Abstract
2-Hydroxytyrosol (2-HT), originally reported as a synthetic compound, was isolated for the first time as a fungal metabolite. 2-HT was found to inhibit mushroom tyrosinase with an IC50 value of 13.0 µmol/L. Furthermore, 2-HT dose-dependently inhibited tyrosinase activity (IC50, 32.5 µmol/L) in the cell-free extract of B16 melanoma cells and α-melanocyte stimulating hormone (α-MSH)-stimulated melanin formation in intact B16 melanoma cells.
KEY WORDS: 2-Hydroxytyrosol, Metarhizium sp., Tyrosinase inhibitor, Melanine formation, B16 melanoma cells
Graphical abstract
2-Hydroxytyrosol (2-HT), was found to inhibit mushroom tyrosinase activity (IC50, 32.5 µmol/L) in the cell-free extract of B16 melanoma cells and α-melanocyte stimulating hormone (α-MSH)-stimulated melanin formation in intact B16 melanoma cells.
1. Introduction
Melanin is essential for protecting human skin against radiation, but the accumulation of abnormal melanin induces pigmentation disorders, such as melasma, freckles, ephelides, and senile lentigines1. Melanogenesis is conducted in melanocytes, located in the basal layer of the epidermis and controlled by tyrosinase2.
Tyrosinase (EC 1.14.18.1), also known as polyphenol oxidase (PPO), is a copper-containing monooxygenase enzyme involved in melanogenesis3. The enzyme is widely distributed in fungi, higher plants and animals4, and is involved in the first two steps of the melanin biosynthesis, in which l-tyrosine is hydroxylated to 3,4-dihydroxyphenylalanine (l-DOPA, monophenolase activity) and the latter is subsequently oxidated to dopaquinone (diphenolase activity)2. A large number of moderate to potent tyrosinase inhibitors from natural and synthetic resources have been reported during the last decade5–9. Tyrosinase inhibitors such as arbutin, kojic acid and hydroquinones have been used as whitening or antihyperpigment agents because of their ability to suppress dermal-melanin production10,11. However, arbutin and kojic acid hardly showed inhibitory activity against pigmentation in intact melanocytes or in a clinical trial12, and hydroquinones are considered to be cytotoxic to melanocytes and potentially mutagenic to mammalian cells11. Therefore, it remains necessary to search for new tyrosinase inhibitors without side effects.
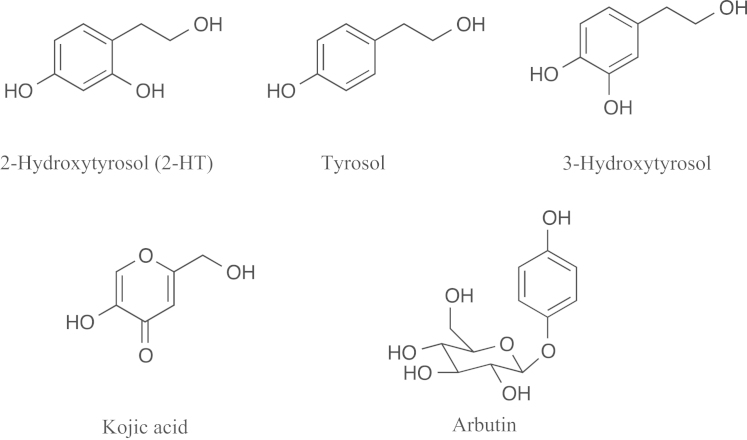
During our course of screening for mushroom tyrosinase inhibitors of microbial origin, 2-hydroxytyrosol (2-HT, Fig. 1) was isolated from the fungal culture broth of Metarhizium sp. OB-0098. 2-HT was originally reported to be a synthetic compound13, but its biological activity has not been reported. In this study, tyrosinase inhibitory activities and melanin formation in mouse B16 melanoma cells of 2-HT were described.
Figure 1.
Structures of 2-hydroxytyrosol (2-HT) and tyrosinase inhibitors.
