOR: soluble erythropoietin receptor; EPO: erythropoietin; hBMSCs: human bone marrow mesenchymal stem cells.
Cell proliferation in the subventricular zone of the ischemic brain after blocking EPO by infusion of sEPOR
Immunohistochemical staining demonstrated that a few BrdU-positive cells were concentrated in the subventricular zone in the heat-denatured sEPOR group. A large number of BrdU-positive cells were detected in the subventricular zone, corpus callosum, and striatum in the hBMSCs + heat-denatured sEPOR group (data not shown). The number of BrdU-positive cells was higher in the hBMSCs + heat-denatured sEPOR group than in the heat-denatured sEPOR group (P < 0.05). The number of BrdU-positive cells was less in the hBMSCs + sEPOR group than in the hBMSC + heat-denatured sEPOR group (P < 0.05; Figure 3).
Figure 3.
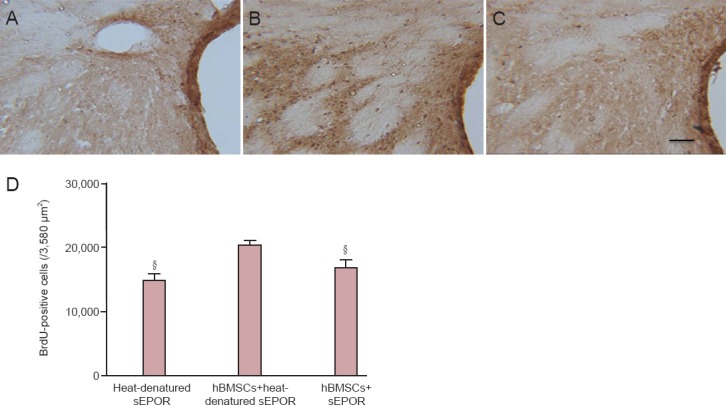
Effects of blocking EPO on cell proliferation in the SVZ of rats with ischemic injury after treatment with hBMSCs.
(A–C) Newborn cells in the SVZ of rats at 14 days after injury. BrdU-positive cells are brown. (A) Heat-denatured sEPOR group; (B) hBMSCs + heat-denatured sEPOR group; (C) hBMSCs + sEPOR group. Scale bar: 20 μm. (D) Number of BrdU-labeled cells in the SVZ. Data are expressed as the mean ± SD (six rats in each group). §P < 0.05, vs. hBMSCs + heat-denatured sEPOR group (one-way analysis of variance). tMCAo: Transient middle cerebral artery occlusion; sEPOR: soluble erythropoietin receptor; SVZ: subventricular zone.
Discussion
In the present study, functional recovery occurs in rats with tMCAo after treatment with hBMSCs, which were consistent with previous results (Chen et al., 2003b; Kurozumi et al., 2005; Koh et al., 2008; Li et al., 2008). BMSCs may promote endogenous plasticity, neurogenesis, and angiogenesis (Zhang et al., 2001, 2003; Chen et al., 2003b; Drury-Stewart et al., 2013; Iskander et al., 2013). However, functional recovery is not remarkably changed after blocking EPO by infusion of sEPOR. These data indicate that endogenous EPO may be associated with functional recovery.
Numerous studies demonstrated that growth factor levels, such as brain-derived neurotrophic factor, vascular endothelial growth factor and hepatocyte growth factor, increased in the ischemic hemisphere of hBMSC-treated rats compared with the tMCAo group (Li et al., 2002, 2008; Hess and Borlongan, 2008; Takahashi et al., 2008; Yasuhara et al., 2010). This study demonstrated that the levels of EPO increased in the ischemic hemisphere of hBMSC-treated tMCAo rats compared with the untreated tMCAo group. Exogenous EPO has been shown to act as a neuroprotectant that can regulate neurogenesis (Studer et al., 2000; Shingo et al., 2001; Wang et al., 2004; Choi et al., 2010). Another study showed that EPO enhanced progenitor cell differentiation and proliferation after a short period of administration (Bahlmann et al., 2004). Exogenous EPO may be needed to substitute for suppressed endogenous EPO and to protect EPOR expressing cells from apoptosis (Brines and Cerami, 2005). Our study demonstrated that hBMSCs treatment can enhance endogenous EPO. hBMSCs may be one of the trophic supports for EPO to ischemic brain.
