Abstract
Background
Iron is an essential micronutrient required by all living organisms including malaria parasites (Plasmodium spp.) for many biochemical reactions, especially growth and multiplication processes. Therefore, malaria parasite needs to take up the iron from outside or/and inside the parasitized red blood cells (PRBC). Iron chelators are widely used for the treatment of thalassaemia-related iron overload and also inhibit parasite growth at levels that are non-toxic to mammalian cells.
Methods
Inhibitory effect of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) and green tea extract (GTE) on the growth of malaria parasite Plasmodium falciparum was compared with standard chelators including desferrioxamine (DFO), deferiprone (DFP) and deferasirox (DFX). A flow cytometric technique was used to enumerate PRBC stained with SYBR Green I fluorescent dye. The labile iron pool (LIP) was assayed using the calcein-acetoxymethyl fluorescent method.
Results
The IC50 values of DFO, GTE, CM1, DFX and DFP against P. falciparum were 14.09, 21.11, 35.14, 44.71 and 58.25 µM, respectively. Importantly, CM1 was more effective in reducing LIP levels in the P. falciparum culture than DFP (p < 0.05).
Conclusions
CM1 and GTE exhibit anti-malarial activity. They could interfere with uptake of exogenous iron or deplete the intracellular labile iron pool in malaria parasites, leading to inhibition of their growth.
Keywords: Plasmodium falciparum, Anti-malarial drug, Iron chelator, Green tea, 3-Hydroxypyrid-4-one
Background
Malaria is one of the most deadly infectious diseases and an enormous public health problem in tropical and subtropical countries. Malaria mortality rates have fallen by more than 25 % globally and by 33 % in the World Health Organization African Region since 2000, where almost every malarial induced death is caused by the highly virulent Plasmodium falciparum. Although anti-malarial drugs are used for treatment of infected patients, resistance to chloroquine (CQ), pyrimethamine (PYR) and sulfadoxine (S) drug is a major public health problem that obstructs the control of malaria [1]. Genetic polymorphisms described in Plasmodium spp. can provide reliable data about the prevalence of drug resistance [2]. Resistance to PYR is primarily conferred by point mutations of the P. falciparum genes encoded two key enzymes in folate pathway, P. falciparum dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) [3].
Plasmodial malaria parasites essentially obtain iron from their hosts for their growth and development, while the hosts have to evolve iron-withholding defence systems to suppress infection. Enhancement of iron withholding is a potential target for the development of novel therapeutic agents. Widespread multiple drug resistance in human malaria has intensified the search for new anti-malarial compounds, particularly iron chelators. The chelators exert their effects by sequestering iron from multiple sources, including transferrin as well as intracellular and extracellular iron [4]. Possibly, the iron that is bioavailable for in the intracellular parasites has originated from non-haem iron rather than the abundant haem iron in erythrocytic cytoplasm. Artemisinin found in the Chinese medicinal plant (Artemisia annua) binds iron to form ferric-dihydroartemisinin complex, resulting in reactive oxygen species (ROS)-mediated potent anti-malarial activity against ring and late stage of CQ-resistant P. falciparum malaria parasites [5].
Paradoxically, iron chelators are cytocidal to the plasmodial parasite despite the abundance of iron within the erythrocytes [6]. Interestingly, many iron chelators such as desferrioxamine (DFO), deferasirox (DFX), alkylthiocarbamates, 8-hydroxyquinoline, 2,3-dihydroxybenzoic acid (2,3-DHB) derivatives, N,N′-bis (o-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid (HBED), N,N′-ethylenebis(o-hydroxyphenylglycine) (EHPG), 1-[N-ethoxycarbonylmethyl-pyridoxy-lidenium]-2-[2′-pyridyl] hydrazine bromide have been shown to be effective anti-malarial agents [7–13]. The compounds could possibly eliminate internal iron pools and interfere with parasite differentiation and growth, but not interfere with biological functions of red blood cells (RBC); nonetheless, some chelators may act by generating free radicals after complexing intracellular iron [14]. 3-Hydroxypyridin-4-one derivatives (e.g. CP20, CP38, CP40, CP51, CP94, and CP96) which are a family of lipophilic, orally effective bidentate iron chelators can suppress malaria in vivo and in vitro [15, 16]. Iron chelators such as DFO and DFP have been tested as chemotherapeutic agents in P. falciparum- or/and Plasmodium vivax-infected patients [13, 17, 18].
A novel orally active bidentate iron chelator named 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one or CM1 (MW = 266), a derivative of deferiprone (DFP) (MW = 139) [19], was synthesized and characterized. CM1 (Kpart = 0.56) is more lipophilic than DFP (Kpart = 0.11), and has a high level of affinity as well as selectivity for iron(III) ions than other metal ions [19]. It is predicted that the CM1 can readily penetrate into mammalian cells to scavenge intracellular iron, forming a neutral Fe(III)-(CM1)3 complex, and to efflux the neutral complex from the cells. Most importantly, CM1 is non-toxic to cultured peripheral blood mononuclear cells as well as primary mouse hepatocytes, and it can also lower levels of plasma non-transferrin bound iron (NTBI), labile plasma iron (LPI), intracellular labile iron pool (LIP) and ferritin-bound iron effectively [19–21]. Green tea extract (GTE) is comprised of polyphenolic compounds, mainly catechins, that can inhibit the iron-catalyzed generation of reactive oxygen species (ROS) [22]. Previous studies have demonstrated that GTE exhibited anti-oxidative, free radical-scavenging, iron-chelating activities in iron-loaded thalassaemic mice, and a renal-protective effect in Plasmodium berghei-infected mice [23, 24].
