Abstract
The enormous progress biotechnology, bioinformatics and nanotechnology made in recent years provides opportunities and scientific framework for development of biomedicine and constitutes a paradigm shift in pharmaceutical R&D and drug innovation. By analyzing the data and related information at R&D level over the past decades, developmental tendency and R&D patterns were summarized. We found that a growing number of biologics in the pipeline of pharma companies with successful products already in the market though, small molecular entities have primarily dominated drug innovation. Additionally, small/medium size companies will continue to play a key role in the development of small molecule drugs and biologics in a multi-channel integrated process. More importantly, modern and effective R&D strategies in biomedicine development to predict and evaluate efficacy and/or safety of 21st century therapeutics are urgently needed. To face new challenges, developmental strategies were proposed, in terms of molecular targeted medicine, generic drugs, new drug delivery system and protein-based drugs. Under the current circumstances, interdisciplinary cooperation mode and policy related to drug innovation in China were deeply discussed as well.
KEY WORDS: Bioeconomy, Biomedicine, Drug development, Innovation, Research and development, Strategy and models
Graphical abstract
The enormous progress biotechnology, bioinformatics and nanotechnology made in recent years provides opportunities and scientific framework for development of biomedicine and constitutes a paradigm shift in pharmaceutical R&D and drug innovation. By analyzing the data and related information at R&D level over the past decades, developmental tendency and R&D patterns were summarized.
1. Introduction
Bioscience is now rapidly expanding in the 21st century. Advances in biology/biotechnology, bioinformatics and nanotechnology provide opportunities and a scientific framework for biomedicine, which can have significant impact on conventional research and development (R&D) and drug innovation, even a revolutionary change. The objective of this article is to further discuss the aforementioned areas by reflecting on the Session on R&D in Drug Innovation during the Bioeconomy 2013 conference in China along with lessons learned and future perspectives, and their implications for the growth of biomedicine in China1–20. Big Pharma׳s challenges which are becoming opportunities for biotech and startup companies include: (1) R&D spending is growing faster than sales growth, (2) drug discovery is lagging relative to industry growth needs, (3) increase presence of large molecules in big pharma׳s pipeline, (4) increasing need for in-licensing products and technologies, and (5) blockbuster drugs are going off patent. As a result of these changes, the number of joint ventures and collaborations between academia, government and industry has exponentially grown in recent years.
Pharmaceutical innovation has led to a decline in industry productivity. Despite the increased investment in R&D by the industry, the number of new molecular entities (NME) achieving marketing authorization is not increasing. Over the past 20 years, the number of Investigational new drugs (INDs) approved by regulatory agency did not increase as anticipated with enhanced quality control level and strict safety assessment as well as many molecular targets identified, while those drugs currently applied in clinical for long time have demonstrated their values, suggesting that high investment, development of technology and “-omics”, such as proteomics and genomics have not reduced the R&D risk effectively and enhance efficiency1,2,8.
In light of these scenarios, various strategies have been adapted in order to increase R&D efficiency and productivity8. At the drug discovery level, increased use of bioinformatics and computer modeling along with accelerated proof of concept studies and enhanced input from commercial and marketing have proved to be useful. The use of biomarkers and translational research in clinical trials is regarded as a powerful tool and used broadly in the pharmaceutical industry. Implementation of risk mitigation strategies and exploitation of outsourcing and strategic partnerships can further improve R&D efficiency and productivity.
