Abstract
In this study, we investigated the effect of an antibody against E3 ubiquitin ligase seven in absentia homolog 1 (SIAH-1) in PC12 cells. 1-Methyl-4-phenylpyridinium (MPP+) treatment increased α-synuclein, E1 and SIAH-1 protein levels in PC12 cells, and it reduced cell viability; however, there was no significant change in light chain 3 expression. Treatment with an SIAH-1 antibody decreased mRNA expression levels of α-synuclein, light chain 3 and SIAH-1, but increased E1 mRNA expression. It also increased cell viability. Combined treatment with MPP+ and rapamycin reduced SIAH-1 and α-synuclein levels. Treatment with SIAH-1 antibody alone diminished α-synuclein immunoreactivity in PC12 cells, and reduced the colocalization of α-synuclein and light chain 3. These findings suggest that the SIAH-1 antibody reduces the monoubiquitination and aggregation of α-synuclein, promoting its degradation by the ubiquitin-proteasome pathway. Consequently, SIAH-1 may be a potential new therapeutic target for Parkinson's disease.
Keywords: nerve regeneration, neurodegeneration, Parkinson's disease, ubiquitin-proteasome system, autophagy, E3 ubiquitin ligase seven in absentia homolog 1, 1-methyl-4-phenylpyridinium, rapamycin, neural regeneration
Introduction
The presence of α-synuclein aggregates is a characteristic of many neurodegenerative diseases, including Parkinson's disease (PD) and Lewy body dementia (Kalia et al., 2013; Tanik et al., 2013). Accumulating evidence shows that α-synuclein misfolding, aggregation and abnormal degradation cause dopaminergic neuron death (Bartels et al., 2011; Gadad et al., 2011; Cremades et al., 2012). This process underlies PD pathogenesis (Venda et al., 2010; Kalia et al., 2013; Ryan et al., 2014). Although monoubiquitinated and polyubiquitinated α-synuclein are present in Lewy bodies, the regulation of α-synuclein ubiquitination and its role in the formation of Lewy bodies are not fully understood (Engelender, 2008; Lee et al., 2008). Recent studies show that the E3 ubiquitin ligase seven in absentia homolog 1 (SIAH-1) mediates α-synuclein monoubiquitination (Engelender, 2008; Lee et al., 2008). SIAH-1 also plays a key role in Lewy body formation and pathogenesis in PD, contributing to α-synuclein aggregation and intracellular inclusion formation. The intracellular inclusions and aggregates cannot be cleared by the ubiquitin-proteasome pathway, and must therefore rely on the autophagy degradation pathway (Engelender, 2008; Lee et al., 2008).
Despite the data linking SIAH-1 to α-synuclein aggregation and degradation, the function of SIAH-1 in the autophagy-lysosomal pathway remains unknown. Furthermore, it is not clear whether SIAH-1-mediated ubiquitination directs α-synuclein to the ubiquitin-proteasome pathway or the autophagy pathway. SIAH-1 may activate or downregulate autophagy via the p53 pathway, thereby promoting or inhibiting the degradation of α-synuclein. To clarify the role of SIAH-1 in α-synuclein degradation, we induced autophagy and inhibited SIAH-1 function using an anti-SIAH-1 antibody. We then examined the effects on SIAH-1 activity, p53 expression and on the ubiquitin proteasome pathway and the autophagy-lysosomal degradation pathway.
Materials and Methods
Cell culture and treatments
The rat pheochromocytoma (PC12) cell line was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (C11995; Life Technologies, Carlsbad, CA, USA). For experiments, cells were seeded in culture flasks, or 24 or 96-well plates until 60–70% confluence. Cells were divided into six groups. In the control group, the normal growth of cells was observed. In the rapamycin (RAPA) group, cells were treated with 0.2 μg/mL RAPA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 24 hours. In the anti-SIAH-1 group, cells were treated with 4 μg/mL anti-SIAH-1 antibody (Santa Cruz Biotechnology) for 24 hours. In the 1-methyl-4-phenylpyridinium (MPP+) group, cells were treated with 0.5 mM MPP+ (Santa Cruz Biotechnology) for 24 hours. In the MPP+ RAPA group, cells were treated with MPP+ for 24 hours then RAPA for 24 hours. In the MPP+ anti-SIAH-1 group, cells were treated with MPP+ for 24 hours then anti-SIAH-1 antibody for 24 hours.
