Abstract
Epidemiological studies and anecdotal evidence show overlap between psychiatric disorders and creativity, but why? A new study shows that genetics are part of the explanation.
Thinkers of the human condition have long associated creativity with psychiatric illness—the ‘mad genius’ archetype. According to Aristotle, “no great genius was without a mixture of insanity.” And there are the oft-repeated anecdotes: the psychotic breaks of Vincent van Gogh and John Nash, the manic and depressive episodes of Virginia Woolf and Ernest Hemingway. There is in fact some empirical evidence that the psychological factors underlying psychiatric disorders are linked to increased creativity. Unaffected relatives of those with bipolar disorder (BD) have greater creativity1 and are over-represented in creative professions2, and similar findings have been reported for schizophrenia (SCZ)2,3.
What these studies have not shown is whether this overlap is due to genetic variation that influences both creativity and BD/SCZ or whether some environmental factor explains the association. For example, highly unstructured rearing environments might contribute to both creativity and risk of the disorders. Understanding whether shared gene variants are responsible for the overlap is important. It can help elucidate the biological underpinnings of these disorders and shine light on the puzzle of why psychiatric diseases persist in the population.
Power et al.4 in work reported in this issue of Nature Neuroscience, asked whether creativity and psychiatric disorders might be associated through common variation in the genome. They used a large discovery sample of 86,000 adults from Iceland and four replication samples totaling 27,000 adults from Sweden and the Netherlands. All had genome-wide SNP genotyping and their professions were known. None of them knowingly suffered from a psychiatric illness. About 1% of them were artists, including actors, dancers, musicians and writers. The authors piggy-backed on recent large genome-wide association studies (GWAS) conducted on SCZ and BD patients and controls, and used the estimated effect on risk of SCZ and BD from thousands of SNPs variants that were associated with either SCZ or BD. They then used the observed genotypes in the healthy people from Iceland, Sweden and the Netherlands and predicted a genetic risk score—the sum of associated risk alleles weighted by their estimated effect sizes. Power et al. found that people at higher genetic risk for SCZ or BD had a higher probability of being employed as an artist or belonging to an artists’ union.
The associations between the genetic risk scores and creativity were highly statistically significant, and the data from the GWAS are completely separate from the samples used here. The associations also showed specificity: the genetic risk scores did not predict any other occupations investigated. This shows how powerful genetic risk scores are for detecting associations such as those reported, in particular in combination with a large sample size. These kinds of population analysis can be performed without knowing anything about the biology underlying the SNP variants that are associated with disease.
The available BD genetic risk score is a less powerful predictor of risk of the corresponding psychiatric disorder, let alone other traits (such as creativity), than the SCZ genetic risk score, and the authors confirm this in the Icelandic data. This is because the GWAS used to select the SNPs for inclusion in the genetic risk score of BD was much smaller (N=16,731) than that for SCZ (N=150,064), and therefore the signal-to-noise ratio in the BD genetic risk score was lower, reducing its apparent strength of association with creativity. Therefore, the observed association between creativity and BD should become stronger once a better genetic risk score for BD becomes available (Figure 1). Hence the biological relationship between psychiatric disorders and creativity is probably even larger for BP than for SCZ.
Figure 1. Estimated probability of being employed in a creative job as a function of genetic risk scores.