2. Innovation trends in biomedicine
2.1. Conventional R&D pattern with high cost, high risk and low productivity is not sustainable
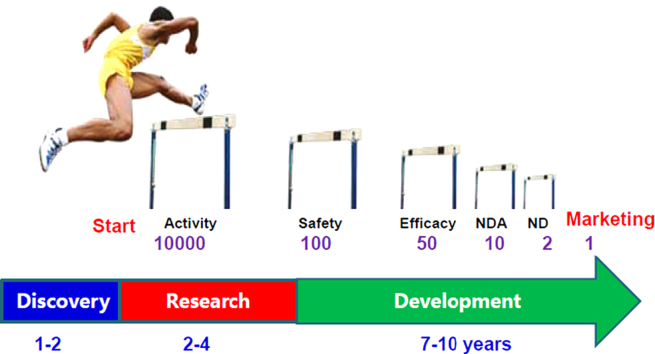
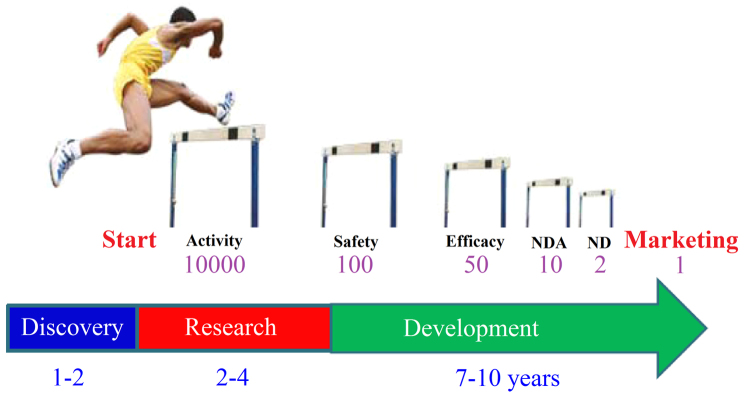
The conventional R&D pattern in drug innovation started in 1960s and it is always accompanied by high cost, high risk and low efficiency. By analyzing the ratio of the number of drugs approved for marketing by Federal Drug Administration (FDA) to that of active ingredients/molecules at the drug discovery stage, it remained at approximately 0.01% (Fig. 1) that is “one in ten-thousand” molecules make it to the market indicating the need for tremendous investment in R&D as a result of the extremely high potential of failure in the course of drug development. High rate of failure in drug development continued despite demands in high drug product quality and safety assessment along with technological advances.
Figure 1.
“One in ten-thousand” model for drug innovation of research and development.
The recently published data sourced from Bristol-Myer–Squibb and other 5 giant pharmaceutical companies revealed that of the 6 drug candidates that were terminated at phase III clinical trial in 2012, 4 were eliminated by efficacy and with the other 2 were safety issues. Efficacy and safety issues were considered as the main causes of failure at the stage of phase III. A molecule is not a drug, neither is active one and “druggability” is a key factor in the systematic process from molecule to drug, while translational research with “risk evaluation” is the decisive element. Unfortunately, this key problem has not been properly considered and addressed by multidimensional investigation through science, technology, policy, regulatory, ethnics and industry, analyzing bottleneck and other issues in common. However, a deep understanding of the issues using traditional models of drug development R&D and focusing on key challenges and opportunities is critical to building and adapting new innovative models of drug R&D.
2.2. Developing small molecule drug is still the mainstream approach
The nature of the pharmaceutical industry is such that the main driver for its growth is innovation21. Biomedicine R&D is becoming increasingly challenged due to lower productivity and thus pharmaceutical companies have opened their R&D organizations to external innovation14,15,20.
Fig. 2 compares new molecular entities and biological applications from 1997 to 2009. R&D spending was increased more than 3-fold since 1997 while NME approvals dropped by 44%. This trend is expected to continue given increased regulatory scrutiny; NME approvals decreased by 4.8% 1997–2009, while R&D spend increased by 10.7%; 45 NMEs and therapeutic biological applications (BLAs) were approved in 1997 which fell to 25 in 2009; in 2009, the industry spent a total of $125 billion (BN) R&D vs. $55BN in 1999, a 128% increase. Small molecule drugs are with obvious advantage. Based on the R&D level and progress made, new molecular entities will still be dominated in drug innovation for the next decade. Perspective of small/medium size enterprises via multichannel integration will be very promising20.
Figure 2.
