Abstract
Therapeutic hypothermia is the most promising non-pharmacological neuroprotective strategy against ischemic injury. However, shivering is the most common adverse reaction. Many studies have shown that dantrolene is neuroprotective in in vitro and in vivo ischemic injury models. In addition to its neuroprotective effect, dantrolene neutralizes the adverse reaction of hypothermia. Dantrolene may be an effective adjunctive therapy to enhance the neuroprotection of hypothermia in treating ischemic stroke. Cortical neurons isolated from rat fetuses were exposed to 90 minutes of oxygen-glucose deprivation followed by reoxygenation. Neurons were treated with 40 μM dantrolene, hypothermia (at 33°C), or the combination of both for 12 hours. Results revealed that the combination of dantrolene and hypothermia increased neuronal survival and the mitochondrial membrane potential, and reduced intracellular active oxygen cytoplasmic histone-associated DNA fragmentation, and apoptosis. Furthermore, improvements in cell morphology were observed. The combined treatment enhanced these responses compared with either treatment alone. These findings indicate that dantrolene may be used as an effective adjunctive therapy to enhance the neuroprotective effects of hypothermia in ischemic stroke.
Keywords: nerve regeneration, ischemic stroke, oxygen-glucose deprivation, fluorescent probe, neurons, flow cytometry, apoptosis, calcium overload, reactive oxygen, neural regeneration
Introduction
Therapeutic hypothermia is a non-pharmacological neuroprotective strategy receiving increasing attention (Wu and Grotta, 2013). The European Stroke Research Network for Hypothermia association has launched an open, randomized, phase III clinical trial of therapeutic hypothermia for adult patients with acute ischemic stroke (Kollmar et al., 2012; Watson, 2012). However, in clinical practice, the side effects of therapeutic hypothermia remain a great concern. Among them, shivering is the most common adverse reaction that needs to be treated by sedatives and muscle relaxants (Kollmar and Schwab, 2012).
Dantrolene, an inhibitor of ryanodine receptors, is an approved medicine for malignant hyperthermia (Hausfater, 2005). Many studies have shown that dantrolene is neuroprotective in in vitro and in vivo ischemic injury models (Yu et al., 2000; Boys et al., 2010; Javed and Bogdanov, 2010). On a cellular level, dantrolene has been shown to block the release of endoplasmic reticulum Ca2+ stores and partially protect neurons from oxygen-glucose deprivation toxicity (Wang et al., 2002). In an animal model of ischemic injury, dantrolene has demonstrated a significant decrease in infarct volume. This inhibitor is also neuroprotective against stroke in this model because it reduces the activation of the endoplasmic reticulum stress-mediated apoptotic signal pathway in the ischemic area (Li et al., 2005). Similar to the results seen in ischemic brain injury, the application of dantrolene has also been shown to help protect spinal cord neurons against ischemia/reperfusion injury in rabbits (Kocogullari et al., 2008; Aslan et al., 2009). In a clinical study, intra-arterial dantrolene induces a sustained improvement in cerebral vasospasm with no observed side effects occurring during or after its infusion (Majidi et al., 2012). In addition to its neuroprotective effect, dantrolene has been shown to accelerate body temperature cooling (Hadad et al., 2005), reduce the incidence of shivering, and elevate the shivering threshold, thereby reducing shivering thermogenesis (Muehlschlegel and Sims, 2009). Overall, dantrolene may be an effective adjunctive therapy to enhance the neuroprotective effect of hypothermia for the treatment of ischemic stroke. In this study, we used oxygen-glucose deprivation (OGD) as a model to mimic ischemic injury in vitro, and determined whether there was a synergistic neuroprotective effect of dantrolene and hypothermia.