3-(4,5-Cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay for cell viability
Cells were cultured in 96-well plates with RPMI-1640 medium containing 10% fetal bovine serum at a density of 1 × 105/mL, in a volume of 200 μL/well. Cells in the exponential phase of growth were incubated with MPP+, RAPA and SIAH-1 antibody for 24 hours. The culture medium was refreshed, and 20 μL MTT solution (final concentration of 0.5 mg/mL) was added to each well. Cells were incubated at 37°C for an additional 4 hours in the dark. After incubation, the medium containing MTT was removed, and 150 μL dimethyl sulfoxide was added to each well to dissolve the formazan dye crystals on a shaker for 15 minutes. The optical density was measured at 492 nm with a microplate reader (Model 680; Bio-Rad, CA, USA). Cell viability was expressed as a percentage of the value in the control group.
Western blot analysis
Western blot analysis was performed as previously described by our group (Cai et al., 2009). Cells were lysed and sonicated in lysis buffer. After electrophoresis, samples were transferred onto a polyvinylidene difluoride membrane (Millipore, Temecula, CA, USA), and then immunoblotted with the following antibodies: goat polyclonal anti-SIAH-1 (1:100; sc-5505; Santa Cruz Biotechnology), rabbit polyclonal anti-α-synuclein (1:1,000; 2642; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal anti-light chain 3 (LC3) (1:1,000; ab62721; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-E1 (1:1,000; 4891S; Cell Signaling Technology), rabbit polyclonal anti-P53 (1:1,000; 2642; Cell Signaling Technology), and mouse monoclonal β-actin (1:1,000; A3854; Sigma-Aldrich, St Louis, MO, USA). Subsequently, the following horseradish peroxidase-conjugated secondary antibodies were added: polyclonal goat anti-rabbit secondary antibody for LC3, α-synuclein and E1; polyclonal donkey anti-goat secondary antibody for SIAH-1; and polyclonal goat anti-mouse secondary antibody for β-actin (1:1,000; Beyotime Biotechnology, Jiangsu, China). Images were captured using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA), and band intensities were calculated by densitometric analysis using Image J software (NIH, Bethesda, MD, USA).
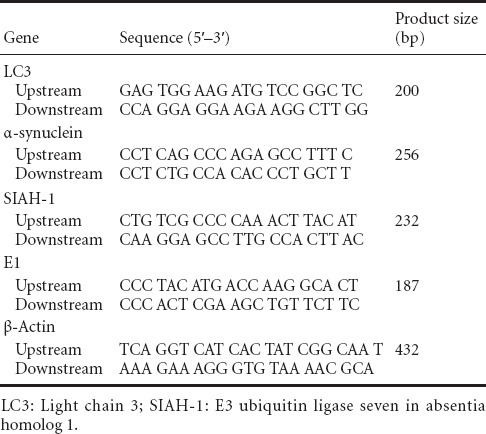
Semi-quantitative analysis of mRNA by reverse transcription (RT)-PCR
Total RNA was extracted from PC12 cells using TRIzol (Life Technologies). First-strand cDNA was synthesized using PrimeScript RT Enzyme Mix I (RR037A; Takara, Otsu, Japan). Primer pairs for the amplification of cDNA for LC3, α-synuclein, SIAH-1 and β-actin were designed (Table 1). cDNA amplification was performed using DyNAmo SYBR green qPCR kits (Finnzymes Oy, Espoo, Finland). Amplification was performed using an iCycler iQ Multicolor Real Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The expression levels of LC3, SIAH-1 and α-synuclein were normalized to that of β-actin.
