Abstract
The aims of this study were to investigate the utility of solid microneedle arrays (150 µm in length) in enhancing transdermal delivery of peptides and to examine the relationship between peptide permeation rates and D2O flux. Four model peptides were used (Gly–Gln–Pro–Arg [tetrapeptide-3, 456.6 Da], Val–Gly–Val–Ala–Pro–Gly [hexapeptide, 498.6 Da], AC–Glu–Glu–Met–Gln–Arg–Arg–NH2 [acetyl hexapeptide-3, 889 Da] and Cys–Tyr–Ile–Gln–Asn–Cys–Pro–Leu–Gly–NH2 [oxytocin, 1007.2 Da]). The influence of microneedle pretreatment on skin permeation was evaluated using porcine ear skin with Franze diffusion cell. Peptide permeation across the skin was significantly enhanced by microneedle pretreatment, and permeation rates were dependent on peptide molecular weights. A positive correlation between D2O flux and acetyl hexapeptide-3 clearances suggests that convective solvent flow contributes to the enhanced transdermal peptide delivery. It is concluded that solid microneedle arrays are effective devices to enhance skin delivery of peptides.
KEY WORDS: Microneedle, Peptide, Transdermal, Convective solvent flow
Graphical abstract
Solid microneedle significantly enhanced the delivery of peptides with low molecular weight through skin and provided a sustained release of the peptides during 24 h. Permeation rate of peptides decreased with the molecular weight increased.
1. Introduction
With rapid advances in biotechnology, a large number of peptides have been developed as therapeutic agents1. For example, topical application of acetyl hexapeptide-3 is used to treat facial wrinkles and may be of value in repairing skin2,3. Acetyl hexapeptide-3 is about 4000 times less toxic than botulin toxin4. In addition, this drug does not require injection, and can be topically used on the skin. However, the hydrophilic nature of these peptides limits the efficacy of these drugs applied topically, because of poor transdermal penetration5. Thus, the major challenges of transdermal peptide delivery are poor permeability, low bioavailability and regulatory issues.
Microneedles have been proposed to be a kind of delivery system which permits entry of small-molecule drugs, therapeutic proteins and vaccines, with minimal skin invasiveness6,7. Although there is a growing interest in the microneedle-based drug delivery system and a significant development in its application, the use of microneedles for delivery of hydrophilic peptides with low molecular weight have not been investigated in detail. Previously, we reported that microneedles significantly increase the transdermal delivery of hydrophilic l-carnitine in comparison to passive diffusion, and enhance substantially the bioavailability of this peptide compared to oral administration8. Microneedles have also been found to increase the skin permeation of calcein, insulin, vaccine and liposome9,10. So far, the solid microneedles have been proved to be an efficient and affordable approach for the delivery of transdermal drug.
Enhancement of transdermal drug delivery is probably related to convective solvent flow. For instance, Manabe et al.11 evaluated the effect of convective flow on iontophoretic skin transport based upon the hydrodynamic pore theory. Morimoto et al.12 investigated the relationship between hydrophilic solute and water transport during ultrasound application, and found that 41 kHz ultrasound can increase the permeation of hydrophilic solutes by inducing convective solvent flow via new routes13. Similarly, microneedles create transient aqueous microchannels which serve as multiple pathways for hydrophilic drug transfer14. Therefore, convective solvent flow may be involved in enhancing skin transport by microneedles, this possibility remains unconfirmed.
The objectives of the present study are: (1) to evaluate the penetration of fluorescent dye into skin after application of microneedles using confocal laser scanning microscopy, (2) to evaluate the effect of pretreatment of puncture by microneedles with 150 μm length on in-vitro skin permeation of hydrophilic peptides of varying molecular weight and (3) to explain the penetration-enhancing effect caused by microneedles based on the relationship between D2O flux and solute transport clearance.
2. Materials and methods
2.1. Chemicals and reagents
Tetrapeptide-3, hexapeptide, acetyl hexapeptide-3 and oxytocin were all purchased from ChuanKangPaiDe Biological Technology (Sichuan, China). HPLC-grade acetonitrile was obtained from Dikma Technology (Beijing, China). All solutions were prepared with ultrapure water (resistivity>18 MΩ cm).
2.2. Skin preparation
Porcine ear skins (from adult pig) were purchased from a local slaughterhouse immediately following death, and the dermatomed skins with a thickness of 800 μm were carefully obtained with a skin grafting knife.