Materials and Methods
Primary cortical neuron culture and identification
The animal experimental protocol was approved by the Animal Ethics Committee of Sun Yat-sen University, China. Pathogen-free and pregnant Sprague-Dawley rats were purchased from Guangdong Experimental Animal Center, Guangzhou, Guangdong Province, China (license No. SCXK (Yue) 2011-0015), and they were sacrificed by cervical dislocation. Fetuses at gestational day 18 were micro-dissected, and primary cortical neurons were cultured, according to our previous study (Xu et al., 2012). In brief, the apical cortex was removed and digested using papain and DNase I (Sigma-Aldrich, St. Louis, MO, USA). The sifted cell suspension was seeded onto 0.1 mg/mL poly-L-lysine-coated culture plate at a density of 50,000 cells per cm2. A 4-hour incubation in high-glucose Dulbecco's Modified Eagle's Medium (HyClone, Logan, UT, USA) allowed the neurons to adhere, and the media was then changed to Neurobasal Medium (Gibco Invitrogen, Carlsbad, CA, USA). Neurons were identified (via immunofluorescence) by Tuj1, a neuron-specific class III β-tubulin III/Tuj1 immunofluorescence (Figure 1). Briefly, cells were incubated with rabbit anti-β-tubulin III (Tuj1) polyclonal antibody (1:1,000; Abcam, Cambridge, UK) for 1 hour at 37°C. Neurons were then co-incubated with the secondary antibody, Alexa Fluor® 488 goat anti-rabbit IgG (1:200; Abcam), for an additional 1 hour at 37°C. The nuclei were labeled with 4,6-diamidino-2-phenylindole. The images were acquired with an inverted fluorescence microscope (Olympus, Tokyo, Japan).
Figure 1.
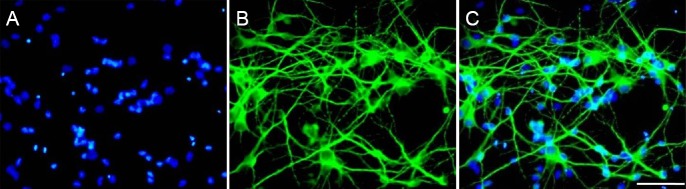
Immunofluorescence identification of primary cortical neurons.
(A) DAPI nuclear staining (blue); (B) skeleton protein staining of neuron-specific β-tubulin III (Tuj1; green); (C) more than 98% of the cells are stained by both Tuj1 and DAPI (merge). Scale bar: 100 μm. DAPI: 4′,6-Diamidino-2-phenylindole.
OGD and hypothermia or dantrolene treatment
Primary neurons were exposed to OGD, according to our previous report (Xu et al., 2014). In brief, neurons were subjected to OGD by replacing the original phenol red-free neurobasal medium with phosphate-buffered saline (PBS) pre-equilibrated with 95% N2 and 5% CO2 (Wise-Faberowski et al., 2001). Cells were then incubated in a humidified anaerobic glove box (Thermo Fisher Scientific, Waltham, MA, USA) with the oxygen concentration below 1%. After 90 minutes of OGD treatment, the neurons were transferred to normal culture conditions (neurobasal medium) and then subjected to hypothermia, hypothermia plus dantrolene (40 μM; Sigma-Aldrich), or dantrolene alone for 12 hours (Wang et al., 2002). Hypothermia was achieved by transferring the cells into a 33°C culture incubator (Thermo Fisher Scientific) (Zhou et al., 2013). DNA fragmentation and annexin V/propidium iodide (PI) labeling was performed 48 hours after OGD treatment to assess cell death. Other parameters were measured 12 hours after hypothermia with or without the administration of dantrolene after OGD, according to our previous report (Xu et al., 2014).
Morphological observations
We first examined morphological changes in OGD-treated neurons and then determined whether hypothermia, dantrolene, or their co-treatment during reoxygenation prevented OGD-induced injury. Three bright fields were randomly chosen for observation under an inverted phase-contrast microscope (Olympus). The investigator was blinded to the allocation.