Hypothetically, iron chelators could deprive the intraerythrocytic iron essential for parasite growth or act directly on the plasmodial functional iron. Here, the inhibitory iron-chelating effects of CM1 and GTE were investigated on growth of cultured P. falciparum. Combined treatments of the compounds with standard anti-malarial drug were also examined.
Methods
Chemicals
Anti-malarial drugs, pyrimethamine (PYR, MW = 249) and dihydroartemisinin (DHA, MW = 284) were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). DFO (desferrioxamine mesylate or Desferal®, MW = 657) was from the Novartis Pharma, Basel, Switzerland, Chiang Mai. DFP (GPO-L-One® or L1, MW = 139) and DFX (Exjade®, MW = 373) were kindly supplied by the Institute of Research and Development, Government Pharmaceutical Organization, Bangkok, Thailand. SYBR Green I nucleic acid gel-stain (10,000× concentrate in DMSO, Cat. No. S9430) was purchased from Invitrogen, Molecular Probes (Eugene, OR, USA). Calcein acetoxymethyl (CA-AM) was purchased from Invitrogen® Corporation (CA, USA). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) and epigallocatechin 3-gallate (EGCG, MW = 458) were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). SYTO® 61 red fluorescent nucleic acid dye (Cat. No. S11343) was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA).
Test compounds
1-(N-Acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1)
The chelator was synthesized from maltol by Dr. Kanjana Pangjit at Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Protocol for CM1 production gave 20 % yield and 98 % purity. CM1’s partition coefficient constant (Kpart), equilibrium constant (pKa) and selectivity constant for iron(III) (pFe3+) values are 0.56, 3.52 ± 0.003 and 9.80 ± 0.002, and 20.3, respectively [19].
Green tea extract (GTE)
GTE was locally prepared from fresh tea (Camellia sinensis) shoots, which had been harvested from tea field in Fang District, Chiang Mai, Thailand by using the microwave method as previously described by Srichairatanakool et al. [25]. Results of HPLC analysis showed that GTE was comprised of many phytochemicals; particularly catechine derivatives of which EGCG was most abundant (24 %, w/w).
Drug solutions
Stock solutions (10 mM) of PYR, DHA, DFO, DFP, DFX, and CM1 were prepared in the suitable solvent, while stock GTE solution (5 %, w/v) was freshly prepared in hot water (80 ℃, 10 min). All the solutions were sterilized by passing through filter membranes (Millipore 0.45 μm, hydrophilic cellulose type). Working solutions were serially diluted in the complete medium to achieve the final concentrations ranging 0–10−5 M.
Plasmodium falciparum culture
Plasmodium falciparum parasites were routinely cultured by using the Trager and Jensen method [26] with minor modifications. P. falciparum (strain TM4/8.2) is routinely maintained in freshly washed non-infected RBC (blood group O, Rh− or Rh+) at 4 % haematocrit (Hct) in 10 ml of RPMI1640 medium supplemented with 25 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), 25 mM NaHCO3, 0.2 % (w/v) d-glucose, 40 mg/ml gentamicin, 50 µg/ml hypoxanthine and 10 % heat inactivated pooled normal human serum in a medium Petri dish. The culture medium comprising P. falciparum-infected RBC pellet (0.2 ml), non-infected RBC pellet (0.6 ml) and the supplemented RPMI1640 (9.2 ml) was incubated in 37 °C incubator with 5 % CO2 for 48 h (one cycle of P. falciparum). Percentage parasitaemia was monitored on Giemsa-stained thin blood smear every 2–3 days and maintained maximally 10–15 %.
Sorbitol synchronization of Plasmodium falciparum-infected RBC
Plasmodiumfalciparum parasites are mostly synchronized at ring stage (but not later than 10–12 h) when the sorbitol treatment is complete [27]. RBC suspension was spun down at 1800g, room temperature for 3 min and the supernatant was discarded. Cell pellet was resuspended in 5 % (w/v) sterile sorbitol solution (15.0 ml) and incubated at 37 ℃ for 10–15 min with shaking. After centrifugation, cell pellet containing only intraeythrocytic late ring- and early trophozoite-stage parasites was resuspended in new culture medium (10.0 ml) to obtain 4 %-Hct RBC suspension and used in experiments.
Drug testing
Single drug treatment
Working drug solutions (100 µl) were dispensed in triplicate test wells and the RBC suspension (90 µl) was transferred to each well of culture plate. The plate was incubated under 5 % CO2 atmospheric condition for 48 h [28]. Afterwards, freshly prepared SYBR Green I solution (100 µl) was added to each well, mixed until complete haemolysis and incubated in the dark at room temperature for 1 h. Finally, fluorescent intensity (FI) (wavelength 530/575 nm) was measured by using a flow cytometer (BD Canto II) [29]. Half maximal inhibitory concentration (IC50) value of each drug was determined from the dose response curve representing used drug concentrations (log scale) on x axis and % parasitaemia (linear scale) on y axis. IC50 value represents the concentration of a compound where 50 % of its maximal inhibitory effect is observed and it is commonly used as a measure of drug’s potency.
