Abstract
The major ingredients of grassleaf sweetflag rhizome are β-asarone and eugenol, which can cross the blood-brain barrier and protect neurons. This study aimed to observe the neuroprotective effects and mechanisms of β-asarone and eugenol, components of the Chinese herb grassleaf sweetflag rhizome, on PC12 cells. First, PC12 cells were cultured with different concentrations (between 1 × 10-10 M and 1 × 10-5 M) of β-asarone and eugenol. Survival rates of PC12 cells were not significantly affected. Second, PC12 cells incubated with amyloid-beta42, which reduced cell survival, were cultured under the same conditions (1 × 10-6 M β-asarone and eugenol). The survival rates of PC12 cells significantly increased, while expression levels of the mRNAs for the pro-apoptotic protein Bax decreased, and those for the anti-apoptotic protein Bcl mRNA increased. In addition, the combination of β-asarone with eugenol achieved better results than either component alone. Our experimental findings indicate that both β-asarone and eugenol protect PC12 cells through inhibiting apoptosis, and that the combination of the two is better than either alone.
Keywords: nerve regeneration, drugs, Chinese herbal, Alzheimer's disease, PC12 cells, Aβ grassleaf sweetflag rhizome, β-asarone, eugenol, apoptosis, neural regeneration
Introduction
Alzheimer's disease (AD), one of the most common degenerative diseases of the central nervous system, is pathologically characterized by excessive deposition of senile plaques, neurofibrillary tangles and degeneration of synapses (Miranda et al., 2000). These pathological characteristics are mainly present in the hippocampus and cerebral cortex, which are associated with the learning and memory capacities of the brain. Currently, the mechanisms leading to AD remain controversial and the present hypotheses include pathogenic roles of amyloid-beta (Aβ) plaques (DeSantis et al., 2012; Liu et al., 2013b; Lu et al., 2013; Kim et al., 2014), tangles of hyperphosphorylated tau (Cohen et al., 2013), apoptosis caused by caspase (Pozueta et al., 2013), decrease of synapses (Kim et al., 2013; Pozueta et al., 2013), and losses of cholinergic neuron (Annunziata et al., 2013; Carvajal et al., 2013; Chen et al., 2013; Kim et al., 2013; Wang et al., 2014). However, the “Aβ hypothesis” is widely accepted as the dominant mechanism of pathogenesis in AD (Ziv et al., 2006; Liu et al., 2013a; Lu et al., 2013). That is, Aβ, the main component of amyloid plaques, aggregates in specific brain regions, produces a neurotoxic effect, and leads to synaptic damage and neuronal death, ultimately leading to the occurrence of AD.
Grassleaf sweetflag rhizome is a perennial herb belonging to the family Araceae. It is often used in traditional Chinese medicine in prescriptions for brain resuscitation and enlightenment because, according to traditional Chinese medicine theory, it has the effects of enlightening and tranquilization (Dong et al., 2014). Previous investigations have shown that the major ingredients of grassleaf sweetflag rhizome with these effects are β-asarone and eugenol (Xue et al., 2014). β-Asarone and eugenol, contained in the volatile oil (Dayer et al., 2005), are the main elements involved in protecting neurons. Both can cross the blood-brain barrier into the brain tissue and provide protective effects (Chen et al., 2014). To observe the mechanisms by which the volatile oil of grassleaf sweetflag rhizome protect against AD and to explore neuroprotective mechanisms of β-asarone and eugenol and a mixture of both, PC12 cell injury models were established using Aβ42 oligomers in this study.
Materials and Methods
Cell culture
The PC12 cell line (Jinan University, Guangzhou, Guangdong Province, China) was cultured in 1640 culture medium (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Hangzhou Sijiqing Bioengineering Co., Ltd., Hangzhou, Zhejiang Province, China) at 37°C with 5% CO2. Culture medium was changed every 2 days and cells were rinsed with 0.01 M PBS. Cells were subcultured when > 90% confluence was reached.