Table 1.
Primer pairs for the amplification of cDNA transcripts
Immunofluorescence microscopy
PC12 cells were seeded onto non-coated 12-mm coverslips and treated with MPP+ (0.5 mM, 24-hour exposure) and goat polyclonal SIAH-1 antibody (4 μg/mL, 24 hours), and then fixed in ice-cold 4% paraformaldehyde for 15 minutes. The cells were incubated with rabbit polyclonal anti-LC3 antibody (1:250), goat polyclonal anti-SIAH-1 antibody (1:500) or rabbit polyclonal anti-α-synuclein antibody (1:250) for 1.5 hours at 37°C. Cells were then treated with polyclonal mouse anti-rabbit secondary antibody for LC3 and α-synuclein or polyclonal mouse anti-goat secondary antibody for SIAH-1 (1:1,000; Beyotime Biotechnology) for 1 hour at 37°C. Thereafter, cells were stained with 4′,6-diamidino-2-phenylindole (0.3 μg/mL) for 15 minutes, and examined with a laser scanning confocal microscope (TCS SP2 CLSM; Leica, Wetzlar, Germany). Images were collected and processed using the imaging software provided with the Leica TCS system.
Statistical analysis
All experiments were performed in triplicate, and the results are presented as the mean ± SD. Statistical analysis was performed using SPSS 12.0 software (SPSS, Chicago, IL, USA). Multiple group comparisons were performed using one-way analysis of variance and Fisher's least significant difference test (equal variances assumed). A value of P < 0.05 was considered statistically significant.
Results
SIAH inhibition increased cell viability
We assessed PC12 cell viability after a 24-hour treatment with RAPA (used to induce autophagy) and SIAH-1 antibody. We found no significant effect on viability under normal growth conditions (P > 0.05). As expected, MPP+-treated cells showed reduced viability. RAPA and SIAH-1 antibody each significantly increased viability in cells treated with MPP+ (P = 0.0008 and 0.0014, respectively). These results indicate that RAPA and SIAH-1 antibody improve viability in MPP+-stressed cells (Figure 1).
Figure 1.
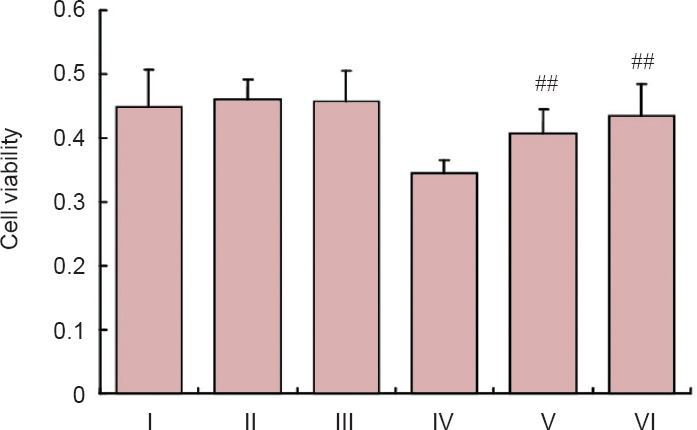
MTT assay for cell viability after treatment with MPP+, RAPA and SIAH-1 antibody.
RAPA and SIAH-1 antibody treatment had no significant effect on cell viability under normal growth conditions (P > 0.05). In the MPP+ group, RAPA and SIAH-1 antibody significantly increased cell viability (##P < 0.01, vs. MPP+ group; mean ± SD, n ≥ 6, one-way analysis of variance followed by Fisher's least significant difference post hoc test). I: Control group; II: RAPA group; III: anti-SIAH-1 group; IV: MPP+ group; V: MPP+ RAPA group; VI: MPP+ anti-SIAH-1 group. MTT: 3-(4,5-Cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; MPP+: 1-methyl-4-phenylpyridinium; RAPA: rapamycin; SIAH-1: E3 ubiquitin ligase seven in absentia homolog 1.