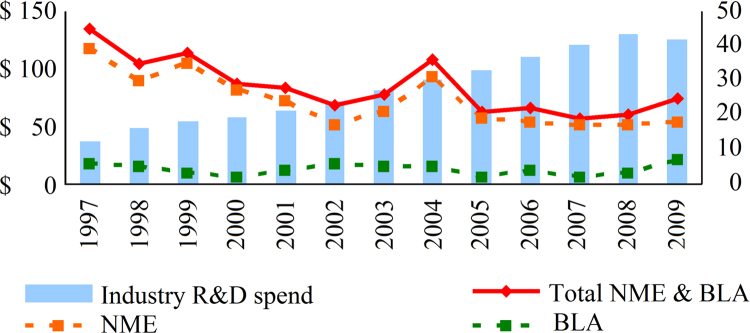
Comparison of new molecular entity and biological application since 1997 (Source from FDA websites, Evaluation Pharm).
In 2011, 35 new drugs were approved by the regulatory agency for marketing, based on long term efforts made. The number of new drugs approved is higher than the average level of approved drugs which is 20 per year. Of the 35 new drugs approved, 25 (71%) were new molecular entities, while new antibody therapeutics was only one (3%), and four kinase inhibitors for anticancer treatment (11%). Similar situation was observed in 2012 with 39 new drugs approved achieving new record for the last decade. The number of new drugs approved was 27 NMEs, 1 antibody and 7 kinase inhibitors, respectively, in terms of category. Since the first antibody therapeutic was developed in 1994, only 42 were approved for marketing up to date, averaging 2–3 new antibodies launched each year. Due to the quality, efficacy and safety issues, in particular the immunogenicity, as well as R&D challenges and heavy demands for biologics, the number of new biologic therapeutics remains low. In addition, treatment costs for government and the patients, as well as reimbursement burden for healthcare providers and medical insurance companies, have contributed to the low market share of biologic therapeutics. In developed countries, for instance in United States, the portion of generic drugs was over 80% in prescription, which limited the use of innovators׳ biotech medicine.
2.3. Small/medium size enterprises, key players in drug innovation
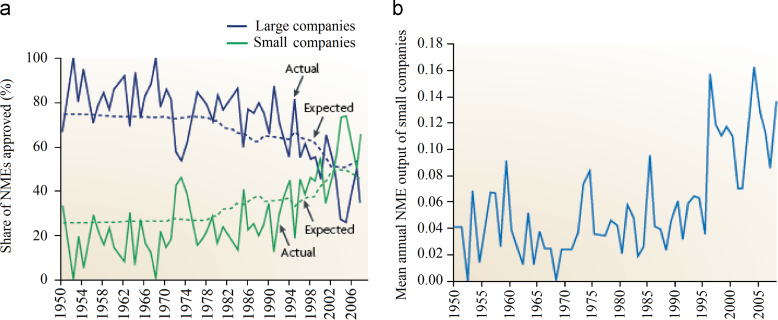
This analysis shows that the rate of production of new drugs by these companies has been constant since they began producing drugs with some variability in rates between companies. It is a fact that the cost per new molecular entity has been steadily increasing over the last decades. A puzzling trend in recent years, however, has been the gradual erosion in the share of innovation that is captured by NMEs discovered by large pharmaceutical companies. Since the early 1980s, their share of NMEs has declined from 75%, a level that had been constant since 1950, to 35% (Fig. 3a). At the same time, the share of NMEs that is attributable to small biotechnology and pharmaceutical companies has almost tripled, from 23% to nearly 70%. Since 2004, small companies have consistently matched or outperformed their larger competitors. The expected share of NMEs generally follows these trends until 2004, when they stabilized at about 50% each22.
Figure 3.
The mean annual new molecular entitie (NME) output of large and small companies. a, actual versus expected shares of NMEs for large and small pharmaceutical companies; b, mean annual NME output for small companies.