Cells cultured with β-asarone and eugenol
PC12 cells in the logarithmic growth phase were incubated in 96-well plates; 200 μL of a cell suspension was added into each well at a density of 1 × 105/mL. Cells were subsequently incubated with β-asarone (National Institutes for Food and Drug Control, Beijing; concentration range 1 × 10-10 M to 1 × 10-5 M in saline), eugenol (National Institutes for Food and Drug Control; concentration range 1 × 10-10 M to 1 × 10-5 M in saline), a mixture of both, or saline for 24 hours. Then, 0.5 g/mL MTT (Sigma, St. Louis, MO, USA) was added to each well to a final concentration of 10%. After 4 hours of incubation at 37°C, the culture medium was replenished with 150 μL of DMSO (Sigma). Optical density in each group was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). A higher optical density indicated better viability.
Establishing the cell injury model
Aβ42 freeze-dried powder (Anaspec, Fremont, CA, USA) was first dissolved in 1% NH4 OH and then added to PBS to a final concentration of 1 × 103 μM, as stock solution. The solution was stored at −20°C before use. In the experiments, Aβ42 stock solution was added to the culture medium to a final concentration of 20 μM (working solution). Cells were firstly incubated at 37°C for at least 24 hours and the culture medium was subsequently changed to another medium containing oligomers of Aβ42 for 24 hours to establish the cell injury model (Togo et al., 2002; Bizzarri et al., 2006).
First, 200 μL of the PC12 cell suspension at a density of 1 × 105/mL was added into each well of a 96-well plate, and cultured with medium containing different concentrations of Aβ42 (0, 10, 20, 30 μM) for 24 hours. Subsequently, MTT (Sigma) was added to each well to a final concentration of 5 mg/mL, and the plate was incubated at 37°C for 4 hours. Then, the medium was replaced with 150 μL of DMSO (Sigma). Cell viability was assessed using the MTT method.
Protective effects of β-asarone and eugenol in the cell injury model
PC12 cells were pre-incubated with β-asarone (1 × 10-6 M), eugenol (1 × 10-7 M), a mixture of both, and PBS as a negative control in 24-well plates for 24 hours. Then, the culture medium was replaced with medium containing Aβ42 (20 μM), and cells were incubated for 12 hours. After two washes in PBS, cells were incubated with proteinase K working solution (Sigma) at 37°C for 8 minutes. Thereafter, TUNEL staining was carried out to assess the level of cell apoptosis in each group. The pre-incubated groups were then treated with TUNEL reactive solution (Abcam, Cambridge, MA, USA, containing 50 μL of TdT and 450 μL of DAB-stained dUTP), while the model group (PC12 cells pre-incubated with PBS as a negative control in 24-well plates for 24 hours) was treated with TUNEL reactive solution (Abcam; containing 450 μL DAB-stained dUTP) at 25°C for 10 minutes and rinsed three times with PBS. Apoptotic cells in each group were counted six times under a light microscope (Olympus, Tokyo, Japan).
Expression of apoptosis-related factors detected by reverse transcription-PCR
The mRNA expression levels of Bax and Bcl-2, which are closely related to cell apoptosis, were measured. Bax mRNA and Bcl-2 mRNA were transcribed in PC12 cells after 0, 1, 3, 6, 12, and 24 hours of incubation with Aβ42 oligomers. β-Asarone (1 × 10-6 M), eugenol (1 × 10-6 M), a mixture of both, or saline were added after 24 hours of incubation with Aβ42 oligomers, and total RNA was extracted using the TRIzol Plus RNA Purification Kit (Invitrogen Life Technologies, Carlsbad, CA, USA). Forward PCR primers (Bax: 5′-GCG AAT TGG AGA TGA ACT GG-3′, Bcl-2: 5′-CTG GTG GAC AAC ATC GCT CTG-3′, β-actin: 5′-ATG CCA TCC TGC GTC TGG ACC TGG C-3′) and reverse PCR primers (Bax: 5′-GTG AGC GAG GCG GTG AGG AC-3′, Bcl-2: 5′-GGT CTG CTG ACC TCA CTT GTG-3′, β-actin: 5′-AGC ATT TGC GGT GCA CGA TGG AGG G-3′) were used for reverse transcription PCR assays. Thereafter, reverse transcription PCR was performed using an MJ Mini Thermal Cycler (Bio-Rad) and the following program: 95°C for 5 minutes, 25 cycles of 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute, and an extension at 72°C for 10 minutes. The reverse transcription PCR products were analyzed by 1% agarose gel electrophoresis. β-Actin was used as an internal reference.