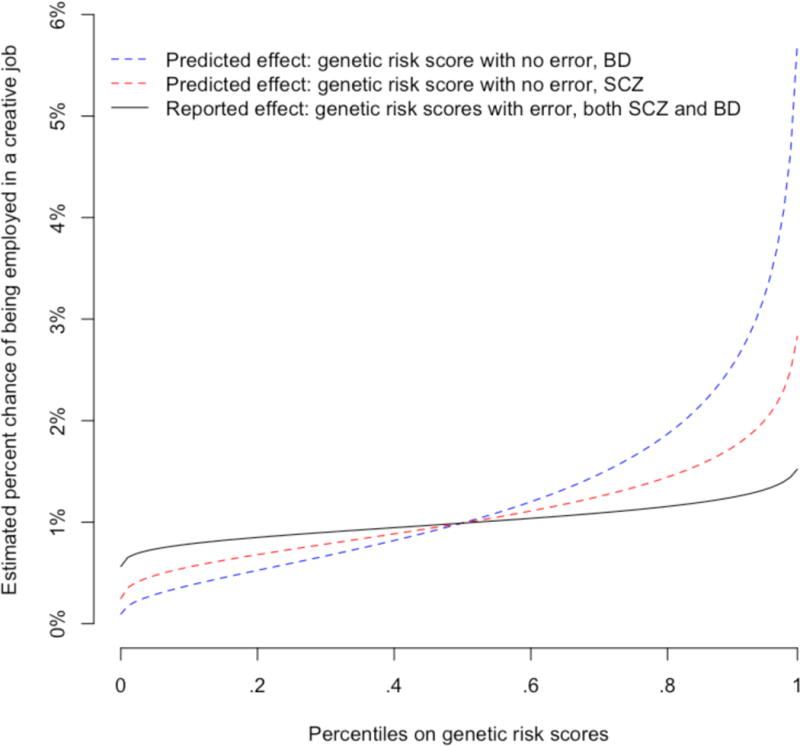
The reported effects4 of the BD and SCZ genetic risk scores on being in a creative job were about the same. These effects have been converted to probabilities as a function of percentiles on either genetic risk score (black line). However, there is more noise in the BD genetic risk score, because of estimation error (see text). The blue (BD) and red (SCZ) lines show what these probabilities are predicted to be if genetic risk scores could be measured without estimation error, which will be approached as GWAS sample sizes grow.
This study is yet another demonstration of the importance of using genome-wide surveys on large numbers of people to answer old questions with new data5–7. The study confirms the polygenicity of human traits (including liability to psychiatric disorders and creative profession) and confirms widespread pleiotropy, in which the same genetic variants influence more than one trait, in the human genome. There are implications of polygenicity and pleiotropy well beyond this study. For example, it implies that genetic models of common human diseases in experimental animals (for example, a single induced mutation in an inbred mouse) are unlikely to fully reflect human biology.
Yet, as with any study, there are important caveats. Creativity is a slippery concept. There is no agreed-upon method for measuring it, and no single metric is likely to capture it fully. In this study, it was not necessarily creativity that was measured, but rather being employed in occupations thought to require creativity. It is possible that having greater genetic risk of these psychiatric disorders leads to other personality traits or tastes that predispose one to certain types of occupations—for example, perhaps ones that require less structure—rather than to creativity per se. In other words, individuals with a higher load of risk alleles may be drawn to these occupations without necessarily possessing greater creativity.
It is also important to recognize that the results from Power et al. apply only to effects of the (mostly common) causal genetic variants that are tagged by SNPs. The effects of rare risk alleles—those carried by less than ~1% of the population—are not well characterized in GWAS and are not represented in the genetic risk scores used by Power et al. It is an open question whether rarer, more penetrant risk alleles also are associated with greater creativity or whether such loci are simply disruptive to all aspects of cognition.
Given that BD and SCZ are associated with moderate to severe social impairment and, at least in modern environments, lower fertility8, it is natural to wonder why natural selection has not eliminated the alleles that predispose to them. One commonly invoked solution to this puzzle is termed antagonistic pleiotropy, which occurs when alleles increase the evolutionary fitness payoff of one trait while simultaneously reducing it for another. Evolutionary genetic theory suggests that fairly restrictive conditions must be met for antagonistic pleiotropy to maintain genetic variation at equilibrium9,10. Nevertheless, it is perfectly plausible that at least some risk alleles’ negative fitness consequences (from increased risk to psychiatric disorders) are partially offset by positive fitness consequences on other traits (for example, from increased creativity). This could cause the net fitness effects of these risk alleles to be closer to neutral, allowing them to “drift” to higher frequencies than would be predicted based solely on their effects on disorders. Therefore, the Power et al. findings may suggest that some of the common genetic variants that increase risk to SCZ or BD have not gone extinct because, averaged across their positive and negative effects, their net effect on fitness is close to zero. Note that this is different than saying that SCZ or BD risk alleles have been selected for, and it is certainly different than saying that the disorders themselves have been positively selected for.