Measurement of cell viability via cell counts
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8 kit, Dojindo, Kumamoto, Japan). According to the manufacturer's instructions, 200 μL of cell culture supernatant (1 × 105 cells) and 20 μL of CCK-8 solution were added to each well of the microtiter plates and incubated at 37°C for 1 hour. The optical density values were measured in a microplate reader (Biotek Instruments, Winooski, Vermont, USA) at 450 nm.
Measurement of intracellular reactive oxygen species (ROS)
The dichlorofluorescin diacetate assay kit (Beyotime, Nantong, Jiangsu, China) was used to measure ROS. Dichlorofluorescin diacetate is a fluorogenic probe that freely penetrates the cell membrane. After entering the neuron, it is oxidized to generate fluorescent dichlorofluorescin, with the fluorescence intensity correlating with the quantity of intracellular ROS levels (Liu et al., 2013). The neurons were digested with 0.25% trypsin-ethylenediamine tetraacetic acid solution (Sigma-Aldrich) and then incubated with 10 μM dichlorofluorescin diacetate at 37°C for 20 minutes. Thereafter, the neurons were analyzed using flow cytometry (BD FACS Canto II, San Jose, CA, USA) at an excitation wavelength (λ ex) of 488 nm and an emission wavelength (λ em) of 535 nm (Li et al., 2008).
Detection of intracellular free calcium
The fluo-3-acetoxymethyl ester assay kit (Beyotime) was used to detect intracellular calcium ion concentrations, according to the manufacturer's instructions. In brief, the harvested neuronal suspension was incubated with 5 μM fluo-3-acetoxymethyl ester in the dark for 1 hour at 37°C before measuring the mean fluorescence intensity by flow cytometry (λ ex: 488 nm; λ em: 535 nm) (Kuang et al., 2010).
Assessment of the mitochondrial membrane potential (ΔΨm)
The JC-1 assay kit (Beyotime) was used to assess ΔΨm, according to manufacturer's instructions. When ΔΨm is high, the JC-1 fluorescence probe accumulates in the mitochondrial matrix to form red fluorescent J-aggregates (λ ex: 520 nm; λ em: 590 nm) (Figure 2A). When ΔΨm is low, the JC-1 forms green fluorescent monomers (λ ex: 490 nm; λ em: 530 nm) (Figure 2B). The ratio of the green-red fluorescence (i.e. JC-1 monomers/J-aggregates) (Figure 2C) reflected the degree of mitochondrial damage, as previously reported (Gao et al., 2014).
Figure 2.

JC-1 fluorescence probe labeling and flow cytometry.
The change in JC-1 fluorescence reflects mitochondrial membrane function and is used as an early indicator of apoptosis. (A) J-aggregates show red fluorescence at a high ΔΨm; (B) JC-1 monomer shows green fluorescence at a low ΔΨm. Scale bars: 50 μm. (C) The mean fluorescence intensity of J-aggregates (upper panel) and the JC-1 monomer (lower panel). ΔΨm: Mitochondrial membrane potential.
Determination of cytoplasmic histone-associated DNA fragments
The photometric enzyme immunoassay kit (Roche, Basel, Switzerland) was used to qualitatively and quantitatively assess cytoplasmic histone-associated DNA fragments, according to manufacturer's instructions. Briefly, the cells were incubated with 100 μL of sample solution in a microplate for 90 minutes at 20°C. After washing, the conjugate solution containing anti-DNA-peroxidase was pipetted into each well and incubated for another 90 minutes at 20°C. Then, 100 μL of substrate was added, and incubation occurred on a plate shaker before the absorbance was measured at 405 nm on a microplate reader (Biotek Instruments).