The increase in the NME output from small companies has been driven primarily by two factors. The first one is a rise in the number of small companies producing NMEs, which nearly doubled from 78 to 145 during the 1980s and 1990s. This was facilitated by the growth of venture capital that has funded much of the “biotech boom”. Second, the mean annual NME output of small companies has increased from 0.04 to 0.12 since 1995, owing to the emergence of new, more efficient companies (Fig. 3b). Conversely, the decline in the output of large companies has been driven by the dwindling number of large pharmaceutical companies, which has decreased by 50% over the past 20 years22.
By comparison of achievement in innovation, seven new drugs were developed by giant pharmaceutical companies in 2011, two products were co-developed. However, the number of new drugs through company merging and collaboration with small/medium size companies were up to 13. Similarly, the number of new drugs from sources mentioned above were 10, 1 and 8, respectively, in 2012. Apparently, small molecule drugs were still favored; also small/medium size companies played a key role in drug innovation.
Although the number of new drugs declined over the past years, the new drugs that giant pharmaceutical companies acquired from small/medium competitors were increasing (Fig. 4). In terms of the amount of new drugs at pre-clinical, phase I, II, III clinical trial stages, small/medium size companies have become major players in drug innovation, particularly with more drugs at phase II, III and on the market and which are originated from small/medium size companies. Their outputs filled the pipeline of giant companies with best-selling drugs14.
Figure 4.
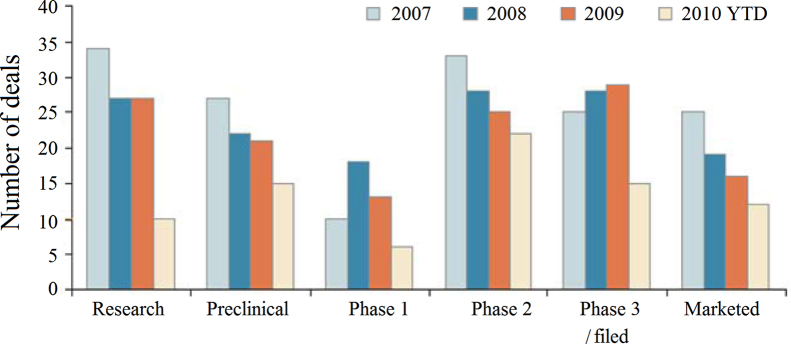
The contribution of small/medium size companies to pharmaceutical industry. (Sourced from Burrill & Co. (San Francisco). 2010 year to date (YTD) is through September 30.
In light of the open innovation spirit, American pharmaceutical companies are integrating into their drug development process the R&D of small/medium size companies, universities/research institutes, contract development and manufacturing organizations (CDMO) and other partners. As presented in Bioeconomy 2013, shortly after Pharmasset was merged with Gilead, an 11 billion United State dollars (USD) deal, the market value of Gilead boosted sharply from 37 to 83 billion USD15, as Sofosbuvir, the orally administrated/non-combined interferon, has been approved by FDA for marketing in 2013 for treatment of HCV. In addition, Zytiga (abiraterone acetate), the CYP17 inhibitor that is able to form non-reversible products to lower its activity by reducing the biosynthesis of androgenic hormone, was initially discovered by British Cancer Institute. The product was then transferred to Cougar Biotech for clinical trials, and eventually purchased by Johnson & Johnson at phase II in 2009 at 1 billion USD. After approval, the sale for first year was over 1 billion. There were also other successful cases in Japan, UK, Canada and Australia.
Sixty six of the 98 companies studied launched only one drug this decade. The costs absorbed by these companies can be taken as a rough estimate of what it takes to develop a single drug. The median cost per drug for these singletons was $350 million (Table 1), but for companies with more drugs approved, the cost per drug went up – until it hit $5.5 billion for companies that have brought to market between eight to 13 drugs over a decade23.
Table 1.
Relationship between number of drugs approved and R&D cost per drug*.
| Number of drugs approved | R&D cost per drug ($MIL) |
|
|---|---|---|
| Median | Mean | |
| 8–13 | 5459 | 5998 |
| 4–6 | 5151 | 5052 |
| 2–3 | 1803 | 2303 |
| 1 | 351 | 953 |
Sources: InnoThink Center for Research in Biomedical Innovation; FactSet Systems.