Statistical analysis
Data are presented as the mean ± SD and were analyzed by one-way analysis of variance followed by the least significant difference test. The threshold for significance was P < 0.05, and statistical analysis was performed using SPSS 22.0 software (IBM, Albany, NY, USA).
Results
Establishment of the Aβ42-induced cell injury model
After addition of different concentrations of Aβ42 to PC12 cells, the MTT assay was applied to assess the viability of PC12 cells (Figure 1). Compared with the control group, significant differences in cell viability were observed in the Aβ 20 μM (P = 0.0067) and 30 μM (P = 0.0008) groups, while there was no significant difference in viability in the 10 μM Aβ group (P = 0.0970).
Figure 1.
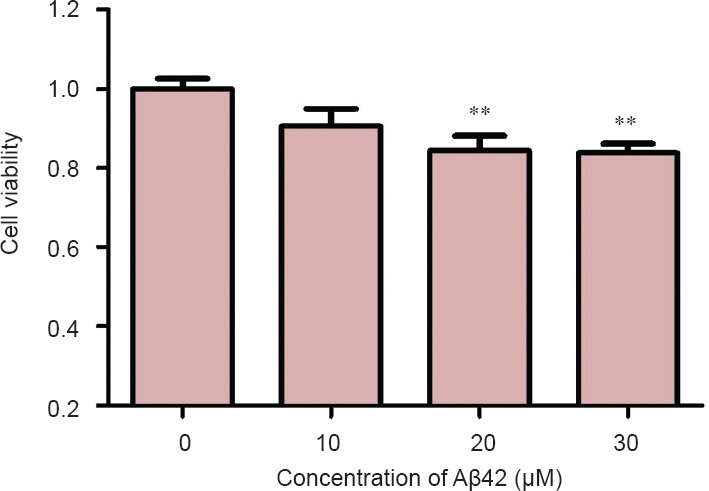
Toxic effect of amyloid-beta(Aβ)42 with different concentrations on the viability of PC12 cells as measured by MTT assay.
Data are presented as the mean ± SD. Results were calculated from six independent experiments. Data were analyzed by one-way analysis of variance combined with the least significant difference test. **P < 0.01, vs. control group (0).
Toxicity of β-asarone and eugenol toward PC12 cells
The viabilities of PC12 cells cultured with β-asarone (1 × 10-10 M to 1 × 10-5 M) and eugenol (1 × 10-10 M to 1 × 10-5 M) were similar to those of cells in the control group (P > 0.05), indicating that there were no toxic effects of β-asarone or eugenol at the experimental concentration range (Figure 2).
Figure 2.
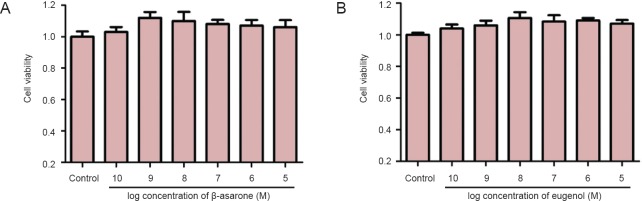
No toxicity of β-asarone (A) and eugenol (B) toward PC12 cells.
Data are expressed as the mean ± SD in all cells. Results were calculated from six independent experiments. Data were analyzed by one-way analysis of variance combined with the least significant difference test.
Effects of β-asarone and eugenol on PC12 cell apoptosis
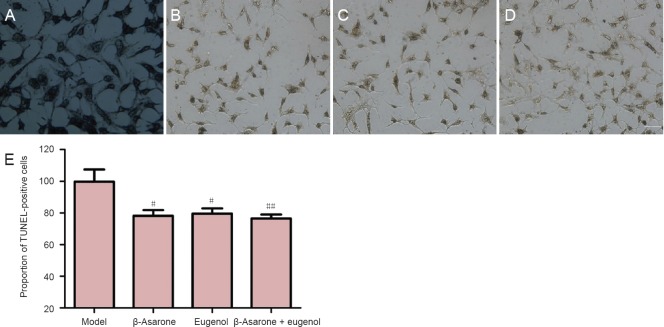
TUNEL staining results showed that, compared with the model group, the rates of apoptosis in PC12 cells incubated with β-asarone, eugenol or a mixture of both were decreased (P < 0.05 or P < 0.01; Figure 3).