Flow cytometry for annexin V/PI
Forty-eight hours after oxygen-glucose deprivation/reoxygenation (OGD/R), neurons were double labeled with annexin V/PI (Invitrogen, Carlsbad, CA, USA) for assessing apoptosis. Briefly, adherent neurons were digested with ethylenediamine tetraacetic acid-free trypsin, then washed and resuspended at 1 × 106/mL in the binding buffer. The cell suspension was incubated with 5 μL annexin V-FITC and 5 μL PI for 10 minutes in the dark before flow cytometry (BD FACS Canto II) (λ ex: 488 nm; λ em: 530 nm). Positive staining was confirmed under fluorescence microscopy (Figure 3). Annexin V is considered to be more suitable for cells in suspension, but less sensitive to detect early apoptosis of adherent cells (Gatti et al., 1998). For this limitation, we excluded early apoptotic cells in our calculation (Quadrant [Q] 4), and only the cells in the Q1 and Q2 were included for statistical analysis. We therefore calculated the percentage of late apoptotic and necrotic cells.
Figure 3.

Annexin V/PI double staining of the neuronal cell suspension.
(A) Annexin V-positive cells (green fluorescence) indicate early apoptosis. (B) PI-positive cells (red fluorescence) indicate necrosis. (C) Both annexin V/PI-positive cells (merge) indicate late apoptosis. Scale bars: 50 μm. PI: Propidium iodide.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed by one-way analysis of variance followed by the least significant difference or Dunnett's test. Statistical analysis was performed using IBM SPSS 19.0 software (IBM Corporation, Armonk, NY, USA).
Results
Effects of dantrolene plus hypothermia on OGD cell morphology
Cell bodies of the normal group (Figure 4A) showed stereoscopic perception and strong refractivity. After OGD and reoxygenation (OGD/R) injury, the neuronal density was reduced. In addition, neurite extension was fractured and unable to form network connections (Figure 4B). Hypothermia and dantrolene treatment alone ameliorated OGD/R-induced morphological changes (Figure 4C, D). Their combined treatment enhanced neuroprotection compared with each treatment alone (Figure 4E).
Figure 4.

Effects of hypothermia plus dantrolene on the morphology of OGD/R cells.
Phase-contrast microscopy of (A) normal group; (B) OGD/R group in which OGD/R induces injury, observed as neuronal loss, axonal rupture, and network connection fracture; (C) hypothermia group; (D) dantrolene group in which hypothermia or dantrolene treatment ameliorates the morphological abnormality; (E) hypothermia + dantrolene group in which the combined treatment enhances neuroprotection compared to either treatment alone. Scale bars: 50 μm. OGD/R: Oxygen-glucose deprivation/reoxygenation.
Dantrolene combined with hypothermia restored cell viability under OGD
OGD/R induced a dramatic (P < 0.05) reduction in cell viability compared to untreated controls (Figure 5A). This effect was markedly (P < 0.05) inhibited by hypothermia or dantrolene treatment, although this reduction was partial (Figure 5A). Furthermore, the combination of hypothermia and dantrolene was more effective in restoring cell viability than each treatment alone (P < 0.05).
Figure 5.
Neuroprotection against OGD-mediated injury occurs with hypothermia, dantrolene, or their combined administration.
DNA fragmentation and annexinV/PI labeling was performed 48 hours after OGD treatment to assess cell death, while other parameters were measured after 12 hours of hypothermia (or dantrolene) administration after OGD. (A) CCK-8: Cell viability. (B) DCFH-DA: ROS. (C) Fluo-3 AM: Free calcium ions. (D) JC-1: Monomers/J-aggregates. (E) Cell death detection: Cytoplasmic histone-associated DNA fragments. (F) Q1 + Q2: Late apoptosis and necrosis cells. Experiments were performed six times. *P < 0.05, vs. normal group; #P < 0.05, vs. OGD group; †P < 0.05, vs. H + D group. I: Normal group; II: OGD/R group; III: Hypothermia group; IV: Dantrolene group; IV: H + D. CCK-8: Cell counting kit-8; DCFH-DA: dichlorofluorescin diacetate; Fluo-3 AM: fluo-3-acetoxymethyl ester; OGD: oxygen-glucose deprivation; OGD/R: oxygen-glucose deprivation/reoxygenation; H + D: hypothermia and dantrolene; ROS: reactive oxygen species.
