Abstract
The clinical effect of electroacupuncture on depression is widely recognized. However, the signal transduction pathways and target proteins involved remain unclear. In the present study, rat models of chronic restraint stress were used to explore the mechanism by which electroacupuncture alleviates depression. Rats were randomly divided into control, model, and electroacupuncture groups. Chronic restraint stress was induced in the model and electroacupuncture groups by restraining rats for 28 days. In the electroacupuncture group, electroacupuncture pretreatment at Baihui (GV20) and Yintang (GV29) acupoints was performed daily (1 mA, 2 Hz, discontinuous wave, 20 minutes) prior to restraint for 28 days. Open field tests and body weight measurements were carried out to evaluate the depressive symptoms at specific time points. On day 28, the crossing number, rearing number, and body weights of the model group were significantly lower than those in the control group. Behavior test results indicated that rat models of depressive-like symptoms were successfully established by chronic restraint stress combined with solitary raising. On day 28, an isobaric tag for a relative and absolute quantitation-based quantitative proteomic approach was performed to identify differentially expressed proteins in hippocampal samples obtained from the model and electroacupuncture groups. The potential function of these differential proteins was predicted through the use of the Cluster of Orthologous Groups of proteins (COG) database. Twenty-seven differential proteins (uncharacteristic proteins expected) were selected from the model and electroacupuncture groups. In addition to unknown protein functions, COG are mainly concentrated in general prediction function, mechanism of signal transduction, amino acid transport and metabolism groups. This suggests that electroacupuncture improved depressive-like symptoms by regulating differential proteins, and most of these related proteins exist in nerve cells.
Keywords: nerve regeneration, traditional Chinese medicine, electroacupuncture, depression, chronic restraint stress, iTRAQ, differential protein, proteomics, multi-target effect, neural regeneration
Introduction
Depression, characterized by negative moods, decreased physical activity, loss of interest in usual activities, feeling of helplessness and suicidal tendencies (Kessler et al., 2003), currently ranks as the fourth leading cause of disability worldwide (Mathers et al., 2006; Moussavi et al., 2007). Depression results from several biological factors, including inflammatory and immune processes, alterations in activation of the hypothalamic-pituitary-adrenal axis, variability in heart rate, increased activity of the sympathoadrenal and pituitary adrenal axis, reduction in circulating endothelial progenitor cells, increased levels of cortisol and catecholamine, altered activity of the autonomic nervous system, and oxidation processes (Compare et al., 2011, 2013; Nemeroff et al., 2012). Depression is also associated with several unhealthy lifestyles, such as increased consumption of tobacco, alcohol and illicit substances, reduced physical activity, overeating and lack of medical adherence.
Various western medicine treatments including antidepressant medication and psychological therapy have a pivotal role in treating depression; however, almost one fourth of patients are unable to achieve favorable effects, especially the improvement of somatic symptoms (Bruce et al., 2005). Therefore, seeking alternative therapy for depression remains an urgent issue.
Electroacupuncture, functioning as a type of bi-directional regulation of positive stress source, has been suggested to be effective in treating both physical and mental disorders (Cabioglu et al., 2007; Yang et al., 2014). However, there have been few basic experimental studies regarding the mechanism of electroacupuncture treatment in depression.
Hippocampal formation is involved in the stress response as a vital part of the negative feedback system regulating circulating glucocorticoids (McEwen, 2000; Liu et al., 2009). Moreover, the hippocampus is coupled to stress through fear and emotional processing via its connections to the amygdala (McIntyre et al., 2003; Roozendaal et al., 2004). Hippocampal formation is believed to be implicated in the etiology of depression, potentially through an important role in stress responses (Sheline, 2000; Nestler et al., 2002; Holm et al., 2010). Based on the role of hippocampal formation in stress and stress-related psychiatric disorders, it might be expected to find markers of stress-susceptibility/resilience in this region of the brain. Chronic psychological stress produces hippocampal-dependent cognitive deficits consistent with structural changes in the hippocampus. Stress-induced changes in hippocampal CA3 neurons are consistent with deficits in hippocampal function (McLaughlin et al., 2007).