2. Results
2.1. Inhibition of mushroom tyrosinase activity by 2-hydroxytyrosol
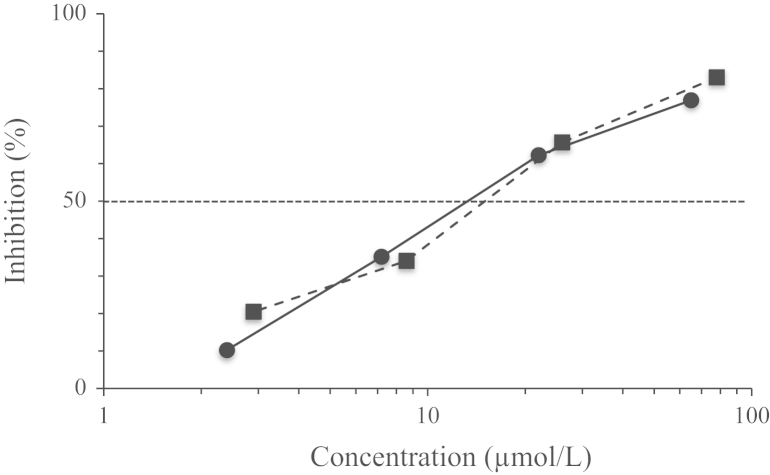
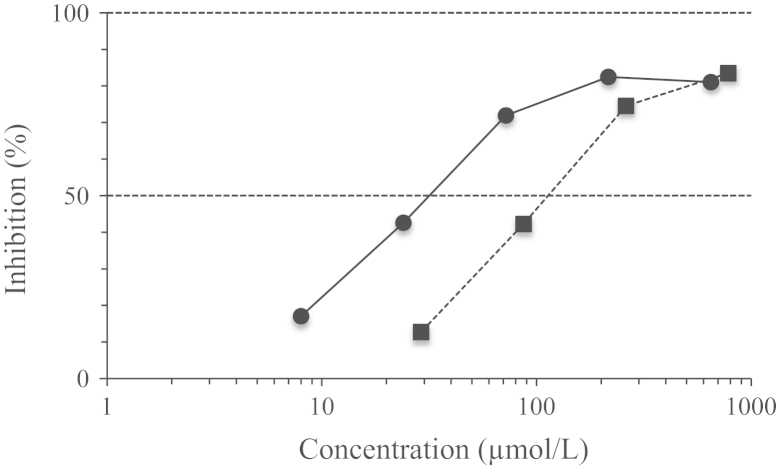
In this assay, the conversion of l-DOPA to dopaquinone by mushroom tyrosinase was observed at 450 nm. As shown in Fig. 2, 2-HT dose-dependently inhibited mushroom tyrosinase activity with an IC50 value of 13.0 µmol/L. Under the same conditions, kojic acid also inhibited the activity with IC50 of 14.8 µmol/L.
Figure 2.
Inhibitory effects of 2-HT(●) and kojic acid (■) against mushroom tyrosinase.
2.2. Inhibition of melanin pigmentation in B16 melanoma cells by 2-hydroxytyrosol
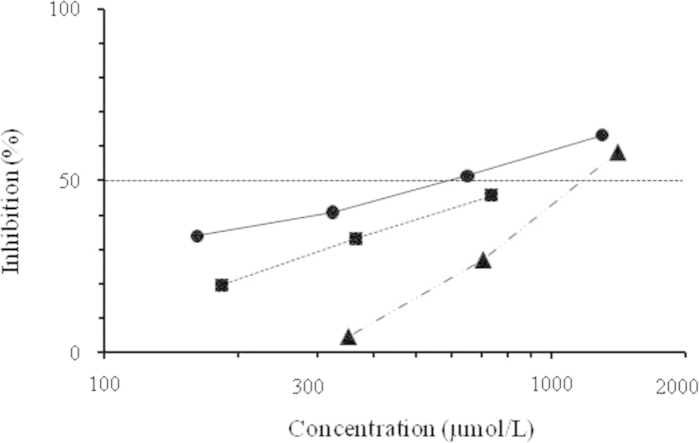
To investigate whether 2-HT inhibited melanogenesis, the effect of 2-HT on melanin pigmentation in intact B16 melanoma cells was studied. α-MSH was added to this assay system, because melanin production was markedly enhanced. 2-HT was found to inhibit the melanin pigmentation of B16 melanoma cells in a dose-dependent manner with IC50 of 571 µmol/L (Fig. 3). Under the same conditions, arbutin inhibited the melanin pigmentation with IC50 of 1130 µmol/L, and kojic acid inhibited it by 45.7% at 735 µmol/L. Furthermore, the cytotoxic effects of these inhibitors on B16 melanoma cells were investigated by the MTT assay. The IC50 values of 2-HT, kojic acid and arbutin were 1.3, 3.0 and 1.8 mmol/L, respectively.