Effects of dantrolene and hypothermia on intracellular ROS and free calcium in OGD cells
Intracellular ROS and free calcium were significantly (P < 0.05) increased after OGD/R treatment compared with controls (Figure 5B, C). Hypothermia or dantrolene treatment alone markedly (P < 0.05) reduced these substances compared with the OGD group. Interestingly, the combination of hypothermia and dantrolene significantly (P < 0.05) enhanced the protective effect against ROS production, but its effects on free calcium overload were comparable to each treatment alone (Figure 5B, C).
Effects of dantrolene combined with hypothermia on ΔΨm
OGD/R significantly (P < 0.05) decreased ΔΨm compared to the normal group. Hypothermia or dantrolene alone significantly (P < 0.05) stabilized ΔΨm compared with the OGD group (Figure 5D). Moreover, the combination of the two treatments markedly (P < 0.05) enhanced the stabilization of ΔΨm compared with either treatment alone (Figure 5D).
Effects of dantrolene and hypothermia on cell death
Compared with the normal group, the optical density value of cytoplasmic histone-associated DNA fragments was significantly (P < 0.05) enhanced after OGD/R treatment (Figure 5E). This response was markedly (P < 0.05) reduced in hypothermia and dantrolene groups compared with the OGD group (Figure 5E). This reduction was significantly (P < 0.05) enhanced with the combined treatment compared with each treatment alone.
Effects of dantrolene plus hypothermia on apoptosis
The anti-apoptotic effects of the combined hypothermia and dantrolene treatment was lastly investigated using flow cytometry after annexin V/PI staining. Compared with the normal group, OGD/R significantly (P < 0.05) increased the number of late apoptotic and necrotic cells. This effect was markedly (P < 0.05) reduced with either hypothermia or dantrolene treatment alone compared to the OGD group (Figures 5F, 6). The combined treatment significantly (P < 0.05) enhanced protection compared with either treatment alone (Figures 5F, 6).
Figure 6.

Detection of annexin V/PI double-labeled neuronal cells via flow cytometry.
(A) Normal group; (B) OGD/R group; (C) hypothermia group; (D) dantrolene group; (E) hypothermia and dantrolene group. Q1, Q2, Q3, and Q4 quadrants represent necrosis, late apoptosis, viable cells, and early apoptotic cells, respectively. PI: Propidium iodide; OGD/R: oxygen-glucose deprivation/reoxygenation.
Discussion
OGD for 30 to 90 minutes induces significant apoptosis of cultured cerebral cortical neurons (Wise-Faberowski et al., 2001). Hypothermia has been shown to suppress a broad range of OGD-induced injury factors (Drury et al., 2010). At 33°C, hypothermia leads to an approximate 50% decrease in morphological changes associated with neuronal death (Xu et al., 2002). Dantrolene has been shown to effectively alleviate ischemia/reperfusion injury to neurons, resulting in reduced neuronal edema and neuronal loss (Kocogullari et al., 2008).
Our results corroborate those of Li et al. (2005), who have shown a significant reduction in ischemia/reperfusion-induced neuronal injury with dantrolene. Moreover, our data revealed a significant protection against OGD/R-induced injury with the combined treatment. To strength our observations of the morphological changes, we investigated changes in several other indicators that are closely associated with ischemic injury.