Therefore, the purpose of this study was to detect alterations of hippocampal protein expression prior to electroacupuncture pretreatment to elucidate the underlying mechanism by which electroacupuncture alleviates depression. According to traditional Chinese medicine, the function of two acupoints Baihui (GV20) and Yintang (GV29) is to replenish qi and boost yang, tranquilize the spirit and calm the mind (Shi, 2007). In the present study, we selected these two acupoints for electroacupuncture and identified differential proteins expressed during depression after electroacupuncture. These differential proteins might be potential targets of the anti-depressive effect of electroacupuncture pretreatment, which provides a reference for further research of depression.
Materials and Methods
Animals
Forty-five specific pathogen free (SPF) Sprague-Dawley male rats, aged 5 weeks, were supplied by Charles River Laboratories of Beijing, China (license No. SCXK (Jing) 2012-0001). Animals were housed at 18–26 °C, humidity 55%, in 12-hour light/dark cycles (light on at 8:00 a.m.), with free access to food and water. The study was performed in rats 3 days after environment acclimatization. All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize animal suffering and to be consistent with the 3R principle of reduction, replacement, and refinement. All experimental procedures received approval from the Animal Ethics Committee of Beijing University of Chinese Medicine (permission No. Kj-dw-18-20131012).
Establishment of animal models of depression and acupuncture administration
All rats were randomly and equally divided into three groups with 15 rats per group. In the control group, herd management, and 6-hour fasting for solids and liquids was performed from 9 a.m. to 3 p.m. per day, lasting for 28 days. In the model group, social isolation and chronic restraint stress were conducted daily for 28 days by the following method: rats were restrained in a self-made cylinder-shaped wire net (20-cm length and 5-cm diameter) from 9 a.m. to 3 p.m. After restraint, they were released with free access to water and food. In the electroacupuncture group, electroacupuncture pretreatment was conducted daily prior to restraint using the same method as for the model group.
During acupuncture administration, rats were maintained within a cloth bag. Baihui (GV20) and Yintang (GV29) acupoints were selected for electroacupuncture. GV20 is located above the apex auriculate, on the midline of the head, and GV29 at the midpoint between the two eyes (Liu et al., 2011). Sterilized disposable stainless steel needles (0.2 × 25.0 mm, Huan Qiu Brand, manufactured by Suzhou Medicine Co., Ltd., Suzhou, China) were inserted obliquely as deep as 5 mm for both points. Following the insertions, electrodes were added to the handle of needles (electroacupuncture apparatus used: SDZ-Velectronic needle therapy instrument, manufactured by Suzhou Medical Supplies Co., Ltd.). Electricity simulation parameters were 1 mA, 2 Hz, discontinuous wave, for 20 minutes.
Body weight measurement
Rat body weights were measured on days 0 and 28 of the study.
Open-field test
At days 0 and 28 of the study, an open-field test was conducted as previously described with modifications (Lu et al., 2011). A wood apparatus, composed of a square arena (80 × 80 cm2) with a 40-cm-high wall, was divided into 25 × 25-cm2 equal squares drawn on the floor. A single rat was gently placed at the center of the floor and allowed to familiarize itself for 3 minutes. The activity of the rat was recorded by a camera installed on top of the lateral high wall. The crossing times (defined as at least three paws in a square) and the rearing times in 3 minutes (defined as the rat standing upright on its hind legs) were monitored by two observers who were blind to the experimental design through a monitor equipped by a camera which was installed 1 meter away from the apparatus. After one rat finished the test, alcohol was applied to clean the floor to exclude the intervention of odor signals.
Tissue collection
The hippocampus was carefully dissected out from 12 male Sprague-Dawley rats on day 28. The specific steps were as follows: (1) cut along the coronal suture and sagittal suture, then pull off both sides of parietal bone and inter parietal bone; (2) open the skull, remove the cerebral cortex covering it, and separate the rest of the hippocampus from the cortex covering it along the surface of the hippocampus towards the ventral part of the hippocampus; and (3) free the hippocampus from the surrounding tissue.