2.4. Development of new biological products and risk assessment
Emerging tumor-targeted medicines opened a new age of clinical application of pharmacogenomics. After the first targeted drug Mabthera was discovered, more products, for example, Glivec, Iressa, Tarceva, Erbitux, Avastin, Crizotinib, Zelboraf, Conmana were developed. Particularly, the product Conmana has strong impact on domestic pharmaceutical industry. Of 4 monoclonal antibody products co-developed by Huahai and OncoBiologics, three are targeted therapeutics. In 2013, Beida–Amgen launched a new project of marketing Vectibix.
Apart from anti-cancer medicines, FDA lists over 100 drugs and 40 biomarkers. In terms of importance of clinical tests, biomarkers were categorized into three levels: (1) mandatory, (2) high risk patient, and (3) recommended. Drugs and biomarkers related, for example, Clopidogrel (CYP2C19), warfarin (CYP2C9+VKOCR) for anti-coagulation, β receptor blockers (CYP2D6), statins: ApoE2, LDL receptor, SLOCR, the anti-infectious/HCV agents, interferon, IL28B gene, HLA gene of HIV and NAT gene of TB, Carbamazepine for psychiatric treatment, HLA-B1502 gene, and Ivacaftor (CFTR gene) are now required to be diagnosed through gene testing for the rational use of the corresponding drugs. Peripheral blood sample, tissues, DNA, SNP, recombinant gene, mRNA, as well as gene expression level are tested for in vitro diagnosis. Development of therapeutic and diagnostic agents is very helpful for enhancing efficacy and safety and, all types of synergies and collaborative efforts should be taken into consideration. Furthermore, emerging challenges, for example, patents on new molecular entities and biomarkers, clinical testing issues, registration of patients in clinical trials, recognition of medical practitioners, redefining the role of pharmacists, cooperation between pharmaceutical and diagnostic companies, and medical insurance/healthcare providers׳ issues, are all associated with science, technology, cooperation, policy and shared profits.
3. Reflecting on R&D in drug innovation
3.1. Higher productivity
The constraints resulting from short-term business decisions, and the harvesting of all “low-hanging fruit”, have been cited as the major causes for the decreased productivity and a change in the preclinical research culture is equally culpable. Current trends in biomedical research have led to a decreased emphasis on the null hypothesis/data-driven approach. A trend toward qualitative rather than quantitative science; an implicit assumption that all targets represent a viable starting point for drug discovery efforts24. Biomedical research efforts directed toward drug discovery, both in academia and industry should prioritize genuine innovation over technology and thus allow efforts in preclinical research to play a key role in the solution to the shortfall in new drug applications24. Pharmaceutical R&D is a very complex organization and requires integration of multiple disciplines, and special skill sets and talent to execute company׳s business strategy and timely achieve objectives. The head of R&D with broad technical skills and management experience is critical to the success of the organization8.
Currently, high cost and risk, as well as low efficiency are main bottlenecks in drug innovation. The strategies on how to enhance efficiency in R&D vary between different companies. The R&D process should be customized based on the position and formulated targets. For instance, the resource and experience can be accumulated through development of generic products. Although, “first-in-class” strategy in drug innovation will be the key approach to lead pharma innovation, “me better” is still the main long-term strategy for most of the domestic pharmaceutical companies. However, the “fast follow-on” pattern will become active in the next decade. Based on generic drugs, a giant pharmaceutical company driven by innovation and internationalization will emerge and expand in China soon.