Combined PYR treatment with CM1 and GTE
Various concentrations of CM1 (0–200 μM) or GTE (0–200 μM EGCG equivalent) including 30 nM PYR were dispensed into triplicate wells. Then, the RBC suspension (90 µl) was transferred to the wells and incubated under the standard condition for 48 h. Afterwards, the SYBR Green I solution (100 µl) was added and incubated for 1 h. Finally, FI was measured as described above.
Measurement of LIP in Plasmodium falciparum-infected RBC
Originally, Breuer and colleagues had developed a method that used calcein, which was derived from the esterase hydrolysis of a non-fluorescent calcein acetoxymethyl ester (CA-AM), as a fluorescent probe to rapidly measure intracellular divalent metals such as Fe(II) and Cu(II) with a flow cytometer [30]. Calcein green fluorescence is quenched by the stoichiometric binding of iron (1:1), leading to a decrease in the FI signal. In this study, the modified method that utilized the calcein probe to assess the LIP of the parasitized RBC and the red fluorescent SYTO 61 dye, were used simultaneously. In the assay, parasitized RBC (5–10 % parasitaemia) were washed twice with phosphate buffered saline (PBS) containing 0.5 % Albumax II (PBS+) solution and inoculated into each well (2 × 106 cells/well). The cells were treated with or without the test compounds (100 μM final concentrations) and incubated as described above. After the incubation period, the cells were labelled with 0.125 μM CA-AM solution for 15 min in the dark to detect the remaining intracellular iron. Then, they were labelled with SYTO 61 red fluorescent dye (5 mM in DMSO) to stain the nucleic acids of the malaria parasites, and were allowed to rest for 15 min. Finally, the FI signals (wavelength 485/530 nm for calcein; wavelength 625/645 nm for SYTO 61) of the cells were analysed through the use of a Cytek-modified FACS-Calibur flow cytometer [31].
Detection of ROS in Plasmodium falciparum-infected RBC
The treated cells were labelled with non-fluorescent DCFH-DA solution (10 μM previously dissolved in methanol) for 30 min, after which the DCFH-DA would be hydrolyzed by plasma membrane esterase into reduced dichlorofluorescein (DCFH). Then, the cells were challenged with the H2O2 solution (125 μM) to convert the forming DCFH to oxidized dichlorofluorescein (DCF) and were then counterstained with the SYTO 61 fluorescent dye as described above. Finally, the FI signals (wavelength 485/530 nm for DCF; wavelength 625/645 nm for SYTO 61) of the cells were analysed through the use of a Cytek-modified FACS-Calibur flow cytometer [32].
Statistical analysis
Data are presented as mean ± SD or/and mean ± SEM, and analysed using SPSS program (IBM SPSS Statistics 20). Statistical significance was determined by using one-way analysis of variance (ANOVA). P value < 0.05 is considered significant difference.
Results
Inhibitory effect of GTE and CM1 on Plasmodium falciparum growth
As shown in Fig. 1, DFO, GTE, CM1, DFX and DFP had different efficiencies in inhibition of P. falciparum growth and development as shown as % parasitaemia. Their IC50 values are 10.09, 21.11, 35.14, 44.71 and 58.25 μM, respectively. In comparison, DHA and PYR (IC50 = 1.930 and 37.89 nM, respectively) were much more potent than these compounds. In combined treatment with 30 nM PYR, CM1 (50–200 μM) significantly enhanced the anti-malarial activity of the PYR per se (mean difference of parasite growth = 24.4, 24.4 and 24.6 %, respectively). Incredibly, GTE (50–200 μM EGCG equivalent) significantly enhanced the PYR activity min a concentration-dependent manner (mean differences of parasite growth = 44.2, 44.9 and 47.7 %, respectively) (Fig. 2). Remarkably, the PYR-GTE synergy seemed to be greater than the PYR-CM1.
Fig. 1.
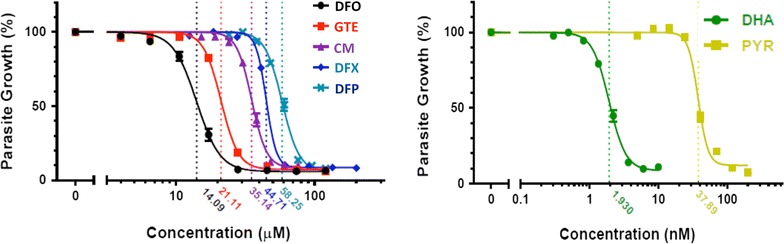
Sensitivity of P. falciparum to anti-malarial drugs, iron chelators and tested compounds. Data are obtained from three independent triplicate experiments and expressed as mean ± SD. Their IC50 values are shown in dotted lines
Fig. 2.