Power et al. discuss the possibility of antagonistic pleiotropy but discount it on the basis of a separate finding in their data that creativity is associated with fewer, not more, offspring in modern Iceland. However, when it comes to understanding the frequencies of alleles today, it is the effect those alleles had on ancestral, not modern, fertility that matters. It is possible that lower fertility among highly creative people in modern Iceland means that creativity was not ancestrally linked to higher fitness, but given modern birth control and changes in mating culture, this is far from certain.
Basic questions remain, of course. For example, what specific aspects of being employed in a creative occupation are most linked to the genetic liability to SCZ and BD? And do the risk alleles that affect other psychiatric disorders similarly affect creativity or other ‘positive’ character traits? For example, among unaffected carriers, are autism risk alleles associated with increased intelligence, obsessive-compulsive risk alleles with attention to detail, or anorexia risk alleles with willpower? The same basic methodology used by Power et al. can be used to investigate these and other fascinating questions once high-powered GWAS results for these disorders are available.
This study does not inform about which specific genetic variants are associated with both risk of psychiatric disease and being employed in a creative occupation, nor does it inform about how such genetic variants function. Nevertheless it is a starting point for pursuing these questions and a reminder that the notion that ‘gene X performs task Y in cell type Z’ is a model inconsistent with empirical observations.
Contributor Information
Matthew C Keller, Email: matthew.c.keller@gmail.com, Department of Psychology and Neuroscience and the Institute for Behavioral Genetics, University of Colorado Boulder, Boulder, Colorado, USA.
Peter M Visscher, Email: peter.visscher@uq.edu.au, Queensland Brain Institute, The University of Queensland, Brisbane, Queensland, Australia and The University of Queensland Diamantina Institute, The Translation Research Institute, Brisbane, Queensland, Australia.
References
- 1.Simeonova DI, Chang KD, Strong C, Ketter TA. Creativity in familial bipolar disorder. J Psychiatr Res. 2005;39:623–31. doi: 10.1016/j.jpsychires.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Kyaga S, et al. Creativity and mental disorder: family study of 300,000 people with severe mental disorder. Br J Psychiatry. 2011;199:373–9. doi: 10.1192/bjp.bp.110.085316. [DOI] [PubMed] [Google Scholar]
- 3.Lauronen E, et al. Links between creativity and mental disorder. Psychiatry. 2004;67:81–98. doi: 10.1521/psyc.67.1.81.31245. [DOI] [PubMed] [Google Scholar]
- 4.Power RA, et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nature Neuroscience. 2015;18:xxx–xxx. doi: 10.1038/nn.4040. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Candia T, et al. Additive genetic variation in schizophrenia risk is shared by populations of African and European descent. American Journal of Human Genetics. 2013;93:463–470. doi: 10.1016/j.ajhg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrieze SI, McGue M, Iacono WG. The interplay of genes and adolescent development in substance use disorders: leveraging findings from GWAS meta-analyses to test developmental hypotheses about nicotine consumption. Hum Genet. 2012;131:791–801. doi: 10.1007/s00439-012-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller M, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404. doi: 10.1017/S0140525X06009095. discussion 405–52. [DOI] [PubMed] [Google Scholar]
- 9.Hedrick PW. Antagonstic pleitropy and genetic polymorphism: A perspective. Heredity. 1999;82:126–132. [Google Scholar]
- 10.Prout T. How well does opposing selection maintain variation? In: Singh RS, Krimbas CB, editors. Evolutionary genetics from molecules to morphology. Cambridge University Press; Cambridge, UK: 1999. pp. 369–392. [Google Scholar]