Figure 3.
Effects of β-asarone and eugenol on PC12 cell apoptosis.
(A–D) Effect of β-asarone and eugenol on the proportion of TUNEL-positive (apoptosis) PC12 cells. (A) Model group; (B) 1 × 10-6 M β-asarone group; (C) 1 × 10-6 M eugenol group; (D) 1 × 10-6 M β-asarone + 1 × 10-6 M eugenol group. Scale bar: 10 μm. (E) Quantification of TUNEL-positive (apoptosis) PC12 cells with each treatment. Data are presented as the mean ± SD. Results were calculated from three independent experiments. Data were analyzed by one-way analysis of variance combined with the least significant difference test. #P < 0.05, ##P < 0.01, vs. model group.
Expression of apoptosis-related factors in Aβ42-induced PC12 cells
The results of reverse transcription PCR showed that, in PC12 cells incubated with Aβ42, Bax mRNA expression increased over time and reached a peak at 24 hours, while Bcl-2 mRNA expression decreased over time and reached the lowest level at 12 and 24 hours (Figure 4).
Figure 4.
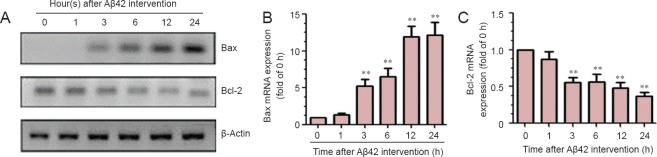
Levels of mRNAs for Bax and Bcl-2 in PC 12 cells incubated with amyloid-beta(Aβ)42.
(A) Representative agarose gel electrophoresis bands for reverse transcription PCR products showing the toxicity of Aβ42 toward PC12 cells. (B) Bax mRNA expression in PC 12 cells incubated with Aβ42. (C) Bcl-2 mRNA expression in PC 12 cells incubated with Aβ42. Data are presented as the mean ± SD. Results were calculated from six independent experiments. Data were analyzed by one-way analysis of variance combined with the least significant difference test. **P < 0.01, vs. control group (0). h: Hour(s).
After PC12 cells were pre-co-incubated with β-asarone (1 × 10-6 M), eugenol (1 × 10-6 M) or a mixture of both, the effects of Aβ42 on the expression levels of apoptosis-related factors, namely Bax and Bcl-2 mRNAs, were all significantly reversed compared with the model group (P < 0.05 or P < 0.01; Figure 5).
Figure 5.
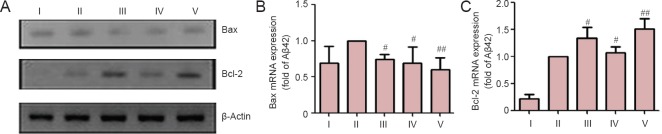
The protective effect of β-asarone, eugenol, and a mixture of both on PC12 cell injury models induced by amyloid-beta(Aβ)42.
(A) Representative agarose gel electrophoresis bands for reverse transcription-PCR products showing the protective effects of β-asarone, eugenol, and a mixture of both on PC12 cells. (B) Protective effect of β-asarone and eugenol in terms of Bax mRNA expression in PC12 cells incubated with Aβ42. (C) Protective effect of β-asarone and eugenol in terms of Bcl-2 mRNA expression in PC12 cells incubated with Aβ42. Data are presented as the mean ± SD. Results were calculated from six independent experiments. Data were analyzed by one-way analysis of variance combined with the least significant difference test. #P < 0.05, ##P < 0.01, vs. model group. I: Control group; II: model group; III: 1 × 10-6 M β-asarone group; IV: 1 × 10-6 M eugenol group; V: 1 × 10-6 M β-asarone + 1 × 10-6 M eugenol group.