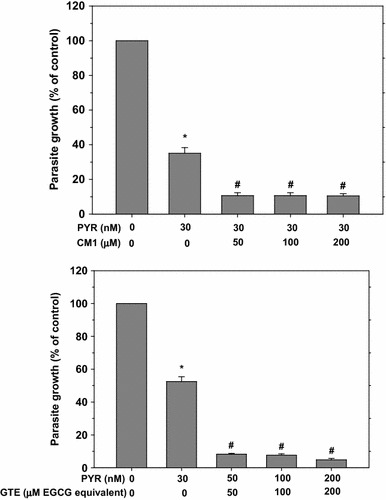
Combined effect of PYR with GTE or CM1 on growth of P. falciparum. Data are obtained from three independent triplicate experiments and expressed as mean ± SD. *p < 0.05 when compared with non-treatment; # p < 0.05 when compared with 30 nM PYR treatment
Efficacy of GTE and CM1 in reduction of LIP in parasitized red blood cells (PRBC)
Similarly to the DFP treatment, CM1 (25–200 μM) significantly removed intracellular non-haem iron in parasitized RBC (PRBC) in a concentration-dependent manner. GTE treatments (25–100 μM EGCG equivalent doses) were also effective in removing LIP in the PRBC; nonetheless, GTE at 200 μM EGCG equivalent was not able to decrease the LIP levels any further (Fig. 3). At equivalent concentrations, CM1 and GTE would probably be more effective in reducing the LIP level in the P. falciparum-infected RBC than DFP.
Fig. 3.
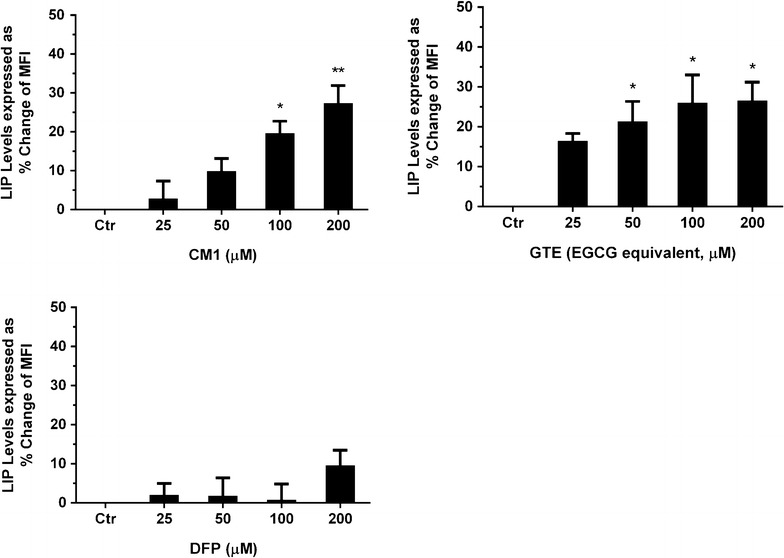
Levels of intracellular LIP in P. falciparum-infected RBC treated with CM1, GTE and DFP. Data are obtained from three independent triplicate experiments and expressed as mean ± SD. **p < 0.01; ***p < 0.001; ****p < 0.0001 when compared with the control
Free-radical scavenging activity of GTE and CM1 in PRBC
DFP and CM1 at any doses did not affect level of ROS in the PRBC. Surprisingly, GTE treatment was able to decrease the ROS level significantly in the PRBC in a concentration-dependent manner (Fig. 4). Possibly, GTE is anti-oxidative natural product that can scavenge persisting ROS and free radicals while DFP and CM1 are not.
Fig. 4.
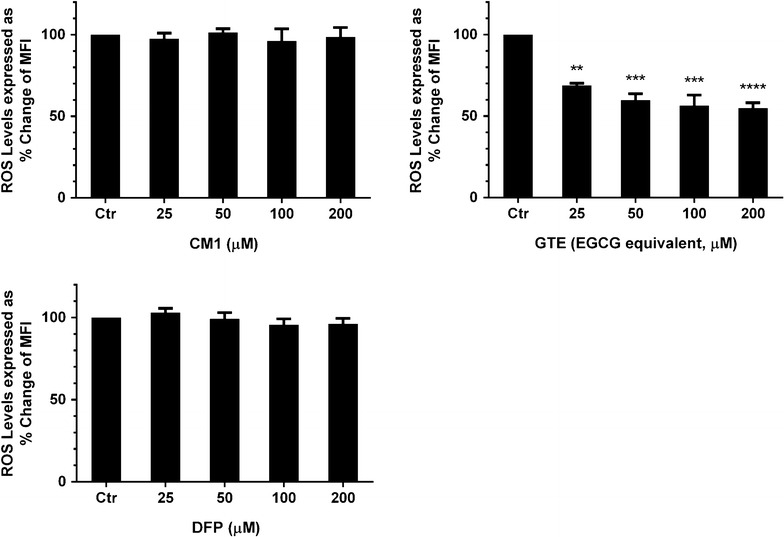
Levels of ROS in P. falciparum-infected RBC treated with CM1, GTE and DFP. Data are obtained from three independent triplicate experiments and expressed as mean ± SD. **p < 0.01; ***p < 0.001; ****p < 0.0001 when compared with the control
Discussion
Iron chelators can deplete cytosolic iron and also interact directly with functional iron in iron-containing enzymes, leading to iron deprivation of intracellular protozoa [7, 9]. In this study, GTE and CM1 showed iron-chelating property by inhibiting growth and development of P. falciparum at micromolar level while the reference anti-malarial drugs PYR and DHA are effective at nanomolar level. Their anti-malarial ability seems to be inversely related with their molecular size in potency of DFO > EGCG > CM1 ≃ DFX > DFP. DFO is a fungal hexadentate iron chelator used clinically for treatment of iron overload in β-thalassaemia patients, and EGCG is a natural hexadentate iron chelator used potentially for treatment of iron accumulation in Parkinson′s disease [33, 34]. Among these chelators, CM1 is the most lipophilic and more efficient than DFP in removing intracellular iron [19], possibly the compound is a more powerful anti-malarial agent. Interestingly, N-methylanthranilic desferrioxamine, which is a more lipophilic DFO derivative (termed reversed siderophore) can permeabilize PRBC by bypassing plasma membrane to bind intracellular iron, leading to inhibition of cultured P. falciparum growth (IC50 values = 30 ± 8 and 3 ± 1 μM, respectively) [35–37]. While losing their membrane selectivity, PRBC allow ions (e.g. Na+, K+, Zn2+, Fe2+ and Ca2+), polar molecules (e.g. amino acids, glucose, purine nucleosides) and even anti-malarial drugs (e.g. mefloquine, chloroquine) to pass into the cells readily [38]. By this way, influx of DFO and green tea EGCG through parasite-encoded transporters or aqueous leaks and/or pores would have occurred as well. The selectivity can be based either on the selective permeation of the chelators into the parasitized cells or on a higher susceptibility of the latter to iron deprivation