Figure 3.
Inhibitory effects of 2-HT, kojic acid and arbutin on α-MSH-induced melanin production in B16 melanoma cells. The pellets were resuspended in 2 mol/L NaOH solution and absorbance of supernatants was measured at 450 nm using a microplate reader. (●) 2-HT, (■) kojic acid and (▲) arbutin.
2.3. Inhibition of B16 cells tyrosinase activity by 2-hydroxytyrosol
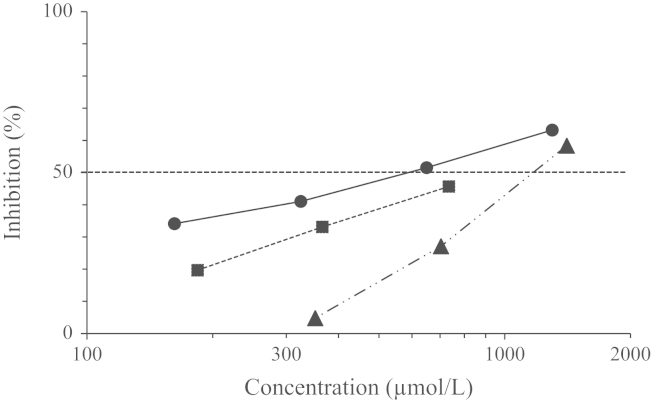
To confirm the inhibition of melanin pigmentation in intact B16 melanoma cells by 2-HT, the effect of 2-HT on tyrosinase activity in B16 cell lysate was examined. As shown in Fig. 4, 2-HT showed inhibitory activity with IC50 of 32.5 µmol/L. Under the same conditions, the IC50 of kojic acid was calculated to be 113 µmol/L.
Figure 4.
Inhibitory effects of 2-HT (●) and kojic acid (■) against tyrosinase in B16 melanoma cell crude lysates.
3. Discussion
In our screening for tyrosinase inhibitors of microbial origin using mushroom tyrosinase, 2-HT was isolated and identified from the fungal culture broth. Although 2-HT was originally reported as a synthetic compound13, we showed for the first time that 2-HT is also a fungal metabolite. Furthermore, 2-HT was found to inhibit tyrosinase in this study. Structurally related tyrosol and 3-hydroxytyrosol were isolated from olive oil as antioxidants (Fig. 1)14.
Kojic acid is a well-known tyrosinase inhibitor. In this study, we showed that the inhibitory activity of 2-HT (IC50, 13 µmol/L) against mushroom tyrosinase is as potent as that of kojic acid (IC50, 14.8 µmol/L), and even more potent against B16 melanoma tyrosinase than kojic acid. Furthermore, we demonstrated that 2-HT suppressed melanin production on α-MSH-treated B16 melanoma cells. 2-HT is a phenolic compound having three hydroxyl groups in the structure, which appears structurally related to l-DOPA, the substrate of tyrosinase. Therefore, it is plausible that 2-HT works competitively with respect to the substrate. These findings suggested that 2-HT is a promising lead compound for the treatment of skin pigmentation disorders.
4. Materials and methods
4.1. General experimental procedure
Various NMR spectra were obtained using an Agilent Technologies XL-400 (400 MHz) spectrometer (Agilent Technologies, Santa Clara, CA, USA). Electronionization mass spectrometry (EI-MS) was conducted on a JEOL JMS-T100LP spectrometer (JEOL, Tokyo, Japan).