Discussion
The importance of Aβ42 oligomers in AD
AD is one of the most common degenerative diseases of the central nervous system, but its specific mechanism of pathogenesis is still unclear. Previous studies have demonstrated that excessive deposition of β-amyloid in brain and excessive phosphorylation of tau are the most important pathogenic mechanisms in AD. The former mechanism, known as the Aβ hypothesis, is widely accepted. Based on this hypothesis, an imbalance between production and clearance of soluble and insoluble Aβ peptide leads to excessive deposition in the hippocampus and cerebral cortex, which are associated with the learning and memory functions of the brain. Excessive deposition usually produces various toxicities toward neurons and finally leads to AD (Lue et al., 1999).
Aβ is derived from the hydrolysis of the amyloid processor protein (APP) by β-secretase and β-secretase enzymes. Two kinds of Aβ, Aβ40 and Aβ42, can form through this pathway (Lu et al., 2013). Aβ42 causes degeneration of synapses and loss of neurons, with toxic effects in the specific brain regions mentioned above (Lu et al., 2013). Several factors are involved in the processing of APP into Aβ, such as caspases and effectors of apoptosis, which are required for apoptosis cascade reactions (Yu et al., 2011; Meesarapee et al., 2014). In addition, processing APP into Aβ is essentially regulated by members of the caspase family. Caspase-3 can cleave APP, influencing the normal metabolism of APP and facilitating the deposition of Aβ (Carvajal et al., 2013; Cetin et al., 2013). Thereafter, caspase-3 receives specific positive feedback from Aβ being stimulated as a result of the toxicity of Aβ. This, in turn, creates a cascade reaction thataccelerates the cleavage of APP and deposition of Aβ, thereby leading to apoptosis of neurons (Niikura et al., 2006; Ghasemi et al., 2014).
An earlier Aβ hypothesis indicated that APP mutations generate Aβ and that increasing levels of Aβ contribute to AD. However, the mechanism underlying the toxicity of Aβ is still unknown (Alvarez-Buylla and Lim, 2004), because there are many forms of Aβ in human brain, including monomers and oligomers, and the deposition of insoluble fibrils has also been shown. Therefore, it is difficult to confirm which factors mediate the pathogenesis of AD (Burdick et al., 1992; Fagan et al., 2006; Wang et al., 2006). Previous studies focused on the toxicities of Aβ contained in senile plaques. However, increasing evidence indicates that Aβ oligomers have a stronger neurotoxicity (Alvarez-Buylla et al., 2000; Kanski et al., 2002; Finder et al., 2010; Nyakas et al., 2011).
Mechanisms underlying the protective effects of β-asarone and eugenol through inhibiting apoptosis-related protein expression
Preliminary studies in our laboratory have shown that volatile oil extracted from grassleaf sweetflag rhizome can significantly improve learning and memory abilities in AD animal models, which indicates that the volatile oil of grassleaf sweetflag rhizome has an anti-AD and neuron protective effect in vivo.
In this study, a PC12 cell injury model was established using Aβ42 to test the protective effects of the main ingredients of grassleaf sweetflag rhizome volatile oil, namely β-asarone and eugenol. Our results showed that β-asarone, eugenol, and mixture of both could prevent the high levels of mRNA expression for the pro-apoptotic protein Bax and the low levels of mRNA expression for the anti-apoptotic protein Bcl-2. The findings in the mixture group were superior to those in either of the single component groups.
Apoptosis-related factors like the pro-apoptotic factors Bax and Bad and the anti-apoptotic factors Bcl-2 and Bcl-XL play important roles in signal transduction during apoptosis (Aimone et al., 2006). Under normal conditions, the Bax/Bcl-2 ratio is a balanced system (Dayer et al., 2005); however, Aβ can facilitate the overexpression of Bax, form a channel in the mitochondrial membrane, and induce cytochrome C transfer into the cytoplasm, which in turn activates the caspase cascade (Matias et al., 2013). That is, apoptosis is induced as a result of disruption of the balance between Bax/Bcl-2. Neuronal apoptosis induced by Aβ primarily operates through the mitochondrial pathway (Zheng et al., 2013; Wang et al., 2014); thus, a certain concentration of Aβ can increase the expression of pro-apoptotic Bax mRNA and decrease the expression of anti-apoptotic Bcl-2 mRNA, consistent with our findings.