2.3. Microneedle arrays
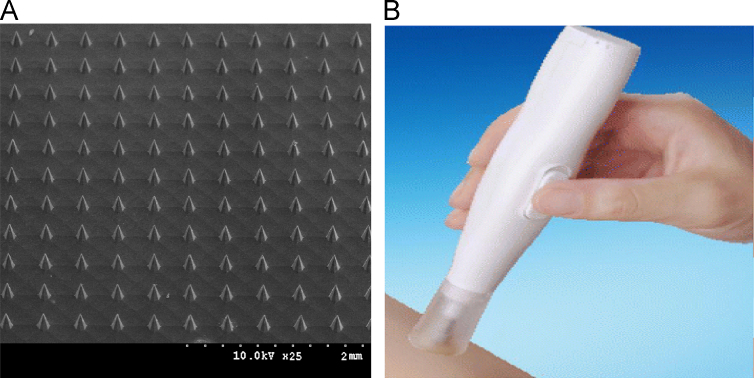
Microneedle arrays and applicator were developed by our group and made by Nasheng Microelectronics (Suzhou) Co. Ltd., China. Arrays of solid microneedle (Fig. 1A) were fabricated by dry and wet etching from silicon wafers. Each microneedle array has 121 needles of 150 μm-length in an area of 4 mm×4 mm. The array was fixed onto a supporting column (5 mm in diameter) of applicator (Fig. 1B), which provided insertion force of nearly 2 N. Microneedle arrays were inserted into the skin with a constant vibration frequency for 20 s.
Figure 1.
The microneedle array and the autoapplicator employed in this study. (A) Microneedle array; (B) autoapplicator.
2.4. In vitro skin permeation studies
The experiments were performed with a system employing Franz vertical diffusion cells. After pretreatment by microneedles for 20 s, the skin samples were clamped in vertical Franz diffusion cells (2.5 mL) with the stratum corneum side facing the donor compartment, giving an effective permeation area of 0.66 cm2. The receptor and donor compartments were filled with phosphate buffer (pH 7.4). After equilibrium for 1 h, the receptor compartment was filled with PBS. The donor compartments were replaced with 300 μL peptides dissolved in PBS prepared with either H2O or D2O. Diffusion cells were stirred by a magnetic bar at 280 rpm at 37 °C. The samples of receptor cell were sampled at predetermined time intervals and the receptor phase was immediately refreshed by equal volume of PBS buffer to keep a constant volume. The samples were centrifuged (4000×g for 7 min), and the supernatant analyzed by HPLC/UV. Results are expressed as the mean±S.D. (n=3–4 independent samples). The osmotic concentration differential was adjusted to 3.08, 0, –3.08 mol/L by adding NaCl to donor or receiver compartments15.
2.5. Microneedle insertion imaging
Confocal laser scanning microscopy (CLSM) was used to observe the microconduits created by microneedle. Skin was pierced by microneedles coated with calcein hydrogel, and removed immediately and placed upon a glass slide. The stratum comeum side at 20 μm was scanned at high speed through the Z axis (Z, defined as perpendicular distance to the skin surface from the dermis) of a ZEISS PASCAL inverted confocal laser scanning microscope (LSM 510 with an attached Zeiss Axiovert 200 M microscope).
2.6. Quantitation of the peptides
The quantitative determination of tetrapeptide-3, hexapeptide, acetyl hexapeptide-3 and oxytocin were conducted by LC-2010 A HPLC/UV (Shimadzu, Japan). The experiments were carried out under gradient elution mode using a mobile phase consisting of 0.1% (v/v) trifluoroacetic acid aqueous solution and 0.1% (v/v) trifluoroacetic acid acetonitrile solution at a flow rate of 1 mL/min. The analysis was performed on a YMC-Pack ODS-A C18 column (250 mm×4.6 mm i.d., 5 μm, YMC Inc., USA). The injection volume was 20 μL. The column eluant was monitored at 220 nm. D2O were analyzed by measuring the intensity of the O-D stretching vibrational band at 2512 cm−1 in infrared spectroscopic spectra (Shimadzu, Japan).
2.7. Data analysis
The accumulative amount (μmol/cm2) of drug permeating across a unit diffusion surface into the receptor compartment was calculated and plotted as a function of time. Steady-state skin permeation rates were determined by linear regression analysis of the amount of peptides penetrated against time. The permeation clearance (CL, μL/h) was calculated using the following equation: CL=(dCR/dt)VR/CD, where CR and CD are the drug concentration in the receiver and donor solution, respectively, VR is the volume of the receiver solution, and t is time.