4.2. Materials
3,4-Dihydroxy-l-phenylalanine (l-DOPA), kojic acid and α-arbutin were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Lyophilized mushroom tyrosinase and α-melanocyte stimulating hormone (α-MSH) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The B16 melanoma cell line, JCRB0202, was obtained from the Health Science Research Resources Bank (Tokyo, Japan).
4.3. Identification of producing fungus OB-0098
Strain fungus OB-0098 was isolated from an unidentified univalve shell collected on Okinawa main island, Okinawa, Japan. The genetic sequence information of the rDNA ITS (including 5.8S rDNA) gene15 was designated as a strain belonging to the genus Metarhizium16.
4.4. Fermentation of Metarhizium sp. OB-0098
A stock culture of strain Metarhizium sp. OB-0098 was grown and maintained on 2.4% potato dextrose agar (Becton, Dickinson and Company, NJ, USA) medium (non-adjusted pH). For the production of 4-(2-hydroxyethyl)-1,3-benzenediol, the seed medium used contained 2.4% potato dextrose broth (PDB) medium (non-adjusted pH). The production medium was composed of 50 g Vialonenano rice (Masi, VR, Italy) and 25 mL of 2.4% PDB (non-adjusted pH).
A loopful of spores of Metarhizium sp. OB-0098 was inoculated into a 500 mL Erlenmeyer flask with 100 mL seed medium and incubated on a rotary shaker at 27 °C for 3 days. The production culture was initiated by transferring 3 mL seed culture into each of fifty 500 mL culture bottles (As one, Osaka, Japan) containing production medium, and the fermentation was carried out at 27 °C for 14 days under stationary conditions.
4.5. Isolation procedure of 2-hydroxytyrosol
The culture (2.5 g) was treated with EtOH (5.0 L) for 2 h, and EtOH extracts were filtered to remove the mycelium and fermentation media. After concentration of the extracts to remove EtOH, the aqueous solution (0.33 L) was extracted with CHCl3. Further, the aqueous layer was adjusted to pH 3.0 and extracted with EtOAc (0.33 L). The organic layer was dried over Na2SO4 and concentrated under reduced pressure to give brown material (0.6 g). The material (75 mg) containing 2-HT was dissolved in a small amount of MeOH and purified by HPLC using a reverse-phase C30 column under the following conditions: column, Develosil C30 (250 mm×10 mm), Nomura Scientific Co., Ltd., (Aichi, Japan); column temperature, 40 °C; mobile phase, 5% CH3CN in 0.05% TFA.; flow rate, 3 mL/min; detection, UV 210 nm. 2-HT was eluted as a peak with a retention time of 16 min. The fraction of the peak was collected and concentrated to dryness to give pure 2-HT (2.73 mg).
4.6. Structure determination of 2-hydroxytyrosol
From the spectral data including 1H NMR, 13C NMR, and MS, and the search results of SciFinder Scholar, 2-HT was identified to be the same as the known synthetic compound 4-(2-hydroxyethyl)-1,3-benzenediol (Fig. 1)13. In this study, 2-HT was named as 2-hydroxytyrosol.
2-hydroxytyrosol: 1H NMR (400 MHz, CD3OD): δ 6.86 (1H, d, J=8 Hz, H-8), δ 6.27 (1H, d, J=2 Hz, H-5), δ 6.21 (1H, dd, J=2, 8 Hz, H-7), δ 3.68 (2H, t, J=7.5 Hz, H-1), δ 2.72 (2H, t, J=7.5 Hz, H-2). 13C NMR (100 MHz, CD3OD): δ 157.9 (s, C-6), δ 157.4(s, C-4), δ 132.2 (d, C-8), δ 117.8 (s, C-3), δ 107.4 (d, C-7), δ 103.6 (d, C-5), δ 63.6 (t, C-1), δ 34.5 (t, C-2). LR-EI-MS m/z: 154 [M]+ HR-EI-MS m/z: [M]+ calcd. for C8H10O3, 154.0630; found, 154.0622.