MPP+ increased α-synuclein expression and SIAH-1 levels
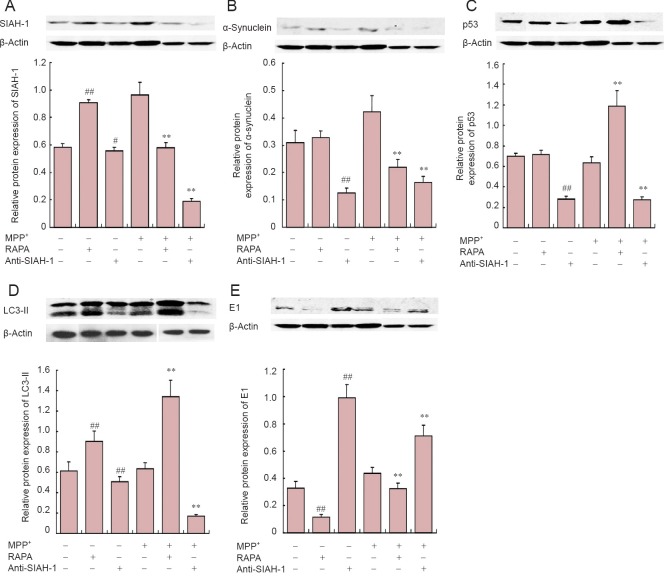
MPP+ treatment not only increased the expression of α-synuclein (P < 0.01) and E1 (P < 0.01), but also that of SIAH-1 (P < 0.01). We found a nonsignificant increase in LC3-II expression (P = 0.17) (Figure 2). In addition, we observed significant α-synuclein aggregation and localization in the cytoplasm after MPP+ treatment. SIAH-1 immunofluorescence staining was significantly increased after 24 hours of MPP+ treatment (Figure 3).
Figure 2.
Effects of SIAH-1 antibody treatment on α-synuclein and the autophagy degradation pathway in PC12 cells.
Western blot assay for protein levels (upper panel) and statistical analysis of optical density measurements (target protein/β-actin; lower panel) in PC12 cells after treatment with MPP+, RAPA and SIAH-1 antibody. (A) SIAH-1 (37 kDa); (B) α-synuclein (19 kDa); (C) p53 (55 kDa); (D) LC3-II (17 kDa); (E) E1 (117 kDa). Values are represented as the mean ± SD (n = 5) (one-way analysis of variance followed by Fisher's least significant difference post hoc test). #P < 0.05, ##P < 0.01, vs. control group (MPP+, RAPA and anti-SIAH-1 are “–”). **P < 0.01, vs. MPP+ group (MPP+ is “+”; RAPA and anti-SIAH-1 are “–”). SIAH-1: E3 ubiquitin ligase seven in absentia homolog 1; LC3: light chain 3; MPP+: 1-methyl-4-phenylpyridinium; RAPA: rapamycin.
Figure 3.
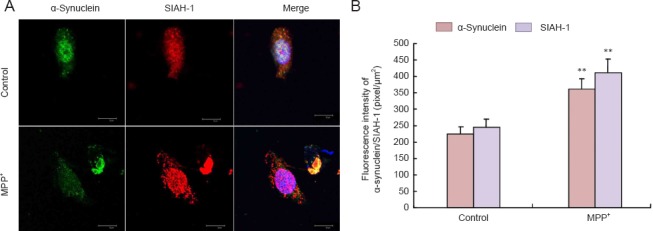
Change in α-synuclein and SIAH-1 in PC12 cells in response to MPP+.