Neuroprotective effects of β-asarone, eugenol, and a mixture of both on PC12 cells
The PC12 cell line grows fast and stably, is unlikely to automatically transform, and has typical features of neuroendocrine cells (Loeb et al., 1991). Therefore, it is usually used as the ideal cell model for studying the mechanisms underlying neuronal injury and the protective effects of drugs on neurons in vitro. In the present study we showed that both β-asarone and eugenol exert protective effects on PC12 cell injury models induced by Aβ42, and that a mixture of both was superior to either single component. This provides evidence that two ingredients of Chinese herbal medicine show efficacy and can potentially be used for the prevention and treatment of AD.
Footnotes
Funding: This study is financially supported by a grant from Guangdong Provincial Science and Technology Plan Program of China, No. 2010B060900085.
Conflicts of interest: None declared.
Copyedited by McGowan D, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res. 2000;127:1–11. doi: 10.1016/s0079-6123(00)27002-7. [DOI] [PubMed] [Google Scholar]
- Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E, Nixon R, d’Azzo A. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat Commun. 2013;4:2734. doi: 10.1038/ncomms3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. doi: 10.1016/j.pharmthera.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992;267:546–554. [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carvajal FJ, Zolezzi JM, Tapia-Rojas C, Godoy JA, Inestrosa NC. Tetrahydrohyperforin decreases cholinergic markers associated with amyloid-β plaques, 4-hydroxynonenal formation, and caspase-3 activation in AβPP/PS1 mice. J Alzheimers Dis. 2013;36:99–118. doi: 10.3233/JAD-130230. [DOI] [PubMed] [Google Scholar]
- Cetin F, Yazihan N, Dincer S, Akbulut G. The effect of intracerebroventricular injection of beta amyloid peptide (1-42) on caspase-3 activity, lipid peroxidation, nitric oxide and NOS expression in young adult and aged rat brain. Turk Neurosurg. 2013;23:144–150. doi: 10.5137/1019-5149.JTN.5855-12.1. [DOI] [PubMed] [Google Scholar]
- Chen QX, Miao JK, Li C, Li XW, Wu XM, Zhang XP. Anticonvulsant activity of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol Pharm Bull. 2013;36:23–30. doi: 10.1248/bpb.b12-00376. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wei G, Nie H, Lin Y, Tian H, Liu Y, Yu X, Cheng S, Yan R, Wang Q, Liu DH, Deng W, Lai Y, Zhou JH, Zhang SX, Lin WW, Chen DF. β-Asarone prevents autophagy and synaptic loss by reducing ROCK expression in asenescence-accelerated prone 8 mice. Brain Res. 2014;1552:41–54. doi: 10.1016/j.brainres.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis ME, Leung EH, Sweeny EA, Jackrel ME, Cushman-Nick M, Neuhaus-Follini A, Vashist S, Sochor MA, Knight MN, Shorter J. Operational plasticity enables hsp104 to disaggregate diverse amyloid and nonamyloid clients. Cell. 2012;151:778–793. doi: 10.1016/j.cell.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Gao Z, Rong H, Jin M, Zhang X. β-asarone reverses chronic unpredictable mild stress-induced depression-like behavior and promotes hippocampal neurogenesis in rats. Molecules. 2014;19:5634–5649. doi: 10.3390/molecules19055634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Zarifkar A, Rastegar K, Maghsoudi N, Moosavi M. Repeated intra-hippocampal injection of beta-amyloid 25-35 induces a reproducible impairment of learning and memory: Considering caspase-3 and MAPKs activity. Eur J Pharmacol. 2014;726:33–40. doi: 10.1016/j.ejphar.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Kanski J, Aksenova M, Butterfield DA. The hydrophobic environment of Met35 of Alzheimer's Aβ(1–42) is important for the neurotoxic and oxidative properties of the peptide. Neurotox Res. 2002;4:219–223. doi: 10.1080/10298420290023945. [DOI] [PubMed] [Google Scholar]