3.2. Integration and rational allocation of resources
Technology innovation is not only the fundamental principle and engine of developing pharmaceuticals and the economy, but also the decisive factor in competition. We should pay more attention to the new thinking in developing new medicines3. For example, discovery of new drug targets in combination with application of genomics is now able to quickly identify and confirm the targets; development of new drugs with recognition of metabolic pathways and genetic differences can be more accurate and efficient; matching drug discovery technologies of drugs and genomics enhance the safety in clinical use; discovery of biomarker improves the accuracy of prediction; application of network pharmacology technology and virtual studies offer new approaches for high-throughput screening of new drugs. In particular, the gene mutant model on a large scale and relevant rules, recognition and studies mentioned in the forum can be beneficial to speed up the innovation process16,17. With the knowledge of new strategies in drug development, integration of various processes can significantly enhance R&D capacity and avoid waste.
Exploration of R&D patterns in drug innovation is the key issue and common to all companies. The pharma industry can rapidly develop through intrinsic growth and/or, peripheral expansion. Domestic and overseas companies should focus on the opportunities for innovative drug products. Cooperation with international healthcare industry investment foundations may be useful for obtaining global resources and funding.
Translational medicine plays a key role and also represents an integration strategy in drug development. In pre-clinical studies of targets and drug candidates, the large scale gene mutant animal model can be directly applied in drug screening to rapidly identify the drug targets. Beyond the good animal model practice, the principle of selection and confirmation may also help to improve the efficiency of translational research at early stage17. Application of translational research with “biology first” pattern into the drug development practice was illustrated in a case study, where productivity and outputs were improved by applying biomarker technology combined with clinical genomics9. Meanwhile, development of targeted therapy and genomics for personalized medicine has important impact in translating drug candidates into clinical treatment, safety and efficacy.
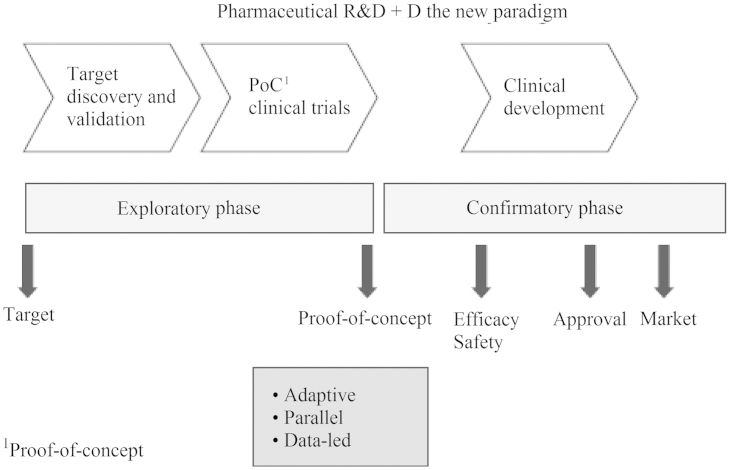
Novel approaches for clinical development and trial design could have a key role in overcoming some of these challenges by improving efficiency and reducing attrition rates25. During the exploratory phase of development, this new model uses all available knowledge and tools (Fig. 5), including biomarkers, modeling and simulation, as well as advanced statistical methodology. Trials are designed to determine proof-of-concept (POC) and to rigorously establish dose selection that will enhance the likelihood of success in the confirmatory phase. The modern designs, tools and knowledge are applied to larger scale studies with the goals of identifying the target patient population in which the drug is efficacious, establishing the benefit/risk ratio and confirming the optimal dose and dosing regimen. The innovative clinical trial designs such as adaptive or seamless studies compress timelines, improve dose and regimen selection, and reduce the number of patients assigned to non-viable dosing regimens.
Figure 5.
A new paradigm for clinical development25.
The scientific community believes that targeted therapy and genomics will offer great opportunities in personalized medicine for patients and the development of companion diagnostics with respect to the investigational drugs and those on market18.
Although, the implemented national project of drug innovation provided sufficient support for the research and development process by optimizing allocation of resources, encouraging innovation and setting policy guidelines, drug innovation remains challenging20. Question has been raised on how to perform integration of resources and rationalize the development pattern and strategy, that is suitable for the specific company. To establish advanced innovation systems for the pharmaceutical industry, there are still many challenges: (1) the capacity of R&D in drug innovation, the specialists and particularly the leading scientists, remain limited; and (2) with respect to industrialization and internationalization, regulatory systems established for the pharmaceutical/biomedical industry and relevant laws and regulations were incomplete.