(A) Immunolabeling for α-synuclein (green) and SIAH-1 (red) or DAPI staining (blue) in PC12 cells. Image overlays are shown in the third column. MPP+ treatment caused an increase in SIAH-1 and α-synuclein fluorescence intensities (laser scanning confocal microscope; scale bars: 30 μm). (B) Quantification of fluorescence intensities of α-synuclein and SIAH-1. At least 30 cells were included for analysis from five images per group. Values are presented as the mean ± SD (n = 5; one-way analysis of variance followed by Fisher's least significant difference post hoc test). **P < 0.01, vs. control group (normal growth of cells). SIAH-1: E3 ubiquitin ligase seven in absentia homolog 1; MPP+: 1-methyl-4-phenylpyridinium; DAPI: 4′,6-diamidino-2-phenylindole.
Effects of autophagy induction on SIAH levels and α-synuclein degradation
Autophagy was induced by rapamycin treatment, and SIAH-1 levels and α-synuclein degradation were measured. LC3-II levels were significantly increased (P < 0.01), while E1 expression was decreased (P < 0.01), and SIAH-1 levels were increased (P < 0.01), with no significant change in P53 or α-synuclein expression (P = 0.536). Additionally, LC3-II and E1 expression levels were increased in the MPP+ group (P < 0.01), while E1 expression was reduced significantly (P < 0.01). RAPA treatment significantly diminished SIAH-1 and α-synuclein levels (P < 0.01; Figure 2).
Decreasing SIAH activity reduced α-synuclein levels and inhibits the autophagy degradation pathway
SIAH activity was inhibited by treating cells with SIAH-1 antibody. LC3-II expression was decreased (P = 0.004), and E1 expression was increased significantly (P < 0.01). Both p53 and α-synuclein levels decreased significantly (P < 0.01). A consistent trend was found in the MPP+ group, where SIAH-1 levels significantly decreased after treatment with SIAH-1 antibody (P < 0.01). Furthermore, LC3-II expression decreased (P < 0.01) and E1 expression increased (P < 0.01). Finally, p53 and α-synuclein levels decreased significantly (P < 0.01; Figure 2).
Inhibition of SIAH-1 altered SIAH-1, E1, LC3-II and α-synuclein mRNA levels
To confirm western blot assay results, we measured mRNA levels of SIAH-1, E1, LC3-II and α-synuclein after SIAH-1 antibody treatment. Consistent with western blot results, we observed decreased mRNA expression levels of SIAH-1, LC3-II and α-synuclein (P < 0.05), and increased E1 mRNA levels (P = 0.0086; data not shown).
MPP+ treatment elevated E1 mRNA levels (P = 0.0302). However, α-synuclein levels increased (P = 0.0030). In addition, MPP+ treatment increased LC3-II and SIAH-1 mRNA levels (P < 0.01). SIAH-1 antibody treatment reversed these trends and significantly reduced SIAH-1 transcript levels (P < 0.0001). In contrast, E1 mRNA expression increased (P = 0.0170; data not shown).
α-Synuclein, SIAH-1 and LC3 colocalized in PC12 cells
We explored the relationship between SIAH-1, α-synuclein and LC3 in the cells after SIAH-1 antibody treatment by examining their localization using immunofluorescence. Immunoreactivities of α-synuclein, SIAH-1 and LC3 decreased after SIAH-1 antibody treatment. In addition, colocalization of α-synuclein and LC3 was lost. However, α-synuclein and SIAH-1 retained their colocalization. Furthermore, α-synuclein aggregates were significantly reduced (Figure 4).
Figure 4.