or antioxidants. CM1 and DFP are orally active bidentate chelators which possess similar physicochemical and biological properties, including log b value for Fe(III) (37.0 and 37.4, respectively), pFe(III) (20.3 and 20.5, respectively), and uncharged free as well as Fe(III)-bound ligands. Extraordinarily, CM1 is more lipophilic (Kpart = 0.53 and 0.11, respectively) and more efficient in chelating hepatic ferritin iron (approximately 18 % and 7 %, respectively) than DFO. The CM1’s log b values at pH 7.4 for Fe3+, Cu2+ and Zn2+ are 20.3, 18.8 and 12.9, respectively; whereas, the selectivity (pM) values for these three metal ions are 20.3, 9.8 and 6.2, respectively, suggesting higher levels in terms of the affinity and selectivity of CM1 for iron ions than other metal ions [19]. The chelator will chelate the cell irons specifically, leading to iron deprivation of the growing malaria parasites. Since malaria-infected individuals tend to be anemic or hypoeferremic, further reduction of iron stores by a non-specific chelator might be deleterious. Moreover, chelators introduced into cultures continuously for days do not quite represent the in vivo situation, as each agent has its own pharmacokinetic profile and parasite susceptibility to the chelators, as they are highly stage dependent. However, IC50 values of cell iron scavenging based on single time point measurement is not necessarily indicative of the chelator’s potency since the latter also depends on the permeation kinetics and pharmacokinetics. Scholl and coworkers support the evidence that malaria parasites utilize labile bioavailable iron pool(s) in red cell cytoplasm rather than haemozoin iron in the food vacuoles in order to synthesize their own haem in the mitochondria and apicomplast, while iron chelators can compete the iron utilization process and kill the parasite based on iron deprivation [6].
GTE contains polyphenols including tannic acid, EGCG, epicatechin, epigallocatechin, gallocatechin-3-gallate, and epicatechin-3-gallate. The compound can chelate irons and inhibit the Fenton reaction-mediated generation of ROS [22, 25]. GTE is indeed a natural iron chelator. Hellmann and colleagues showed that green tea EGCG inhibited P. berghei sporozoite motility and liver cell infection efficiently and also synergized with digitonin for these two activities [39]. ROS and oxidant drugs are toxic to biomolecules of cell components and can cause oxidative cell damage. The oxidants such as t-butyl hydroperoxide and artemisinin-catalyzed epoxide can damage malaria parasites and PRBC [40, 41]. Potent antioxidant GTE significantly decreased plasma levels of blood urea nitrogen and creatinine significantly in P. berghei ANKA-infected mice and consequently reversed their kidney function caused by oxidative stress condition [23, 42]. Controversially, EGCG and M30 (an iron chelator) did not improve survival of P. berghei ANKA-infected mice [43]. Similarly, hot water extract of the tea (Artemisia annua) containing many polyphenolic acids such as mono-caffeoylquinic acids, tri-caffeoylquinic acid, artemisinic acid, arteannuin B and rosmarinic acid exhibited synergistic anti-plasmodial activity with artemisinin in the CQ- sensitive strain of P. falciparum [44].
Taken together, the authors are confident CM1 and EGCG in green tea are plasmodicidal agent and function as iron-chelating agents rather than antioxidants to limit the iron supply to the malaria parasite for its growth and development. The possible iron sources are transferrin-bound iron (TBI) and non-transferrin bound iron (NTBI) in the plasma compartment [45–48], labile/transient iron [31] and ferritin iron [49] in red cell cytoplasm.