4.7. Assay for mushroom tyrosinase activity
Tyrosinase inhibitory activity was measured spectrophotometrically according to the method of Masamoto et al.17 with some modifications. First, 10 µL solution of 2-HT (2.4–65 µmol/L) in DMSO was added to a 96-well microplate and mixed with 60 µL 50 mmol/l phosphate buffer (pH 6.8) on ice. Then, 20 µL 0.9 mg/mL l-DOPA in phosphate buffer was added. Finally, 10 µL mushroom tyrosinase (500 U/mL in phosphate buffer) was added and the assay mixture was then incubated at 27 °C for 10 min. Following incubation, the amount of dopachrome production in the reaction mixture was determined spectrophotometrically at 450 nm (OD450) in a microplate reader. Kojic acid (2.9–77 µmol/L) dissolved in 50 mmol/L phosphate buffer was used as a positive control. The concentration for 50% inhibition (IC50) was determined. Each measurement was performed at least in duplicate.
4.8. Cell culture
The murine melanoma B16 cell line, JCRB020218 (obtained from the National Institute of Biomedical Innovation) was maintained in Minimum Essential Medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 units/mL)/streptomycin (100 mg/mL) and cultured at 37 °C in a humidified atmosphere with 5% CO2. All experiments were performed in triplicate and were repeated 3 times to ensure reproducibility.
4.9. Cell proliferation
The cell viability assay of B16 cells was performed using the 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay19. The cells were grown in 96-well plates at a density of 7.5×103 cells/well. After 22 h, the cells were then incubated in the presence of 100 nmol/L α-MSH and treated with various concentrations of sample. After 48 h of incubation, the cells were rewashed and 10 µL MTT solution (5 µg/mL) was added and incubated for approximately 3 h. After the cells were lysed with 40% N,N-dimethylformamide, 20% sodium dodecyl sulfate (SDS), 2.0% acetic acid, and 0.03% hydrochloric acid, the amount of formazan salt was quantified by measuring the absorbance at 570 nm with a microplate reader.
4.10. Measurement of melanin pigmentation on B16 melanoma cells
The melanin content was determined in accordance with the procedure described by Komiyama et al.20 with some slight modifications. The B16 melanoma cells were seeded at a density of 2.5×103 cells/well in 6-well culture plates and then incubated for 5 days. The cells were washed twice in phosphate-buffered saline and treated with various concentrations of sample in the presence of 100 nmol/L α-MSH. After incubation for 3 days, the cells were washed in phosphate-buffered saline and was removed from the bottom of the well with 0.25% trypsin-EDTA solution (1 mL/well), and the solution containing the cells was transferred to a 1.5 mL microtube. After centrifugation for 10 min at 10,000×g, the pellets were dissolved in 2 mol/L NaOH (50 µL) for 15 min at 60 °C. The absorbance of the sample at 450 nm was measured with a microplate reader.
4.11. Assay for tyrosinase activity in B16 melanoma cell crude lysates
Crude tyrosinase was prepared by the method of Ohguchi et al.21 with some slight modifications. Aliquots of B16 melanoma cells (5.0×106 cells/mL) in 100 mmol/L phosphate buffer (pH 6.8) containing 0.1% Triton X-100 (13.4 mL) were homogenized using a sonicator (Bioruptor; Cosmo Bio Co., Ltd., Tokyo, Japan). After centrifugation (11,000×g, 30 min, 4 °C), the supernatant was used as a crude tyrosinase enzyme solution. Protein in this crude enzyme solution was measured by the Lowry method, using bovine serum albumin as a standard.
Tyrosinase activity in B16 cell crude lysates was estimated using a modified mushroom tyrosinase assay. First, 50 µL 50 mmol/L phosphate buffer (pH 6.8), 20 µL l-DOPA (0.9 mg/mL, dissolved in 50 mmol/L phosphate buffer, pH 6.8) and 10 µL 2-HT or kojic acid were mixed. Then 20 µL crude lysates (2500 µg/mL) was added, and the amount of dopachrome in the reaction mixture was determined by optical density at 450 nm after 5 h at 37 °C.