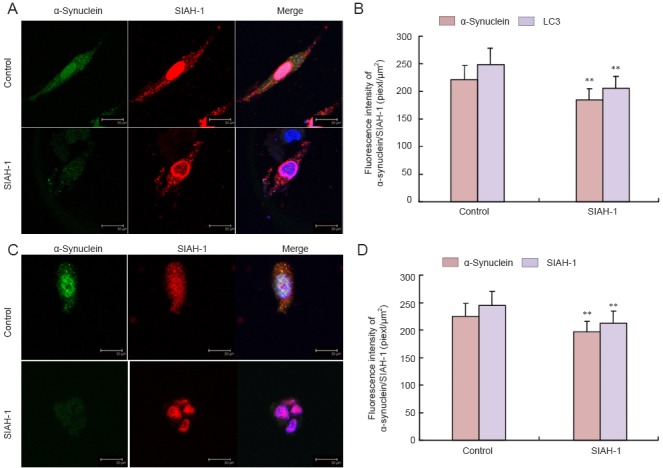
Colocalization of α-synuclein, SIAH-1 and microtubule-associated protein LC3 in PC12 cells after SIAH-1 antibody treatment.
(A, C) Laser scanning confocal microscope; scale bars: 30 μm. Immunoreactivity for α-synuclein, SIAH-1 and LC3 decreased after treatment with SIAH-1 antibody, and colocalization of α-synuclein and LC3 was lost, but α-synuclein and SIAH-1 retained their colocalization. (A) Immunostaining for α-synuclein (green), microtubule-associated protein LC3 (red) and DAPI (blue). (B) Quantification of immunostaining results in A. (C) Immunostaining for α-synuclein (green), SIAH-1 (red) and DAPI (blue). (D) Quantification of immunostaining results in C. Values are presented as the mean ± SD (n = 5; one-way analysis of variance followed by Fisher's least significant difference post hoc test). **P < 0.01, vs. control group (normal growth of cells). SIAH-1: E3 ubiquitin ligase seven in absentia homolog 1; LC3: light chain 3; DAPI: 4′,6-diamidino-2-phenylindole.
Discussion
The ubiquitin-proteasome and autophagy-lysosomal pathways are two main pathways for clearing proteins and organelles in eukaryotic cells (Jimenez-Sanchez et al., 2012; Kuang et al., 2013; Nixon, 2013). The proteasome is a barrel-shaped multi-protein complex that degrades short-lived nuclear and cytosolic proteins (Jimenez-Sanchez et al., 2012; Sadanandom et al., 2012; Gu and Enenkel, 2014). Proteasome substrates are forced to linearize and travel through the narrow cylindrical pore of the proteasome, preventing the clearance of oligomers and aggregates (Verhoef et al., 2002; Mizushima and Komatsu, 2011). The E3 ubiquitin ligase SIAH-1 monoubiquitinates α-synuclein and promotes aggregation and toxicity in cells (Rott et al., 2008, 2011; Engelender, 2012). The α-synuclein monoubiquitination leads to α-synuclein aggregation and the formation of intracellular inclusions in dopaminergic neurons (Szargel et al., 2009; Alvarez-Castelao and Castano, 2011). Therefore, we hypothesized that the inhibition of SIAH-1 would suppress α-synuclein monoubiquitination and aggregation, leading to increased levels of monomers. The monomers would then likely be selectively degraded by the ubiquitin-proteasome pathway, but not by the autophagy pathway.
Several in vitro studies (Kalivendi et al., 2004; Su et al., 2011; Park et al., 2014), including our previous study (Cai et al., 2009), have shown that MPP+ induces α-synuclein expression and aggregation. This results in symptoms similar to those in Parkinson's disease in experimental animals and humans (Sherer et al., 2002; Yang et al., 2009), and appear to be induced by, among others, increased levels of oxidative stress and apoptosis (Fiskum et al., 2003; Bernstein and O’Malley, 2013; Schildknecht et al., 2013). However, it is unclear how oxidative stress alters SIAH activity or its function in α-synuclein degradation.