3.3. Developmental strategy of molecular targeted medicines
Drug discovery must be driven not only by medical need and commercial potential, but also by the areas in which new science is creating therapeutic opportunities, such as target identification and the understanding of disease mechanisms26. Development of anti-cancer drugs has evolved from conventional drugs with cytotoxicity to molecular targeted medicine. Since Gleevec, the first small molecule targeted medicine, was approved for marketing in 1997, there are over 30 molecular targeted drugs on the market with respect to various types of signaling pathways. Molecular targeted anti-cancer drugs became a reality as multiple signaling molecules and pathways have been confirmed as useful targets for therapy. In the 21st century, the developmental strategy of anti-cancer drugs focuses on molecular targeted drugs with strong cytotoxicity. As previously mentioned, the discovery of drug targets provides opportunities for drug innovation, rational use of drugs, personalized medicine and reduction in the side effects.
Discovery and validation of new targets is the focus of global competition in research and development. The sustained breakthrough in screening assays and evaluation technologies is based on updated theories and technologies of genomics, proteomics, bioinformatics, system biology, modern detection technologies etc. The trend in the anti-cancer drug R&D is the design of multi-targets as well as the discovery of new targets. However, these opportunities are always accompanied with issues and challenges: (1) the features of the target, signaling pathways and interaction between the two might not be accurate, leading to uncertain efficacy; (2) broad spectrum of targeting and side effects are controversial, particularly the complicated toxicity caused by inmunogenicity of antibody drugs, for example the immuno-mediated hepatitis; and (3) to overcome acquired drug resistance, combination use of drugs has become the leading edge in the study of molecular targeted drugs20. Regardless of the development of antibody drugs or antibody–drug conjugates (ADC), the drug resistance issue has not yet been properly solved. Thus, study of targeted medicine and relevant molecular biology fundamentals should be strengthened. Recognition of the features of multiple targets and regulatory process for most solid tumors, as well as the current level of translational research cannot explain all clinical aspects. Based on biomarkers, personalized medicine will be the primary target of treatment. Development of molecular targeted medicine and companion diagnostics will have significant impact on clinical trials and treatment. The challenge, however, is how to identify the biomarker and accurately select signaling molecules for the rational use of a particular drug.
In recent years, nanotechnology has been increasingly applied in drug development throughout the drug development value chain27–29. The development and approval of nanoparticle-based therapeutics, has strengthen the dialogue between drug developers and regulatory agencies to accelerate the growth of this important field. Nanoparticle-based therapeutics can confer the ability to overcome biological barriers, effectively deliver drugs and biologics, and preferentially target sites of disease28,29. The complexity of nanoparticles as multi-component three dimensional constructs requires careful design and engineering, detailed characterization methods, and reproducible scale-up and manufacturing process to achieve a consistent product with the intended physicochemical characteristics, biological behavior, and pharmacological profile27–29. However, despite the potential advantages of nanoparticles, only a relatively small number of nanoparticle-based medicines have been approved and marketed for clinical use, while numerous challenges and hurdles exist with nanoparticulate drugs that are at different stages of development. The safety and efficacy of nanomedicines can be influenced by minor variations in multiple parameters and need to be carefully examined and controlled in preclinical and clinical studies, particularly in reference to their biodistribution, pharmacokinetics and potential toxicity27–29.