Oxidative damage and calcium overload are two important factors contributing to ischemic neuronal injury (Szydlowska and Tymianski, 2010). They are also the most studied targets for clinical intervention (Xu and Pan, 2013). Our results are consistent with previous findings that OGD elevated intracellular production of ROS (Suh et al., 2008; Velly et al., 2009) and calcium overload (Larsen et al., 2005), both of which contribute to endoplasmic reticulum injury, mitochondrial damage, and eventually neuronal death (Pivovarova et al., 2004). Hypothermia or dantrolene alone has been shown to inhibit ischemia-mediated production of ROS and calcium overload (Aslan et al., 2009; Staats et al., 2012; Tissier et al., 2013). However, the combination of hypothermia and dantrolene fails to show a great protective effect and suggests that each treatment alone may reach their “ceiling effect” in reducing calcium overload in OGD/R-treated neurons. Our data confirmed that calcium ion concentrations between the hypothermia alone group and the combined treatment group remained unchanged, while cell death was suppressed in combined treatment group. These results suggest that dantrolene may exert its neuroprotective effect via a specific, yet unidentified, mechanism that does not involve calcium.
The neuronal mitochondrial membrane is deformed or ruptured after 30–90 minutes of OGD (Pivovarova et al., 2004). Mitochondrial transmembrane potential injury is considered as an early indicator of apoptosis (Iijima, 2006). Hypothermia has been shown to protect mitochondria against oxidative injury (Huang et al., 2009). Our observations are consistent with these previous reports. Hypothermia-mediated mitochondrial protection is also associated with a reduction in the opening of the mitochondrial permeability transition pore, the release of pro-apoptotic substances from mitochondria, and the cleavage of caspase-3 (Gong et al., 2013). In light of these effects, our data may therefore indicate that dantrolene does not only act as a calcium antagonist in protecting against OGD/R injury. In this study, the mechanisms underlying this additional protection in stabilizing the ΔΨm were not investigated. The enrichment of histone-associated DNA fragments is caused by DNA degeneration, which occurs early before the breakdown of the plasma membrane. Our results showed that the combination of hypothermia and dantrolene significantly stabilized mitochondria in the early apoptotic stage. We then further confirmed that the combined treatment induced a greater anti-apoptotic effect than with either treatment alone.
There are a couple of limitations to this study. Firstly, although our data verify a synergistic neuroprotective effect of the combined treatment in some experimental parameters, the mechanisms underlying this effect remain unclear. Secondly, because results from an in vitro model may not necessarily be reflected in an in vivo model, OGD/R in primary neuronal cultures may have posed as a limitation in this study. Therefore, more work is needed to further verify our findings in animal models of ischemic stroke.
In conclusion, the combination of dantrolene and hypothermia may provide significant neuroprotection against OGD/R injury by restoring cell viability, inhibiting ROS production, stabilizing ΔΨm, and reducing cytoplasmic histone-associated DNA fragments and apoptosis. However, dantrolene may mediate its synergistic neuroprotective effect by mechanisms other than inhibiting calcium. These findings indicate that the combination of hypothermia and dantrolene may be a potential therapeutic strategy against ischemic neuronal injury. Rather, similar to a hypothermic compound neuroprotective solution, it may involve multi-target therapy. However, this aspect needs further in-depth study to determine the best multi-target components.
Acknowledgments
We thank the Shenzhen Key Laboratory of Neurosurgery (ZDSYS20140509173142601) and Shenzhen Brain Injury & Repair Research Center in China for support.
Footnotes
Funding: This work was supported by a grant from the Guangdong Science & Technology Plan Program in China, No. 2014A020212043; the a grant from the Shenzhen Science & Technology Plan Program in China, No. JCYJ20140414170821242; the a grant from Shenzhen Collaborative Innovation Plan Program in China, No. GJHZ20120614154914623; a grant from the Science & Technology Project of Shanxi Health and Family Planning Commission in China, No. 201201060.
Conflicts of interest: None declared.