Conclusions
GTE and CM1 could inhibit growth and development of P. falciparum culture, possibly by depriving the iron nutrient, which is essential for the fast-dividing malaria parasites. Predominantly, EGCG-enriched green tea would be beneficial for treatment of malaria-infected patients and amelioration of their haemolytic oxidative stress. Suggestively, green tea and CM1 may be used as therapeutic/preventive agents per se or in adjunction with PYR to increase efficacy of and eliminate resistance to anti-malarial drug. Prospectively, the effect of green tea on antioxidant defense mechanism and inflammation crisis of the patients needs to be further investigated.
Authors’ contributions
SS, CU and PT designed the study and experiments. PT performed the experiments and data analysis. SS and CU wrote the manuscript. SS, CU, SK and WT helped conceive the study and reviewed drafts of the manuscript. SS, CU, SK, PT and WT read and approve the final manuscript. SS revised the manuscript and corresponded with the editor and reviewers.
Acknowledgements
The work was supported by Thailand Graduate Institute of Science and Technology (TGIST), National Science and Technology Development Agency (NSTDA), and Faculty of Medicine, Chiang Mai University, Chiang Mai Thailand. We also thank Professor Robert C. Hider, Ph.D., Institute of Pharmaceutical Science, King’s College London, UK, for giving useful scientific comments and for English proof-reading.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- ANOVA
one-way analysis of variance
- CA-AM
calcein acetoxymethyl
- CQ
chloroquine
- DCF
oxidized dichlorofluorescein
- DCFH
reduced dichlorofluorescein
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DFO
desferrioxamine
- DFP
deferiprone
- DFX
deferasirox
- DHA
dihydroartemisinin
- 2,3-DHB
2,3-dihydroxybenzoic acid
- DHFR
dihydrofolate reductase
- DHPS
dihydropteroate synthase
- EGCG
epigallocatechin 3-gallate
- EHPG
N,N′-ethylenebis(o-hydroxyphenylglycine)
- FI
fluorescent intensity
- GTE
green tea extract
- HBED
N,N′-bis (o-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid
- IC50
half maximal inhibitory concentration
- Kpart
partition coefficient
- LIP
labile iron pool
- LPI
labile plasma iron
- NTBI
non-transferrin bound iron
- PBS
phosphate buffered saline
- pKa
equilibrium constant
- PRBC
parasitized red blood cells
- PYR
pyrimethamine
- RBC
red blood cells
- ROS
reactive oxygen species
- S
sulfadoxine
Contributor Information
Phitsinee Thipubon, Phone: +66 53945322, Email: pedassy@hotmail.com.
Chairat Uthaipibull, Email: chairat@biotec.or.th.
Sumalee Kamchonwongpaisan, Email: sumaleek@biotec.or.th.
Wachiraporn Tipsuwan, Email: tipsuwanw@hotmail.com.
Somdet Srichairatanakool, Phone: +66 53945322, Email: ssrichai@med.cmu.ac.th.
References
- 1.Noranate N, Durand R, Tall A, Marrama L, Spiegel A, Sokhna C, et al. Rapid dissemination of Plasmodium falciparum drug resistance despite strictly controlled antimalarial use. PLoS One. 2007;2:e139. doi: 10.1371/journal.pone.0000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jovel IT, Mejia RE, Banegas E, Piedade R, Alger J, Fontecha G, et al. Drug resistance associated genetic polymorphisms in Plasmodium falciparum and Plasmodium vivax collected in Honduras, Central America. Malar J. 2011;10:376. doi: 10.1186/1475-2875-10-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks F, Evans J, Meyer CG, Browne EN, Flessner C, von Kalckreuth V, et al. High prevalence of markers for sulfadoxine and pyrimethamine resistance in Plasmodium falciparum in the absence of drug pressure in the Ashanti region of Ghana. Antimicrob Agents Chemother. 2005;49:1101–1105. doi: 10.1128/AAC.49.3.1101-1105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heppner DG, Hallaway PE, Kontoghiorghes GJ, Eaton JW. Antimalarial properties of orally active iron chelators. Blood. 1988;72:358–361. [PubMed] [Google Scholar]
- 5.Sibmooh N, Udomsangpetch R, Kujoa A, Chantharaksri U, Mankhetkorn S. Redox reaction of artemisinin with ferrous and ferric ions in aqueous buffer. Chem Pharm Bull (Tokyo) 2001;49:1541–1546. doi: 10.1248/cpb.49.1541. [DOI] [PubMed] [Google Scholar]
- 6.Scholl PF, Tripathi AK, Sullivan DJ. Bioavailable iron and heme metabolism in Plasmodium falciparum. Curr Top Microbiol Immunol. 2005;295:293–324. doi: 10.1007/3-540-29088-5_12. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch G, Treumer J, Spira DT, Jung A. Plasmodium vinckei: suppression of mouse infections with desferrioxamine B. Exp Parasitol. 1985;60:171–174. doi: 10.1016/0014-4894(85)90020-7. [DOI] [PubMed] [Google Scholar]
- 8.Raventos-Suarez C, Pollack S, Nagel RL. Plasmodium falciparum: inhibition of in vitro growth by desferrioxamine. Am J Trop Med Hyg. 1982;31:919–922. doi: 10.4269/ajtmh.1982.31.919. [DOI] [PubMed] [Google Scholar]