- Kim J, Yang Y, Song Seung S, Na JH, Oh Kyoung J, Jeong C, Yu Yeon G, Shin YK. Beta-amyloid oligomers activate apoptotic BAK pore for cytochrome c release. Biophys J. 2014;107:1601–1608. doi: 10.1016/j.bpj.2014.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Frost JL, Sun J, Fu H, Grimes S, Blackburn P, Lemere CA. MER5101, a novel Aβ1-15: DT conjugate vaccine, generates a robust anti-Aβ antibody response and attenuates Aβ pathology and cognitive deficits in APPswe/PS1ΔE9 transgenic mice. J Neurosci. 2013a;33:7027–7037. doi: 10.1523/JNEUROSCI.5924-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao X, Zeng X, Bossers K, Swaab DF, Zhao J, Pei G. β-arrestin1 regulates γ-secretase complex assembly and modulates amyloid-β pathology. Cell Res. 2013b;23:351–365. doi: 10.1038/cr.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb DM, Maragos J, Martin-Zanca D, Chao MV, Parada LF, Greene LA. The trk proto-oncogene rescues NGF responsiveness in mutant NGF-nonresponsive PC12 cell lines. Cell. 1991;66:961–966. doi: 10.1016/0092-8674(91)90441-z. [DOI] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias AC, Manieri TM, Cipriano SS, Carioni VM, Nomura CS, Machado CM, Cerchiaro G. Diethyldithiocarbamate induces apoptosis in neuroblastoma cells by raising the intracellular copper level, triggering cytochrome c release and caspase activation. Toxicol In Vitro. 2013;27:349–357. doi: 10.1016/j.tiv.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Meesarapee B, Thampithak A, Jaisin Y, Sanvarinda P, Suksamrarn A, Tuchinda P, Morales NP, Sanvarinda Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother Res. 2014;28:611–616. doi: 10.1002/ptr.5036. [DOI] [PubMed] [Google Scholar]
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC. The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer's disease. Prog Neurobiol. 2000;62:633–648. doi: 10.1016/s0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Niikura T, Tajima H, Kita Y. Neuronal cell death in Alzheimer's disease and a neuroprotective factor humanin. Curr Neuropharmacol. 2006;4:139–147. doi: 10.2174/157015906776359577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakas C, Granic I, Halmy LG, Banerjee P, Luiten PG. The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-β42 with memantine. Behav Brain Res. 2011;221:594–603. doi: 10.1016/j.bbr.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Ribe EM, Troy CM, Arancio O, Shelanski M. Caspase-2 is required for dendritic spine and behavioural alterations in J20 APP transgenic mice. Nat Commun. 2013;4:1939. doi: 10.1038/ncomms2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Muralidhara Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res. 2013;38:330–345. doi: 10.1007/s11064-012-0924-9. [DOI] [PubMed] [Google Scholar]
- Roberts SK, McAinsh M, Cantopher H, Sandison S. Calcium dependence of eugenol tolerance and toxicity in Saccharomyces cerevisiae. PLoS One. 2014;9:e102712. doi: 10.1371/journal.pone.0102712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang C, Shu Z, Chan K, Huang S, Li Y, Xiao Y, Wu L, Kuang H, Sun X. Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J Ethnopharmacol. 2014;153:318–325. doi: 10.1016/j.jep.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Zhou HD, Zhou XF. Clearance of amyloid-beta in Alzheimer's disease: progress, problems and perspectives. Drug Discov Today. 2006;11:931–938. doi: 10.1016/j.drudis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wei G, Chen YB, Chen DF, Lai XP, Liu DH, Deng RD, Zhou JH, Zhang SX, Li YW, Lii H, Liu LF, Wang Q, Nie H. β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. J Alzheimers Dis. 2013;33:863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- Xue Z, Guo Y, Zhang S, Huang L, He Y, Fang R, Fang Y. Beta-asarone attenuates amyloid beta-induced autophagy via Akt/mTOR pathway in PC12 cells. Eur J Pharmacol. 2014;741:195–204. doi: 10.1016/j.ejphar.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Yu W, Mechawar N, Krantic S, Quirion R. α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem. 2011;119:848–858. doi: 10.1111/j.1471-4159.2011.07466.x. [DOI] [PubMed] [Google Scholar]
- Zheng M, Liu J, Ruan Z, Tian S, Ma Y, Zhu J, Li G. Intrahippocampal injection of Aβ1–42 inhibits neurogenesis and down-regulates IFN-γ and NF-κB expression in hippocampus of adult mouse brain. Amyloid. 2013;20:13–20. doi: 10.3109/13506129.2012.755122. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]