Copyedited by Mark F, Maxwell R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y, Cosar M. Dantrolene can reduce secondary damage after spinal cord injury. Eur Spine J. 2009;18:1442–1451. doi: 10.1007/s00586-009-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boys JA, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart brain, liver, and kidney. J Invest Med. 2010;58:875–882. doi: 10.231/JIM.0b013e3181e5d719. [DOI] [PubMed] [Google Scholar]
- Drury PP, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Semin Fetal Neonatal Med. 2010;15:287–292. doi: 10.1016/j.siny.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Gao XY, Huang JO, Hu YF, Gu Y, Zhu SZ, Huang KB, Chen JY, Pan SY. Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury. Sci Rep. 2014;4:7091. doi: 10.1038/srep07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti R, Belletti S, Orlandini G, Bussolati O, Dall’Asta V, Gazzola GC. Comparison of annexin V and calcein-AM as early vital markers of apoptosis in adherent cells by confocal laser microscopy. J Histochem Cytochem. 1998;46:895–900. doi: 10.1177/002215549804600804. [DOI] [PubMed] [Google Scholar]
- Gong P, Hua R, Zhang Y, Zhao H, Tang Z, Mei X, Zhang M, Cui J, Li C. Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. J Cereb Blood Flow Metab. 2013;33:928–934. doi: 10.1038/jcbfm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad E, Cohen-Sivan Y, Heled Y, Epstein Y. Clinical review: Treatment of heat stroke: should dantrolene be considered? Crit Care. 2005;9:86–91. doi: 10.1186/cc2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausfater P. Dantrolene and heatstroke: a good molecule applied in an unsuitable situation. Crit Care. 2005;9:23–24. doi: 10.1186/cc2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Chen HW, Tsai MS, Hsu CY, Peng RH, Wang TD, Chang WT, Chen WJ. Antiapoptotic cardioprotective effect of hypothermia treatment against oxidative stress injuries. Acad Emerg Med. 2009;16:872–880. doi: 10.1111/j.1553-2712.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res. 2006;55:234–243. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Javed M, Bogdano v A. Oral dantrolene and severe respiratory failure in a patient with chronic spinal cord injury. Anaesthesia. 2010;65:855–856. doi: 10.1111/j.1365-2044.2010.06409.x. [DOI] [PubMed] [Google Scholar]
- Kocogullari CU, Emmiler M, Cemek M, Sahin O, Aslan A, Ayva E, Tur L, Buyukokuroglu ME, Demirkan I, Cekirdekci A. Can dantrolene protect spinal cord against ischemia/reperfusion injury? An experimental study. The Thoracic and cardiovascular surgeon. 2008;56:406–411. doi: 10.1055/s-2008-1038731. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Schwab S. Hypothermia and ischemic stroke. Curr Treat Options Neurol. 2012;14:188–196. doi: 10.1007/s11940-012-0164-y. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Gebhardt B, Schwab S. EuroHYP-1 trial: EU-funded therapy study on the effectiveness of mild therapeutic hypothermia for acute ischemic stroke. Nervenarzt. 2012;83:1252–1259. doi: 10.1007/s00115-012-3533-6. [DOI] [PubMed] [Google Scholar]
- Kuang CY, Yu Y, Guo RW, Qian DH, Wang K, Den MY, Shi YK, Huang L. Silencing stromal interaction molecule 1 by RNA interference inhibits the proliferation and migration of endothelial progenitor cells. Biochem Biophys Res Commun. 2010;398:315–320. doi: 10.1016/j.bbrc.2010.06.088. [DOI] [PubMed] [Google Scholar]
- Larsen G, Skjellegrind H, Moe M, Vinje M, Berg-Johnsen J. Endoplasmic reticulum dysfunction and Ca 2+ deregulation in isolated CA1 neurons during oxygen and glucose deprivation. Neurochem Res. 2005;30:651–659. doi: 10.1007/s11064-005-2753-6. [DOI] [PubMed] [Google Scholar]