3. Results
3.1. Fluorescence microscopic observations
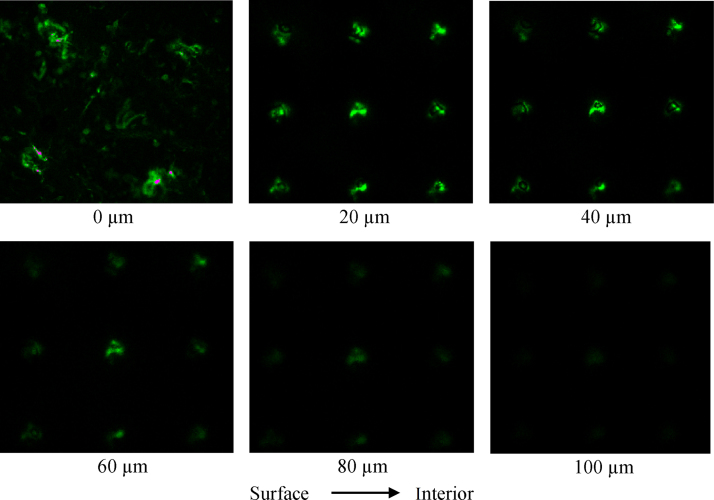
In order to directly verify the formation of microconduits in the skin, microneedles with 150 μm length were used with an insertion system designed to pierce the skin at a constant speed. Fig. 2 shows the distribution of calcien fluorescence at skin depths of 0, 20, 40, 60, 80 and 100 μm after microneedle pretreatment. Confocal images confirmed the formation of microconduits by microneedle pretreatment. Calcein fluorescence was confined to microconduits, while dots correspond to sites of microconduits from the array. Calcein could diffuse into deeper skin layers. At Z=80 μm, the fluorescence of calcein around microconduits are still visible, but at Z=100 μm, there is trace amount of calcein fluorescence and microconduits is disappeared.
Figure 2.
Calcein fluorescence across the porcine ear skin at Z=0, 20, 40, 60, 80 and 100 μm following microneedle pretreatment. Z direction is defined as perpendicular distance to the skin surface from the dermis. Fluorescence-emission signal of calcein is represented by green color.
3.2. Permeation studies with hydrophilic peptides varying in molecular weight
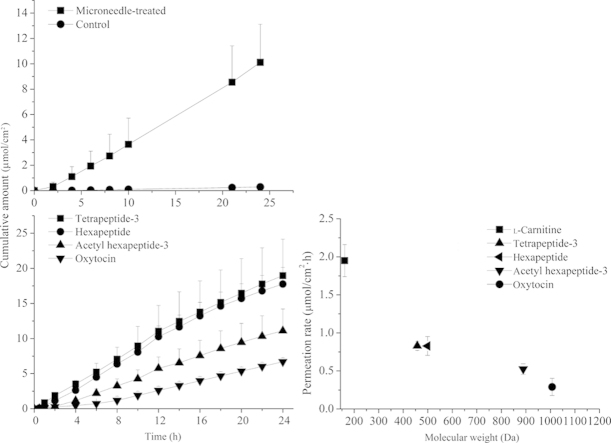
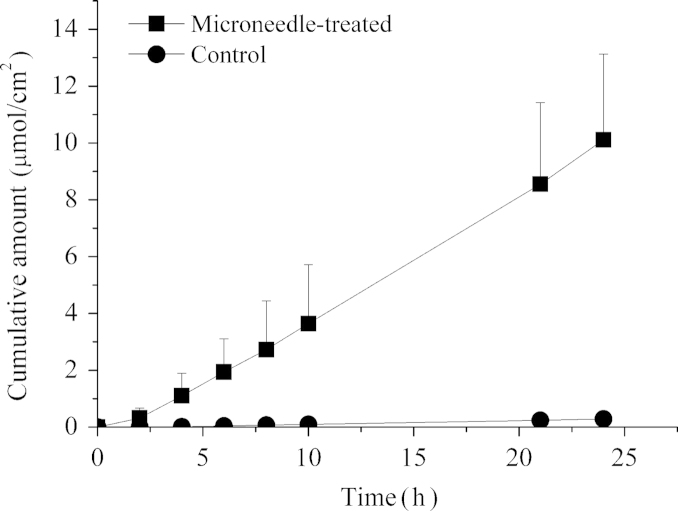
To assess whether microneedle pretreatment resulted in an increased skin transport of hydrophilic peptides, the permeabilities of acetyl hexapeptide-3 through untreated and microneedle-treated porcine ear skin were compared. Experiments were conducted under mode of microneedle application (auto) and the acetyl hexapeptide-3 solution (90 mmol/L). Fig. 3 shows the cumulative amount permeated through porcine ear skin during 24 h. The passive flux of acetyl hexapeptide-3 across untreated skin was 0.014±0.002 μmoL/cm·h. After microneedle treatment, the flux increased to 0.44±0.12 μmoL/cm·h, an enhancement of more than 31-fold.