Protein preparation
Hippocampal samples were ground to a powder in liquid nitrogen and lysed with lysis buffer (7 M urea, 2 M thiourea, 4%CHAPS, 40 mM Tris-HCl, pH 8.5) containing 1 mM phenylmethyl sulfonylfluoride and 2 mM phenylmethyl sulfonylfluoride (final concentration). After 5 minutes, 10 mM dithiothreitol (final concentration) was added to the samples. The resulting suspension was sonicated at 200 W for 15 minutes, and then centrifuged at 4°C, 30,000 × g for 15 minutes. The supernatant was mixed well with a 5× volume of chilled acetone containing 10% (v/v) trichloroacetic acid and incubated at −20°C overnight. After centrifugation at 4°C, 30, 000 × g, the supernatant was discarded. The precipitate was washed with chilled acetone three times. The pellets were air-dried and dissolved in lysis buffer (7 M urea, 2 M thiourea, 4% NP40, 20 mM Tris-HCl, pH 8.0–8.5). The suspension was sonicated at 200 W for 15 minutes, and centrifuged at 4°C, 30,000 × g for 15 minutes. The supernatant was transferred to another tube to reduce disulfide bonds in proteins of the supernatant, 10 mM dithiothreitol (final concentration) was added and incubated at 56°C for 1 hour. Subsequently, 55 mM iodoacetamide (final concentration) was added to block cysteines, and incubated for 1 hour in a dark room. The supernatant was mixed well with a 5× volume of chilled acetone for 2 hours at −20°C to precipitate proteins. After centrifugation at 4°C, 30,000 × g, the supernatant was discarded, the pellets were air-dried for 5 minutes, dissolved in 500 μL of 0.5 M triethylammoniumbicarbonate (Applied Biosystems, Milan, Italy), and sonicated at 200 W for 15 minutes. Finally, samples were centrifuged at 4°C, 30, 000 × g for 15 minutes, and the supernatant was transferred to a new tube and quantified. The proteins in the supernatant were kept at −80°C for further analysis.
iTRAQ™ labeling and SCX fractionation
Total protein (100 μg) was extracted from each sample solution and then digested with Trypsin Gold (Promega, Madison, WI, USA) with a ratio of protein:trypsin of 30:1 at 37°C for 16 hours. After trypsin digestion, peptides were dried by vacuum centrifugation. Peptides were reconstituted in 0.5 M triethylammoniumbicarbonate and processed according to the manufacturer's protocol for 8-plex iTRAQ reagent (Applied Biosystems). Briefly, one unit of iTRAQ reagent was thawed and reconstituted in 24-μL isopropanol. Samples were labeled with the iTRAQ tags as follows: Sample H45 (No.117), and Sample H50 (No.116). After incubation at room temperature for 2 hours, the isobaric-tag-labeled peptides were pooled and dried by vacuum centrifugation.
SCX chromatography was carried out using a LC-20AB HPLC Pump system (Shimadzu, Kyoto, Japan). The iTRAQ labeled peptides were reconstituted with 4-mL buffer A (25 mM NaH2PO4 in 25% ACN, pH 2.7) and loaded onto a 4.6 × 250-mm2 Ultremex SCX column containing 5-μm-sized particles (Phenomenex, Torrance, CA, USA). The peptides were eluted at a flow rate of 1mL/min with a gradient of buffer A for 10 minutes, 5–60% buffer B (25 mM NaH2 PO4, 1 M KCl in 25% ACN, pH 2.7) for 27 minutes, and 60–100% buffer B for 1 minute. The system was then maintained with 100% buffer B for 1 minute before equilibrating with buffer A for 10 minutes prior to the next injection. Absorbance at 214 nm was measured, and fractions were collected every minute. The eluted peptides were pooled into 20 fractions, desalted with a Strata X C18 column (Phenomenex) and vacuum-dried.