- 9.Singh C, Arif AJ, Mathur PD, Chandra S, Sen AB. Effect of a specific iron chelator, desferrioxamine on the host biochemistry and parasitaemia in mice infected with Plasmodium berghei. Indian J Malariol. 1985;22:35–44. [PubMed] [Google Scholar]
- 10.Scheibel LW, Stanton GG. Antimalarial activity of selected aromatic chelators. IV. Cation uptake by Plasmodium falciparum in the presence of oxines and siderochromes. Mol Pharmacol. 1986;30:364–369. [PubMed] [Google Scholar]
- 11.Pollack S, Rossan RN, Davidson DE, Escajadillo A. Desferrioxamine suppresses Plasmodium falciparum in Aotus monkeys. Proc Soc Exp Biol Med. 1987;184:162–164. doi: 10.3181/00379727-184-42461. [DOI] [PubMed] [Google Scholar]
- 12.Goudeau C, Loyevsky M, Kassim OO, Gordeuk VR, Nick H. Assessment of antimalarial effect of ICL670A on in vitro cultures of Plasmodium falciparum. Br J Haematol. 2001;115:918–923. doi: 10.1046/j.1365-2141.2001.03159.x. [DOI] [PubMed] [Google Scholar]
- 13.Thuma PE, Olivieri NF, Mabeza GF, Biemba G, Parry D, Zulu S, et al. Assessment of the effect of the oral iron chelator deferiprone on asymptomatic Plasmodium falciparum parasitemia in humans. Am J Trop Med Hyg. 1998;58:358–364. doi: 10.4269/ajtmh.1998.58.358. [DOI] [PubMed] [Google Scholar]
- 14.Iheanacho EN, Samuni A, Avramovici-Grisaru S, Sarel S, Spira DT. Inhibition of Plasmodium falciparum growth by a synthetic iron chelator. Trans R Soc Trop Med Hyg. 1990;84:213–216. doi: 10.1016/0035-9203(90)90259-H. [DOI] [PubMed] [Google Scholar]
- 15.Hershko C, Theanacho EN, Spira DT, Peter HH, Dobbin P, Hider RC. The effect of N-alkyl modification on the antimalarial activity of 3-hydroxypyridin-4-one oral iron chelators. Blood. 1991;77:637–643. [PubMed] [Google Scholar]
- 16.Pattanapanyasat K, Thaithong S, Kyle DE, Udomsangpetch R, Yongvanitchit K, Hider RC, et al. Flow cytometric assessment of hydroxypyridinone iron chelators on in vitro growth of drug-resistant malaria. Cytometry. 1997;27:84–91. doi: 10.1002/(SICI)1097-0320(19970101)27:1<84::AID-CYTO11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Mabeza GF, Biemba G, Gordeuk VR. Clinical studies of iron chelators in malaria. Acta Haematol. 1996;95:78–86. doi: 10.1159/000203953. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty D, Ghosh K, Pathare AV, Karnad D. Deferiprone (L1) as an adjuvant therapy for Plasmodium falciparum malaria. Indian J Med Res. 2002;115:17–21. [PubMed] [Google Scholar]
- 19.Pangjit K, Banjerdpongchai R, Phisalaphong C, Fucharoen S, Xie YY, Lu ZD, et al. Characterisation of a novel oral iron chelator: 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one. J Pharm Pharmacol. 2015;67:703–713. doi: 10.1111/jphp.12373. [DOI] [PubMed] [Google Scholar]
- 20.Kulprachakarn K, Chansiw N, Pangjit K, Phisalaphong C, Fucharoen S, Hider RC, et al. Iron-chelating and anti-lipid peroxidation properties of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in long-term iron loading beta-thalassemic mice. Asian Pac J Trop Biomed. 2014;4:663–668. doi: 10.12980/APJTB.4.2014APJTB-2014-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chansiw N, Pangjit K, Phisalaphong C, Porter JB, Evans P, Fucharoen S, et al. Effect of a novel oral active iron chelator: 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in iron-overloaded and non-overloaded mice. Asian Pac J Trop Med. 2014;7S1:S155–S161. doi: 10.1016/S1995-7645(14)60223-6. [DOI] [PubMed] [Google Scholar]
- 22.Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/S0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 23.Somsak V, Jaihan U, Srichairatanakool S, Uthaipibull C. Protection of renal function by green tea extract during Plasmodium berghei infection. Parasitol Int. 2013;62:548–551. doi: 10.1016/j.parint.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Ounjaijean S, Thephinlap C, Khansuwan U, Phisalapong C, Fucharoen S, Porter JB, et al. Effect of green tea on iron status and oxidative stress in iron-loaded rats. Med Chem. 2008;4:365–370. doi: 10.2174/157340608784872316. [DOI] [PubMed] [Google Scholar]
- 25.Srichairatanakool S, Ounjaijean S, Thephinlap C, Khansuwan U, Phisalpong C, Fucharoen S. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin. 2006;30:311–327. doi: 10.1080/03630260600642666. [DOI] [PubMed] [Google Scholar]