Figure 3.
The permeation profiles of acetyl hexapeptide-3 across porcine ear skin with and without pretreatment by microneedles.
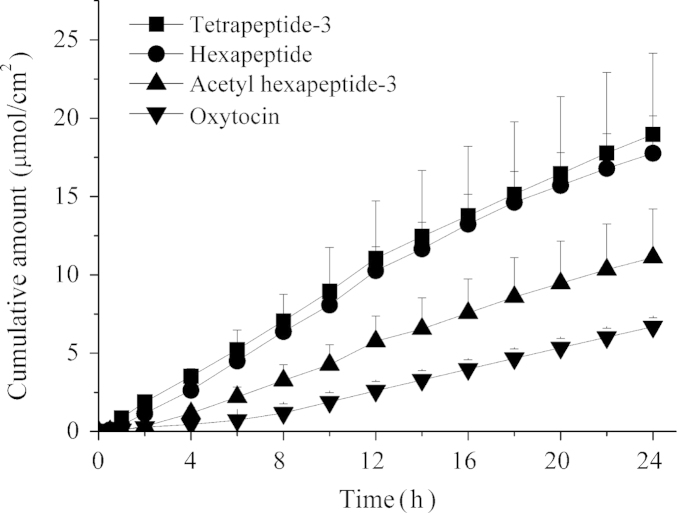
Four peptides (tetrapeptide-3, hexapeptide, acetyl hexapeptide-3 and oxytocin, 0.09 mol/L) were used to study the relationship between permeability rate and molecular weight with and without microneedle pretreatment. All of the peptides have been widely used in medical and cosmetic industries. Table 1 summarizes the in vitro cumulative delivery of peptides across porcine ear skin over 24 h. Microneedle pretreatment significantly enhanced the penetration of all peptides.
Table 1.
Transdermal accumulation of peptides with and without microneedle pretreatment.
| Peptides | Cumulative amount (μmol/cm2) |
||
|---|---|---|---|
| Microneedle | Passive diffusion | Ratio | |
| Oxytocin | 6.69±0.57 | 0.12±0.008 | 55.75 |
| Acetyl hexapeptide-3 | 11.11±3.1 | 0.29±0.073 | 38.31 |
| Hexapeptide | 17.76±2.38 | 0.49±0.12 | 36.24 |
| Tetrapeptide-3 | 18.96±5.19 | 0.58±0.16 | 32.69 |
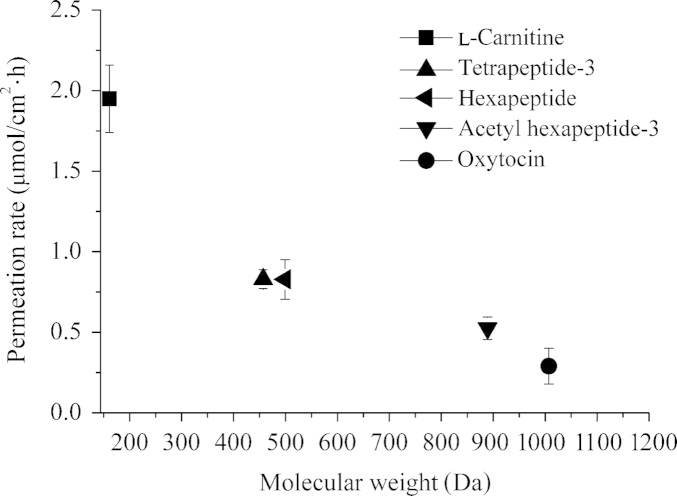
Fig. 4 shows the cumulative amount of these peptides after application of the microneedles during 24 h. Besides the dipeptides studied here, transport for l-carnitine (161.2 Da) was also studied. The permeation rates of l-carnitine, tetrapeptide-3, hexapeptide, acetyl hexapeptide-3 and oxytocin were 1.95±0.21, 0.90±0.09, 0.84±0.11, 0.42±0.14, 0.16±0.05 μmoL/cm·h, respectively. Fig. 5 shows an inverse relationship between permeability and molecular weight of the peptides.