LC-ESI-MS/MS analysis based on Triple TOF 5600
Each fraction was re-suspended in buffer A (5% ACN, 0.1%FA) and then centrifuged at 20,000 × g for 10 minutes, and the mean peptide concentration was 0.5 μg/μL. A 10-μL volume of supernatant was loaded onto a 2-cm C18 trap column on a LC-20AD nanoHPLC (Shimadzu, Kyoto, Japan) through the use of autosampler. Then, the peptide samples were eluted onto a homemade 10-cm analytical C18 column (inner diameter 75 μm) and loaded at 8 μL/min for 4 minutes. Thereafter, a 35-minute gradient was run at 300 nL/min starting from 2–35% buffer B (95%ACN, 0.1%FA), followed by a 5-minute linear gradient to 60%, 2-minute linear gradient to 80%, maintenance at 80% buffer B for 4 minutes, and finally returning to 5% in 1 minute. Data acquisition was performed with a Triple TOF 5600 System (AB SCIEX, Concord, ON, USA) fitted with a Nanospray IIIsource (AB SCIEX) and a pulled quartz tip as the emitter (New Objectives, Woburn, MA, USA). Data were acquired using the following parameters: ion spray voltage 2.5 kV, curtain gas 30 psi, nebulizer gas 15 psi, and interface heater temperature 150°C. The MS was operated with a reflection pattern of greater than or equal to 30,000 FWHM for TOF MS scans. For information associated with data collection (IDA), survey scans were acquired in 250 ms and as many as 30 product ion scans were collected if exceeding a threshold of 120 counts per second (counts/s) and with a 2+ to 5+ charge-state. Total cycle time was fixed to 3.3 seconds. The Q2 transmission window was 100 Da for 100%. Four time bins were summed for each scan at a pulse frequency value of 11 kHz through monitoring of the 40-GHz multichannel TDC detector with four-anode channel detection. A sweeping collision energy setting of 35 ± 5 eV coupled with iTRAQ adjust rolling collision energy was applied to all precursor ions for collision-induced dissociation. Dynamic exclusion was set for 1/2 of peak width (15 seconds) and then the precursor was refreshed off the exclusion list.
Data analysis
The raw files were converted to MGF files with Proteome Discoverer 1.2 software (PD 1.2; Thermo; 5600-msconverter) and the MGF files were searched. Protein identification was conducted using Mascot search engine (Matrix Science, London, UK; version 2.3.02) against a database containing 39,925 sequences. For protein identification, a mass tolerance of 0.1 Da (ppm) was permitted for intact peptide masses and 0.05 Da for fragmented ions, with allowance for one missed cleavage in the trypsin digests. Gln-> pyro-Glu (N-term Q), Oxidation (M), and Deamidated (NQ) were the potential variable modifications, and Carbamidomethyl (C), iTRAQ8 plex (N-term), and iTRAQ8plex (K) were the fixed modifications. The charge states of peptides were set to +2 and +3. Specifically, an automatic decoy database search was performed in Mascot by choosing the decoy checkbox in which a random sequence of database was generated and tested for raw spectra as well as the real database. To reduce the probability of false peptide identification, only peptides with significance scores (≥ 20) at the 99% confidence interval by Mascot probability analysis greater than “identity” were counted as “identified”. The identification of each confident protein involved at least one unique peptide. For protein quantitation, a protein should contain at least two unique peptides. The quantitative protein ratios were weighted and normalized by the median ratio in Mascot. Only ratios with P values < 0.05 were used, and only fold changes >1.2 were considered statistically significant.
The differential proteins were further analyzed by pathway enrichment to determine which main biochemical pathways and signaling transduction pathways they were part of.
Statistical analysis
The behavior test results are expressed as the mean ± SD and were analyzed statistically using SPSS 17.0 software (SPSS, Chicago, IL, USA). All data conformed to normal distribution and variance. One-way analysis of variance, followed by the least significance difference test, was performed to compare the differences between groups. A level of P < 0.05 was considered statistically significant.
Results
Body weight changes during restraint stress period
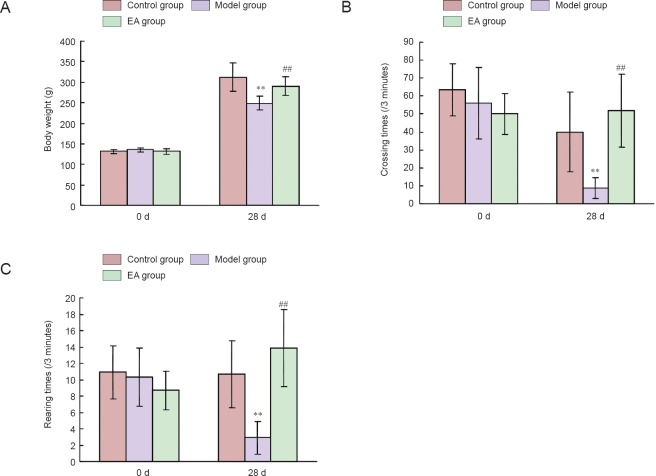
As shown in Figure 1A, there was no significant difference in bone weight among the three groups at the beginning of the study. After 28 days of induction, rat body weights were significantly decreased in the model group compared with the control group (P < 0.01), and rat body weight was significantly increased in the electroacupuncture group compared with the model group (P < 0.01).
Figure 1.