In this study, SIAH-1 protein and mRNA expression levels were significantly increased in MPP+-treated cells. Hara et al. (2006) showed that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment produces GAPDH/SIAH protein complexes in the corpus striatum, triggering the NO/GAPDH/SIAH cell death cascade. Furthermore, suppressing SIAH-1 markedly reduces cell death (Ortiz-Ortiz et al., 2010). These findings are consistent with our result that SIAH-1 antibody treatment increases cell viability in MPP+-treated cells. This suggests that the SIAH-1 antibody alleviates cellular stress. However, it remains unclear how the SIAH-1 antibody prevents the MPP+-induced reduction in cell viability.
Recent studies show that SIAH-1-mediated α-synuclein monoubiquitination leads to the formation of α-synuclein aggregates and inclusions (Rott et al., 2008; Szargel et al., 2009). SIAH-1 mediates the ubiquitination of α-synuclein at a single lysine, promoting aggregation and cytotoxicity (Engelender, 2008; Rott et al., 2008). Our results show that treatment with SIAH-1 antibody reduces α-synuclein levels in normal and MPP+-treated cells. In addition, our immunofluorescence results revealed that α-synuclein aggregates were significantly reduced as well by the antibody. However, the mechanisms by which SIAH-1 antibody reduces α-synuclein levels and plays a protective role in cells remain to be elucidated.
In summary, in this study, we found that MPP+ increases SIAH activity and impairs the clearance of α-synuclein by inducing the upregulation and aggregation of α-synuclein. Co-treatment with SIAH-1 antibody clearly had neuroprotective effects. Our findings suggest that SIAH-1 plays a key role in the pathogenesis of Parkinson's disease. Furthermore, SIAH-1 may be a potential new therapeutic target for neurodegenerative diseases characterized by α-synuclein aggregates.
Acknowledgments
We are very grateful to the staff from Cyrus Tang Hematology Center and the Institute of Neuroscience at Soochow University in China for their helps. We also thank Dr. Austin Cape for careful reading and insightful suggestions.
Footnotes
Funding: This study was supported by the China Postdoctoral Science Foundation, No. 1630; the Natural Science Foundation of Jiangsu Province in China, No. BK2011402; the Jiangsu Province Postdoctoral Research Foundation in China, No. 1301174C; the Jiangsu Province Health Department Foundation in China, No. H201361.
Conflicts of interest: None declared.
Copyedited by Patel B, Hindle A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alvarez-Castelao B, Castano JG. Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell Mol Life Sci. 2011;68:2643–2654. doi: 10.1007/s00018-010-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein AI, O’Malley KL. MPP + -induces PUMA- and p53-dependent, but ATF3-independent cell death. Toxicol Lett. 2013;219:93–98. doi: 10.1016/j.toxlet.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZL, Shi JJ, Yang YP. MPP + impairs autophagic clearance of alpha-synuclein by impairing the activity of dynein. Neuroreport. 2009;20:569–573. doi: 10.1097/WNR.0b013e32832986c4. [DOI] [PubMed] [Google Scholar]
- Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S. Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease. Autophagy. 2008;4:372–374. doi: 10.4161/auto.5604. [DOI] [PubMed] [Google Scholar]
- Engelender S. alpha-Synuclein fate: proteasome or autophagy. Autophagy. 2012;8:418–420. doi: 10.4161/auto.19085. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Starkov A, Polster BM, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson's disease. Ann N Y Acad Sci. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Gadad BS, Britton GB, Rao KS. Targeting oligomers in neurodegenerative disorders: lessons from alpha-synuclein, tau, and amyloid-beta peptide. J Alzheimers Dis 24 Suppl. 2011;2:223–232. doi: 10.3233/JAD-2011-110182. [DOI] [PubMed] [Google Scholar]