Figure 4.
In vitro transdermal permeation of a series of peptides in solution across porcine ear skin 24 h after pretreatment by microneedles.
Figure 5.
Relationship between the permeation rate of five model peptides and their corresponding molecular weight.
3.3. Effect of microneedles on the permeability of D2O and hydrophilic drugs
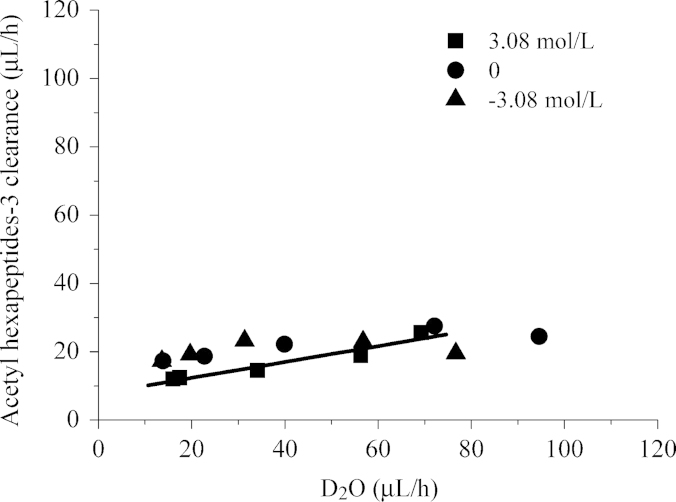
The enhancing effects of ultrasound and iontophoresis on skin permeation of hydrophilic compound can be explained by inducing convective solvent flow13. In the present study, we analyzed how the transport of the hydrophilic compound acetyl hexapeptide-3 was improved by microneedles based on the relationship between hydrophilic solute and vehicle (D2O) transport. To assess the skin permeability of D2O and acetyl hexapeptide-3, the time course of D2O flux and permeation clearance of acetyl hexapeptide-3 under various osmotic concentrations were determined (Fig. 6). A good correlation was observed between the acetyl hexapeptide-3 clearance and the D2O flux (r=0.96, slope of the regression line: 0.23±0.037). The results suggest that convection generated by microneedles plays an important role in skin permeability of hydrophilic drugs.
Figure 6.
Relationship between D2O flux and permeation clearance of acetyl hexapeptide-3.
4. Discussion
The present results found that pretreatment with solid microneedles with a length of 150 μm create visible microconduits in a “poke and patch” approach. The presence of microconduits created by microneedles was in accordance with our intended purpose, i.e. to puncture the epidermis without reaching nerves in the dermis. In the “poke and patch” approach, it is very important for the microconduits to remain open at least 72 h under occlusive conditions16. Many microneedle-base studies have shown that the permeability for different compounds is increased up to four orders in magnitude17,18. As observed from Fig. 4, transdermal permeation of all peptide was sustained for 24 h after treatment of microneedles. Therefore, solid microneedles could be conveniently incorporated into a transdermal patch to prolong peptide release.