Effects of electroacupuncture (EA) on rat body weight and locomotor activity in the open-field test at 0 and 28 days (d) of treatment.
(A) Body weight changes during the restraint stress period. (B, C) Open-field test changes during the restraint stress period. **P < 0.01, vs. control group; ##P < 0.01, vs. model group. Data are expressed as the mean ± SD (n = 15; one-way-analysis of variance followed by the least significance difference test).
Open-field test changes during the restraint stress period
The exploratory and locomotor activities of rats were determined using the open-field test. As shown in Figure 1B, no difference in crossing numbers was observed among the three groups on day 0. After 28 days of the restraint stress procedure, the crossing numbers in the model group were significantly lower compared with the control group (P < 0.01). The crossing numbers in the electroacupuncture group were significantly higher compared with the model group (P < 0.01). On day 0, there was no significant difference in rearing numbers among the three groups (P > 0.05). Figure 1C shows that restraint stress reduced the rearing numbers of rats in the model group compared with the control group (P < 0.01). The rearing numbers were significantly increased in the electroacupuncture group compared with the model group (P < 0.01). According to the behavior comparison between control and model groups, rats in the model group exhibited a depressive-like state.
Differential proteins
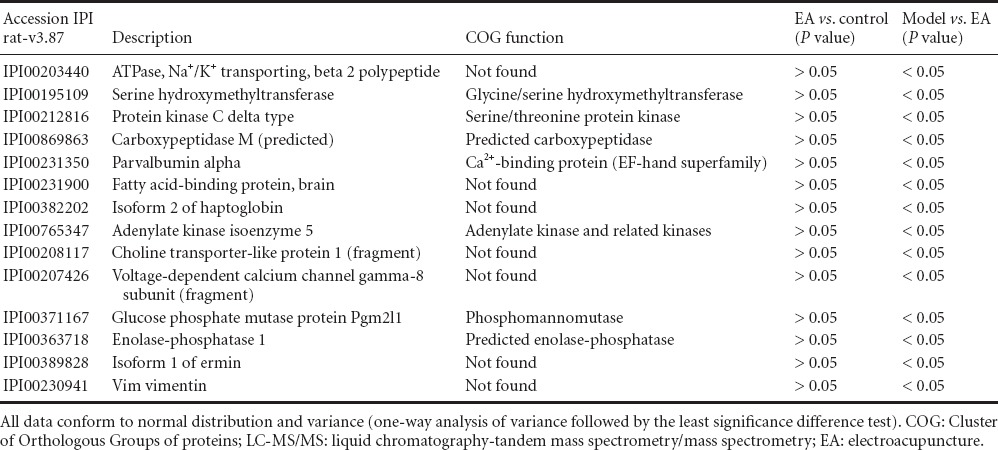
Immunodepleted protein fractions were then profiled using iTRAQ coupled to a liquid chromatography-tandem mass spectrometry/mass spectrometry (LC-MS/MS)-based approach. The identified proteins in the depressed animals were used for both chronic restraint stress and electroacupuncture pretreatment comparisons. A total of 4,240 and 4,352 proteins were identified separately by two computers using the Mascot software. Differentially regulated proteins (fold change > 1.2 with P < 0.05) were selected. These cut-offs were selected based on literature investigating the reproducibility of iTRAQ quantification (Moulder et al., 2010). The potential function of these differential proteins was predicted using the COG database. Thirteen proteins were downregulated in the model group compared with the electroacupuncture group, and two differential proteins were downregulated in the electroacupuncture group compared with the control group (Table 1). Fourteen proteins were upregulated in the model group compared with the electroacupuncture group, and there were no differential proteins between electroacupuncture and control groups (Table 2).
Table 1.