- Gu ZC, Enenkel C. Proteasome assembly. Cell Mol Life Sci. 2014;71:4729–4745. doi: 10.1007/s00018-014-1699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Sanchez M, Thomson F, Zavodszky E, Rubinsztein DC. Autophagy and polyglutamine diseases. Prog Neurobiol. 2012;97:67–82. doi: 10.1016/j.pneurobio.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivendi SV, Cunningham S, Kotamraju S, Joseph J, Hillard CJ, Kalyanaraman B. Alpha-synuclein up-regulation and aggregation during MPP + -induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J Biol Chem. 2004;279:15240–15247. doi: 10.1074/jbc.M312497200. [DOI] [PubMed] [Google Scholar]
- Kuang E, Qi J, Ronai Z. Emerging roles of E3 ubiquitin ligases in autophagy. Trends Biochem Sci. 2013;38:453–460. doi: 10.1016/j.tibs.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Wheeler TC, Li L, Chin LS. Ubiquitination of alpha-synuclein by Siah-1 promotes alpha-synuclein aggregation and apoptotic cell death. Hum Mol Genet. 2008;17:906–917. doi: 10.1093/hmg/ddm363. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz MA, Moran JM, Ruiz-Mesa LM, Bravo-San PJM, Fuentes JM. Paraquat exposure induces nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the activation of the nitric oxide-GAPDH-Siah cell death cascade. Toxicol Sci. 2010;116:614–622. doi: 10.1093/toxsci/kfq146. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shin JY, Kim HN, Oh SH, Lee PH. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol Aging. 2014;35:1920–1928. doi: 10.1016/j.neurobiolaging.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Rott R, Szargel R, Haskin J. Monoubiquitylation of alpha-synuclein by seven in absentia homolog (SIAH) promotes its aggregation in dopaminergic cells. J Biol Chem. 2008;283:3316–3328. doi: 10.1074/jbc.M704809200. [DOI] [PubMed] [Google Scholar]
- Rott R, Szargel R, Haskin J, Bandopadhyay R, Lees AJ, Shani V, Engelender S. alpha-Synuclein fate is determined by USP9X-regulated monoubiquitination. Proc Natl Acad Sci U S A. 2011;108:18666–18671. doi: 10.1073/pnas.1105725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BJ, Lourenco-Venda LL, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R. alpha-Synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med. 2014;67:58–68. doi: 10.1016/j.freeradbiomed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandom A, Bailey M, Ewan R, Lee J, Nelis S. The ubiquitin-proteasome system: central modifier of plant signalling. New Phytol. 2012;196:13–28. doi: 10.1111/j.1469-8137.2012.04266.x. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Gerding HR, Karreman C, Drescher M, Lashuel HA, Outeiro TF, Di MDA, Leist M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies. J Neurochem. 2013;125:491–511. doi: 10.1111/jnc.12226. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Shi JJ, Yang YP, Li J, Zhang YL, Chen J, Hu LF, Liu CF. HDAC6 regulates aggresome-autophagy degradation pathway of alpha-synuclein in response to MPP + -induced stress. J Neurochem. 2011;117:112–120. doi: 10.1111/j.1471-4159.2011.07180.x. [DOI] [PubMed] [Google Scholar]
- Szargel R, Rott R, Eyal A, Haskin J, Shani V, Balan L, Wolosker H, Engelender S. Synphilin-1A inhibits seven in absentia homolog (SIAH) and modulates alpha-synuclein monoubiquitylation and inclusion formation. J Biol Chem. 2009;284:11706–11716. doi: 10.1074/jbc.M805990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem. 2013;288:15194–210. doi: 10.1074/jbc.M113.457408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. alpha-Synuclein and dopamine at the crossroads of Parkinson's disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef LG, Lindsten K, Masucci MG, Dantuma NP. Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum Mol Genet. 2002;11:2689–2700. doi: 10.1093/hmg/11.22.2689. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Zhang S, Ding JH, Zhou F, Hu G. Iptakalim protects against MPP + -induced degeneration of dopaminergic neurons in association with astrocyte activation. Int J Neuropsychopharmacol. 2009;12:317–327. doi: 10.1017/S1461145708009243. [DOI] [PubMed] [Google Scholar]