Among various therapeutic peptide-delivery techniques, microneedle-assisted peptide delivery is currently believed to be the most efficient method. Most published studies have focused on the high molecular weight compounds, such as protein, vaccine and insulin19–23. Hitherto, there are few studies examining whether microneedles could deliver peptides with various molecular weights, especially those with low molecular weight. Even less is known about the permeation mechanisms for these compounds. In the present study, in vitro results on the permeation of peptides with different molecular weights indicate that microneedles can remarkably enhance the transdermal delivery of all hydrophilic peptides. The skin permeation of peptides depends on their molecular weight and decreases as the molecular weight increases. In addition, the enhanced skin permeation of peptides produced by microneedle pretreatment may be caused by the generation of convection. This study demonstrates that microneedles provide an attractive route to deliver low molecular weight peptides to the skin. The transdermal administration of such compounds is of significant current interest in clinical medicine and the cosmetic industry.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Herwadkar A., Banga A.K. Peptide and protein transdermal drug delivery. Drug Discov Today Technol. 2012;9:e147–e154. doi: 10.1016/j.ddtec.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Chirita R.I., Chaimbault P., Archambault J.C., Robert I., Elfakir C. Development of a LC-MS/MS method to monitor palmitoyl peptides content in anti-wrinkle cosmetics. Anal Chim Acta. 2009;641:95–100. doi: 10.1016/j.aca.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Choi C.M., Berson D.S. Cosmeceuticals. Semin Cutan Med Surg. 2006;25:163–168. doi: 10.1016/j.sder.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Zhou W.L., Wang P.G., Krynitsky A.J., Rader J.I. Rapid and simultaneous determination of hexapeptides (Ac-EEMQRR-amide and H2N-EEMQRR-amide) in anti-wrinkle cosmetics by hydrophilic interaction liquid chromatography-solid phase extraction preparation and hydrophilic interaction liquid chromatography with tandem mass spectrometry. J Chromatogr A. 2011;1218:7956–7963. doi: 10.1016/j.chroma.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 5.Henchoz Y., Abla N., Veuthey J.L., Carrupt P.A. A fast screening strategy for characterizing peptide delivery by transdermal iontophoresis. J Control Release. 2009;137:123–129. doi: 10.1016/j.jconrel.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Brown K., Siebenaler K., Determan A., Dohmeier D., Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29:170–177. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S.H., Qin G.J., Wu Y., Gao Y.H., Qiu Y.Q., Li F. Enhanced bioavailability of l-carnitine after painless intradermal delivery vs. oral administration in rats. Pharm Res. 2011;28:117–123. doi: 10.1007/s11095-010-0109-7. [DOI] [PubMed] [Google Scholar]
- 9.Guo L., Chen J.M., Qiu Y.Q., Zhang S.H., Xu B., Gao Y.H. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int J Pharm. 2013;447:22–30. doi: 10.1016/j.ijpharm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Qin G.J., Gao Y.H., Wu Y., Zhang S.H., Qiu Y.Q., Li F. Simultaneous basal-bolus delivery of fast-acting insulin and its significance in diabetes management. Nanomedicine. 2012;8:221–227. doi: 10.1016/j.nano.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Manabe E., Numajiri S., Sugibayashi K., Morimoto Y. Analysis of skin permeation-enhancing mechanism of iontophoresis using hydrodynamic pore theory. J Control Release. 2000;66:149–158. doi: 10.1016/s0168-3659(99)00265-5. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto Y., Mutoh M., Ueda H., Fang L., Hirayama K., Atobe M. Elucidation of the transport pathway in hairless rat skin enhanced by low-frequency sonophoresis based on the solute–water transport relationship and confocal microscopy. J Control Release. 2005;103:587–597. doi: 10.1016/j.jconrel.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Tang H., Mitragotri S., Blankschtein D., Langer R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J Pharm Sci. 2001;90:545–568. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Hatanaka T., Manabe E., Sugibayashi K., Morimoto Y. An application of the hydrodynamic pore theory to percutaneous absorption of drugs. Pharm Res. 1994;11:654–658. doi: 10.1023/a:1018911926190. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri H., Banga A.K. Formation and closure of microchannels in skin following microporation. Pharm Res. 2011;28:82–94. doi: 10.1007/s11095-010-0122-x. [DOI] [PubMed] [Google Scholar]
- 17.Banks S.L., Paudel K.S., Brogden N.K., Loftin C.D., Stinchcomb A.L. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm Res. 2011;28:1211–1219. doi: 10.1007/s11095-011-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banga A.K. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6:343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- 19.Hiraishi Y., Hirobe S., Iioka H., Quan Y.S., Kamiyama F., Asada H. Development of a novel therapeutic approach using a retinoic acid-loaded microneedle patch for seborrheic keratosis treatment and safety study in humans. J Control Release. 2013;171:93–103. doi: 10.1016/j.jconrel.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Ling M.H., Chen M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9:8952–8961. doi: 10.1016/j.actbio.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y.C., Song J.M., Lipatov A.S., Choi S.O., Lee J.W., Donis R.O. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur J Pharm Biopharm. 2012;81:239–247. doi: 10.1016/j.ejpb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbaan F.J., Bal S.M., van den Berg D.J., Groenink W.H., Verpoorten H., Lüttge R. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117:238–245. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Wu X.M., Todo H., Sugibayashi K. Effects of pretreatment of needle puncture and sandpaper abrasion on the in vitro skin permeation of fluorescein isothiocyanate (FITC)-dextran. Int J Pharm. 2006;316:102–108. doi: 10.1016/j.ijpharm.2006.02.046. [DOI] [PubMed] [Google Scholar]