Differential (downregulated) proteins identified by iTRAQ coupled with LC-MS/MS
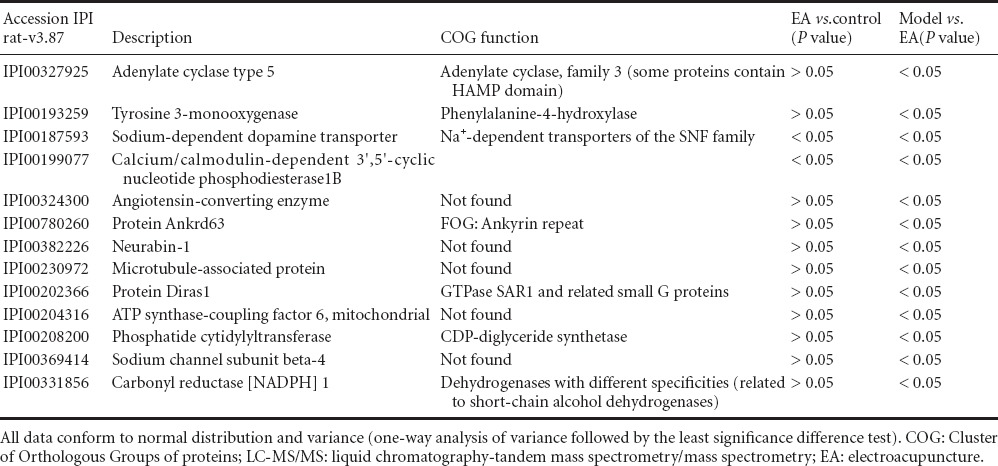
Table 2.
Differential (upregulated) proteins identified by iTRAQ coupled with LC-MS/MS
Discussion
iTRAQ technology was used in this study to quantitatively analyze proteomics of the hippocampus of chronic restrained rats and electroacupuncture pretreated rats. iTRAQcan be used for parallel comparison of protein abundance by determining the peak intensity of reporter ions released by iTRAQ-tagged peptides and is an important tool in the area of quantitative proteomic study.
iTRAQ has been used in a study to determine the mechanism involved in the anti-depressive effects of electroacupuncture pretreatment. Using this method, 27 proteins related to emotional diseases were successfully identified. To identify potential curative mechanisms of early intervention depressive state, the current study preliminarily explored proteins that were associated with depression by comparing chronic restraint stress rats with electroacupuncture pretreated rats.
Intriguingly, a recent study suggested that dopamine played a critical role in the anti-inflammatory potential of electroacupuncture (Torres-Rosas et al., 2014). These findings offer novel evidence for the involvement of electroacupuncturein modulating immune responses via the neural-immune network. The present study identified proteins related to depression or stress, such as dopamine transporter (also dopamine active transporter, DAT, SLC6A3) (Meyeret al., 2001; Yang et al., 2008) and protein kinase C (Coull et al., 2000). A large body of literature showed that dopamine insufficiency might be a susceptible factor for depression and treatment targeting dopamine had favorable effects (Liang et al., 2011). This indicates that neurotransmitters such as dopamine might mediate the antidepressant-like effects of electroacupuncture by regulating immune responses; meanwhile, they serve as a direct effector per se in treating depression. These results are consistent with the present findings.
The neurobiological mechanisms underlying the pathophysiology and therapeutics of bipolar disorders are still unknown. In recent years, protein kinase C has emerged as a potentially important factor in mania (Abrial et al., 2012). Protein kinase C inhibitors were recently reported to ease moods (Einat, 2014). In the present study, protein kinase Cδ showed a tendency to be increased after chronic restraint stress, which was consistent with the results of a previous study (Coull et al., 2000). Protein kinase C can activate the cascade system, leading to mitogen-activated protein kinase (MAPK) phosphorylation, induction of the protein phosphorylation of the MAPK gene and regulation of the phosphorylation of the Elk-1protein. Protein kinase C activates Elk-1 protein and binds with serum reaction components, followed by serum response factors, to regulate gene expression. In the present study, protein kinase Cδ was shown to be related to the neurotrophin signaling pathway, vascular smooth muscle contraction and tight junction pathway after chronic restraint stress. A genetic polymorphism in the DAT gene (DAT1), known as a Variable Number of Tandem Repeats (VNTR), is associated with dopamine-related disorders, and influences the amount of proteins expressed. In addition, protein kinase C and MAPK can adjust the DAT transit speed of dopamine or cause DAT internal conformational changes (Vandenbergh et al., 1992).
This study initially investigated delineating primary comparative protein profiles in the model and electroacupuncture groups using iTRAQ, then confirmed interesting candidate biomarkers on a larger sample of depression (caused by chronic stress). Some of the “traditional” methods such as western blot or ELISA using samples of hippocampus in addition to serum samples/supernatant should be performed for further verification.
In the present study, electroacupuncture pretreatment was implemented in rats under slightly restrained conditions. A previous study (Liang, 2012) demonstrated no significant difference in serum adrenocorticotropic hormone and corticosterone between normal rats with or without acupuncture. This finding suggests that acupuncture administration does induce a stress response.