Acknowledgment
This work was supported by Kitasato Research Project for Lactic Acid Bacteria from Kitasato University.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hearing V.J. Determination of melanin synthetic pathways. J Invest Dermatol. 2011;131:8–11. doi: 10.1038/skinbio.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parveen I., Threadgill M.D., Moorby J.M., Winters A. Oxidative phenols in forage crops containing polyphenol oxidase enzymes. J Agric Food Chem. 2010;58:1371–1382. doi: 10.1021/jf9024294. [DOI] [PubMed] [Google Scholar]
- 4.van Gelder C.W., Flurkey W.H., Wichers H.J. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry. 1997;45:1309–1323. doi: 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 5.Khan M.T.H. Novel tyrosinase inhibitors from natural resources – their computational studies. Curr Med Chem. 2012;19:2262–2272. doi: 10.2174/092986712800229041. [DOI] [PubMed] [Google Scholar]
- 6.Bao K., Dai Y., Zhu Z.B., Tu F.J., Zhang W.G., Yao X.S. Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg Med Chem. 2010;18:6708–6714. doi: 10.1016/j.bmc.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.J., Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solano F., Briganti S., Picardo M., Ghanem G.H. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Melanoma Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang T.S. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda K., Fukuda M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J Sic Cosmet Chem. 1991;42:361–368. [Google Scholar]
- 11.Jimbow K., Obata H., Pathak M.A., Fitzpatrick T.B. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62:436–449. doi: 10.1111/1523-1747.ep12701679. [DOI] [PubMed] [Google Scholar]
- 12.Curto E.V., Kwong C., Hermersdörfer H., Glatt H., Santis C., Virador V. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem Pharmacol. 1999;57:663–672. doi: 10.1016/s0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki Y., Mochida K., Kim S.W. Aromatic annelation with α-phenylsulfinyl-γ-butyrolactones. A novel route to 4-(2-hydroxyalkyl)-1,3-benzenediols. Chem Pharm Bull. 1987;35:1790–1795. [Google Scholar]
- 14.Franconi F., Coinu R., Carta S., Urgeghe P.P., Ieri F., Mulinacci N. Antioxidant effect of two virgin olive oils depends on the concentration and composition of minor polar compounds. J Agric Food Chem. 2006;54:3121–3125. doi: 10.1021/jf053003+. [DOI] [PubMed] [Google Scholar]
- 15.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand R.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; New York: 1990,. pp. 315–332. [Google Scholar]
- 16.Driver F., Milner R.J., Trueman J.W.H. A taxonomic revision of Metarhizium based on a phylogeneticanalysis of rDNA sequence data. Mycol Res. 2000;104:134–150. [Google Scholar]
- 17.Masamoto Y., Ando H., Murata Y., Shimoishi Y., Tada M., Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem. 2003;67:631–634. doi: 10.1271/bbb.67.631. [DOI] [PubMed] [Google Scholar]
- 18.Silagi S. Control of pigment production in mouse melanoma cells in vitro. Evocation and maintenance. J Cell Biol. 1969;43:263–274. doi: 10.1083/jcb.43.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Komiyama K., Takamatsu S., Takahashi Y., Shinose M., Hayashi M., Tanaka H. New inhibitors of melanogenesis, OH-3984 K1 and K2. I. Taxonomy, fermentation, isolation and biological characteristics. J Antibiot. 1993;46:1520–1525. doi: 10.7164/antibiotics.46.1520. [DOI] [PubMed] [Google Scholar]
- 21.Ohguchi K., Tanaka T., Iliya I., Ito T., Iinuma M., Matsumoto K. Gnetol as a potent tyrosinase inhibitor from genus. Gnetum. Biosci Biotechnol Biochem. 2003;67:663–665. doi: 10.1271/bbb.67.663. [DOI] [PubMed] [Google Scholar]