Results from this study demonstrated that proliferation was enhanced by the application of hBMSCs, which was consistent with previous results that hBMSCs promoted endogenous neurogenesis (Chen et al., 2003b; Li et al., 2008; Yang et al., 2009; Shen et al., 2010). After blocking EPO by infusion of sEPOR, we found fewer BrdU-positive cells. These findings indicate that EPO can promote neurogenesis. Further studies are needed to evaluate whether the increased BrdU-positive cells are the result of increased endogenous proliferation.
This study has some limitations. We did not establish an exogenous EPO treatment group, or compare neurogenesis in the hBMSCs-treated groups to any EPO-treated groups. However, the present study focused on the effect of endogenous EPO on cell proliferation. Further studies are needed to evaluate whether the increased BrdU-positive cells are the result of increased neurogenesis.
In conclusion, hBMSCs can enhance cell proliferation in the ischemic region and this potential is associated with released EPO.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81371258; a grant from the TCM General Research Project of Zhejiang Province of China, No. 2015ZA061; a grant from the Education of Zhejiang Province of China, Y201431639.
Conflicts of interest: None declared.
Copyedited by Dawes EA, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Andrews EM, Tsai SY, Johnson SC, Farrer JR, Wagner JP, Kopen GC, Kartje GL. Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol. 2008;211:588–592. doi: 10.1016/j.expneurol.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bao X, Wei J, Feng M, Lu S, Li G, Dou W, Ma W, Ma S, An Y, Qin C, Zhao RC, Wang R. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12:621–628. doi: 10.1038/jcbfm.1992.86. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Li WY, Moon GJ, Lee PH, Ahn YH, Lee G, Bang OY. Enhancing trophic support of mesenchymal stem cells by ex vivo treatment with trophic factors. J Neurol Sci. 2010;298:28–34. doi: 10.1016/j.jns.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Drury-Stewart D, Song M, Mohamad O, Guo Y, Gu X, Chen D, Wei L. Highly efficient differentiation of neural precursors from human embryonic stem cells and benefits of transplantation after ischemic stroke in mice. Stem Cell Res Ther. 2013;4:93. doi: 10.1186/scrt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif 41 Suppl. 2008;1:94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NRS, Bagher-Ebadian H, Ali MM, Arbab AS, Janic B. Intravenous administration of human umbilical cord blood-derived AC133 + endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl Med. 2013;2:703–714. doi: 10.5966/sctm.2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, Kim HT, Kim SH. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu H, Liu Y, Li Q, Lu S, Feng M, Xu Y, Huang L, Ma C, An Y, Zhao RC, Wang R, Qin C. Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macaca fascicularis. Brain Res. 2010;1334:65–72. doi: 10.1016/j.brainres.2010.03.080. [DOI] [PubMed] [Google Scholar]
- Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res. 1992;575:238–246. doi: 10.1016/0006-8993(92)90085-n. [DOI] [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Löwl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chopp M. Astrocytes mediate bone marrow stromal cell transplantation enhanced glial cell derived neurotrophic factor (GDNF) production in the ischemic boundary zone after stroke in adult rats. Glia. 2010;58:1074–1081. doi: 10.1002/glia.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HK, Gavins FNE. The potential of stem cell therapy for stroke: is PISCES the sign? FASEB J. 2012;26:2239–2252. doi: 10.1096/fj.11-195719. [DOI] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, Takeuchi A, Yano A, Kurozumi K, Agari T, Miyoshi Y, Kinugasa K, Date I. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res. 2008;1234:172–182. doi: 10.1016/j.brainres.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Yang T, Tsang KS, Poon WS, Ng HK. Neurotrophism of bone marrow stromal cells to embryonic stem cells: noncontact induction and transplantation to a mouse ischemic stroke model. Cell Transplant. 2009;18:391–404. doi: 10.3727/096368909788809767. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM, Masuda T, Yu SJ, Bae EK, Hayashi T, Matsukawa N, Kaneko Y, Kuzmin-Nichols N, Ellovitch S, Cruz EL, Klasko SK, Sanberg CD, Sanberg PR, Borlongan CV. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–921. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]