- 26.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 27.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 28.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somsak V, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S, Uthaipibull C. Flow cytometric enumeration of Plasmodium berghei-infected red blood cells stained with SYBR Green I. Acta Trop. 2012;122:113–118. doi: 10.1016/j.actatropica.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am J Physiol. 1995;268(6 Pt 1):C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- 31.Clark M, Fisher NC, Kasthuri R, Cerami Hand C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br J Haematol. 2013;161:262–269. doi: 10.1111/bjh.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Tilley L, Kenny S, Klonis N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytometry A. 2010;77:253–263. doi: 10.1002/cyto.a.20856. [DOI] [PubMed] [Google Scholar]
- 33.Mounsey RB, Teismann P. Chelators in the treatment of iron accumulation in Parkinson′s disease. Int J Cell Biol. 2012;2012:983245. doi: 10.1155/2012/983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter JB, Rafique R, Srichairatanakool S, Davis BA, Shah FT, Hair T, et al. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: implications for clinical use. Ann N Y Acad Sci. 2005;1054:155–168. doi: 10.1196/annals.1345.018. [DOI] [PubMed] [Google Scholar]
- 35.Loyevsky M, Lytton SD, Mester B, Libman J, Shanzer A, Cabantchik ZI. The antimalarial action of desferal involves a direct access route to erythrocytic (Plasmodium falciparum) parasites. J Clin Invest. 1993;91:218–224. doi: 10.1172/JCI116174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lytton SD, Mester B, Dayan I, Glickstein H, Libman J, Shanzer A, et al. Mode of action of iron (III) chelators as antimalarials: I. Membrane permeation properties and cytotoxic activity. Blood. 1993;81:214–221. [PubMed] [Google Scholar]
- 37.Lytton SD, Cabantchik ZI, Libman J, Shanzer A. Reversed siderophores as antimalarial agents. II. Selective scavenging of Fe(III) from parasitized erythrocytes by a fluorescent derivative of desferal. Mol Pharmacol. 1991;40:584–590. [PubMed] [Google Scholar]
- 38.Sherman IW. The Wellcome Trust lecture. Mechanisms of molecular trafficking in malaria. Parasitology. 1988;96(Suppl):S57–S81. doi: 10.1017/S003118200008598X. [DOI] [PubMed] [Google Scholar]
- 39.Hellmann JK, Munter S, Wink M, Frischknecht F. Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility. PLoS One. 2010;5:e8682. doi: 10.1371/journal.pone.0008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark IA, Hunt NH, Cowden WB, Maxwell LE, Mackie EJ. Radical-mediated damage to parasites and erythrocytes in Plasmodium vinckei infected mice after injection of t-butyl hydroperoxide. Clin Exp Immunol. 1984;56:524–530. [PMC free article] [PubMed] [Google Scholar]
- 41.Clark IA, Cowden WB, Hunt NH, Maxwell LE, Mackie EJ. Activity of divicine in Plasmodium vinckei-infected mice has implications for treatment of favism and epidemiology of G-6-PD deficiency. Br J Haematol. 1984;57:479–487. doi: 10.1111/j.1365-2141.1984.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 42.Audomkasok S, Singpha W, Chachiyo S, Somsak V. Antihemolytic activities of green tea, safflower, and mulberry extracts during Plasmodium berghei infection in mice. J Pathog. 2014;2014:203154. doi: 10.1155/2014/203154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francischetti IM, Gordon E, Bizzarro B, Gera N, Andrade BB, Oliveira F, et al. Tempol, an intracellular antioxidant, inhibits tissue factor expression, attenuates dendritic cell function, and is partially protective in a murine model of cerebral malaria. PLoS One. 2014;9:e87140. doi: 10.1371/journal.pone.0087140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—possible synergistic and resistance mechanisms. PLoS One. 2013;8:e80790. doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aremu CY. Changes in serum transferrin and iron concentrations in humans suffering from malaria with parasitaemia. Ann Trop Med Parasitol. 1989;83:517–520. doi: 10.1080/00034983.1989.11812380. [DOI] [PubMed] [Google Scholar]
- 46.Hurrell RF. Safety and efficacy of iron supplements in malaria-endemic areas. Ann Nutr Metab. 2011;59:64–66. doi: 10.1159/000332140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peto TE, Thompson JL. A reappraisal of the effects of iron and desferrioxamine on the growth of Plasmodium falciparum ‘in vitro’: the unimportance of serum iron. Br J Haematol. 1986;63:273–280. doi: 10.1111/j.1365-2141.1986.tb05550.x. [DOI] [PubMed] [Google Scholar]
- 48.Pollack S, Fleming J. Plasmodium falciparum takes up iron from transferrin. Br J Haematol. 1984;58:289–293. doi: 10.1111/j.1365-2141.1984.tb06087.x. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs P, Wood L, Bird AR. Erythrocytes: better tolerance of iron polymaltose complex compared with ferrous sulphate in the treatment of anaemia. Hematology. 2000;5:77–83. doi: 10.1080/10245332.2000.11746490. [DOI] [PubMed] [Google Scholar]