- Li F, Hayashi T, Jin G, Deguchi K, Nagotani S, Nagano I, Shoji M, Chan PH, Abe K. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res. 2005;1048:59–68. doi: 10.1016/j.brainres.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Li Y, Bao Y, Jiang B, Wang Z, Liu Y, Zhang C, An L. Catalpol protects primary cultured astrocytes from in vitro ischemia-induced damage. Int J Dev Neurosci. 2008;26:309–317. doi: 10.1016/j.ijdevneu.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Liu C, Duan W, Xu S, Chen C, He M, Zhang L, Yu Z, Zhou Z. Exposure to 1800 MHz radiofrequency electromagnetic radiation induces oxidative DNA base damage in a mouse spermatocyte-derived cell line. Toxicol Lett. 2013;218:2–9. doi: 10.1016/j.toxlet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Majidi S, Grigoryan M, Tekle W, Qureshi A. Intraarterial dantrolene for refractory cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2012;17:245–249. doi: 10.1007/s12028-012-9737-6. [DOI] [PubMed] [Google Scholar]
- Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care. 2009;10:103–115. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci. 2004;24:5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats KA, Van Rillaer M, Scheveneels W, Verbesselt R, Van Damme P, Robberecht W, Van Den Bosch L. Dantrolene is neuroprotective in vitro, but does not affect survival in SOD1G93A mice. Neuroscience. 2012;220:26–31. doi: 10.1016/j.neuroscience.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Tissier R, Chenoune M, Pons S, Zini R, Darbera L, Lidouren F, Ghaleh B, Berdeaux A, Morin D. Mild hypothermia reduces perischemic reactive oxygen species production and preserves mitochondrial respiratory complexes. Resuscitation. 2013;84:249–255. doi: 10.1016/j.resuscitation.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Velly LJ, Canas PT, Guillet BA, Labrande CN, Masmejean FM, Nieoullon AL, Gouin FM, Bruder NJ, Pisano PS. Early anesthetic preconditioning in mixed cortical neuronal-glial cell cultures subjected to oxygen-glucose deprivation: the role of adenosine triphosphate dependent potassium channels and reactive oxygen species in sevoflurane-induced neuroprotection. Anesth Analg. 2009;108:955–963. doi: 10.1213/ane.0b013e318193fee7. [DOI] [PubMed] [Google Scholar]
- Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab. 2002;22:206–214. doi: 10.1097/00004647-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Watson R. European research is launched into hypothermia stroke treatment. BMJ. 2012;344:e2215. doi: 10.1136/bmj.e2215. [DOI] [PubMed] [Google Scholar]
- Wise-Faberowski L, Raizada MK, Sumners C. Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth Analg. 2001;93:1281–1287. doi: 10.1097/00000539-200111000-00051. [DOI] [PubMed] [Google Scholar]
- Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12:275–284. doi: 10.1016/S1474-4422(13)70013-9. [DOI] [PubMed] [Google Scholar]
- Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22:21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Xu SY, Pan SY. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med Sci Monit Basic Res. 2013;19:37–45. doi: 10.12659/MSMBR.883750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Wu YM, Ji Z, Gao XY, Pan SY. A modified technique for culturing primary fetal rat cortical neurons. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/803930. 803930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Hu YF, Li WP, Wu YM, Ji Z, Wang SN, Li K, Pan SY. Intermittent hypothermia is neuroprotective in an in vitro model of ischemic stroke. Int J Biol Sci. 2014;10:873–881. doi: 10.7150/ijbs.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Zucchi R, Ronca-Testoni S, Ronca G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic Res Cardiol. 2000;95:137–143. doi: 10.1007/s003950050175. [DOI] [PubMed] [Google Scholar]
- Zhou T, Jiang J, Zhang M, Fu Y, Yang Z, Jiang L. Protective effect of mild hypothermia on oxygen-glucose deprivation injury in rat hippocampal neurons after hypoxia. Mol Med Rep. 2013;7:1859–1864. doi: 10.3892/mmr.2013.1410. [DOI] [PubMed] [Google Scholar]