Depression induced by chronic restraint stress is an ideal model to mimic human depression symptoms. Chronic restraint stress affects multiple related proteins and through the modulation of these proteins, electroacupuncture can improve depressive symptoms. The specific targets identified in this study warrant further investigation.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81373729.
Conflicts of interest: None declared.
Copyedited by Croxford L, Raye W, Wang J, Li CH, Song LP, Zhao M
References
- Abrial E, Etievant A, Bétry C, Scarna H, Lucas G, Haddjeri N, Lambás-Señas L. Protein kinase C regulates mood-related behaviors and adult hippocampal cell proliferation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;3:40–48. doi: 10.1016/j.pnpbp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Bruce AE. Efficacy and tolerability of tricyclic antidepressants and ssris compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med. 2005;3:449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabioglu MT, Ergene N, Tan U. Electroacupuncture treatment of obesity with psychological symptoms. Int J Neurosci. 2007;117:579–590. doi: 10.1080/00207450500535545. [DOI] [PubMed] [Google Scholar]
- Compare A, Germani E, Proietti R, Janeway D. Clinical psychology and cardiovascular disease: an up-to-date clinical practice review for assessment and treatment of anxiety and depression. Clin Pract Epidemiol Ment Health. 2011;7:148–156. doi: 10.2174/1745017901107010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compare A, Bigi R, Orrego PS, Proietti R, Grossi E, Steptoe A. Type D personality is associated with the development of stress cardiomyopathy following emotional triggers. Ann Behav Med. 2013;45:299–307. doi: 10.1007/s12160-013-9474-x. [DOI] [PubMed] [Google Scholar]
- Coull MA, Lowther S, Katona CL, Horton RW. Altered brain protein kinase C in depression: a post-mortem study. Eur Neuropsychopharmacol. 2000;10:283–288. doi: 10.1016/s0924-977x(00)00084-5. [DOI] [PubMed] [Google Scholar]
- Einat H. Partial effects of the protein kinase C inhibitor chelerythrine in a battery of tests for manic-like behavior in black Swiss mice. Pharmacol Rep. 2014;66:722–725. doi: 10.1016/j.pharep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2010;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Liang J. Beijing: Beijing University of Chinese Medicine; 2012. Diversity research on neuron protection mechanism of depression by acupuncture and paroxetine. [Google Scholar]
- Liang Y, Wang CX, Fang JQ, Shao XM, Ying XM. Effect of pre-electroacupuncture at “Zusanli” (ST 36) on DA and 5-HT contents and their ratio in hypothalamus and striatum in exercise rats (Chin) Zhongguo Zhen Jiu. 2012;31:1101–1105. [PubMed] [Google Scholar]
- Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25:237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. J Affect Disord. 2011;128:309–311. doi: 10.1016/j.jad.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Lu J, Liang J, Wang JR. Acupuncture activates ERK-CREB Pathway in rats exposed to chronic upredictable mild stress. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/469765. 469765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Krüger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12:4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Moulder R, Lonnberg T, Elo LL, Filen JJ, Rainio E, Corthals G, Oresic M, Nyman TA, Aittokallio T, Lahesmaa R. Quantitative proteomics analysis of the nuclear fraction of human CD4 + cells in the early phases of IL-4-induced Th2 differentiation. Mol Cell Proteomics. 2010;9:1937–1953. doi: 10.1074/mcp.M900483-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–539. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Shi XM. Beijing: China Press of Traditional Chinese Medicine; 2007. Science of Acupuncture and Moxibustion. [Google Scholar]
- Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Yang L, Yue N, Zhu X, Han Q, Li B, Liu Q, Wu G, Yu J. Electroacupuncture promotes proliferation of amplifying neural progenitors and preserves quiescent neural progenitors from apoptosis to alleviate depressive-like and anxiety-like behaviours. Evid Based Complement Alternat Med. 2004;2014:872568. doi: 10.1155/2014/872568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YK, Yeh TL, Yao WJ, Lee IH, Chen PS, Chiu NT, Lu RB. Greater availability of dopamine transporters in patients with major depression a dual-isotope SPECT study. Psychiatry Res. 2008;162:230–235. doi: 10.1016/j.pscychresns.2007.08.008. [DOI] [PubMed] [Google Scholar]