3.4. Developmental strategy of new drug delivery systems
As the investment into R&D sharply declined, many internal R&D organizations have been downsized or eliminated. Special consideration has been given to novel drug delivery systems (DDS) and it is estimated that the market size of novel drug delivery system has reached 153 billion USD in 2011 with an average annual growth rate of 16% over the past five years. Development of novel DDS is more practical than that of a new molecular entity, in terms of efficiency, cost/risk and timeline. For example, Rapamune®, Emend®, Tricor® 145, Megace® ES, Invega® Sustenna® nanocrystalline drug products developed by Elan through nanotechnology have been approved by FDA28. Several preparations of Shandong Green leaves pharmaceutical and products developed by other companies have been approved by either FDA or State Food and Drug Administration (SFDA) for clinical trials20.
During the past decade, the functions and roles of FDA in USA have evolved from “legislation, administration of justice” to “encourage science and innovation to ensure product quality, safety and efficacy”. Laws/regulations related to drug registration revealed that for drug safety and efficacy evaluation of raw materials, pharmaceutical excipients, new technologies and novel formulations can be beneficial. Most importantly, clinical assessment has become the key in drug product review. Several nano-drug products as discussed earlier have been approved for marketing and small/medium size companies may profit from novel formulations19. Development of novel DDS particularly those incorporating nanotechnology is limited by theory, technology, excipients and manufacturing process and equipment. Cooperation patterns in the nanotechnology field will be further explored and discussed at the global summit on scientific regulation 2013.
3.5. Developmental strategy of generic drugs
According to the national strategy and needs, all major pharmaceutical companies have seriously considered the development of generic drugs as to lower financial burden and medical expenses. The development and use of generic drugs is encouraged by regulatory agencies and relevant authorities. Top pharmaceutical companies that are focused on innovative drugs have joined forces with other companies for the development of generic drug. In addition, approximately 40% of patents owned by top 20 pharmaceutical companies are set to expire during 2009–2013.
Development of generic drugs is still dominating in China at this stage. However, combination of generic and innovative drugs has become the main approach in developing advanced generic products. The issue has been raised on how to identify new indications of currently used medicine, discover new drugs from failed drug candidates and new pathways of unsuccessful investigational drugs. Deep understanding and investigation of patents of innovative drugs, in particular their chemical entity, polymorphism, manufacture, intermediates, formulations and indications may be useful for patent innovation and protect the interests of products on markets. For example, generic products can be launched shortly after patent of innovative chemical entity expired, if patent of polymorphism can be thoroughly understood. Or new formulation or indications are developed in advance via technology innovation. Optimization of purity and safety of innovative products is another approach for domestic industry to get involved.
3.6. Developmental strategy of protein-based drugs
Up to date, Therapeutic biological applications approved by regulatory agency for marketing remained limited, since first antibody was launched in 1994. Protein-based drugs sourced from bio-tech and engineering, however, are undoubtedly a new class of revolutionary medicine, covering many scientific fields such as genetic engineering, recombinant protein technology, industrial fermentation, microbiology30. For example, antibody-based therapies succeeded in treatment of cancer. Thirteen monoclonal antibodies (mAbs) have been approved for clinical use in EU and US, as well as hundreds of mAbs, including bispecific mAbs and multispecific fusiong proteins in trials. Efforts have been made on humanizing the antibody protein and expanding of the target antigen repertoire. Antibody–drug conjugates (ADCs) and peptide–drug conjugates (PDCs) have been developed for delivering potent anti-cancer drugs. Deeper understanding of action of mechanism is still needed to overcome main issues limiting applications of biologically sourced medicine, including resistance to therapy, access to targets, complexity of biological systems, individual variation, cancer cell specificity, conjugation chemistry, tumor penetration, product heterogeneity and manufacturing31.
Additionally, absorption, distribution, metabolism and excretion properties (ADME) of ADCs in trials should be fully characterized to understand their safety and efficacy. Even the linker existing in ADCs may have significant impact on pharmacokinetic behavior, observed catabolites in tumor and liver tissues32,33. Also, physicochemical, biopharmaceutical and/or pharmacokinetic properties played a key role in developing novel protein-based drugs. Oral absorption and aqueous solubility improved, and increasing lipophilicity may enhance active transportation, as well as achieve site-selective delivery. For instance, drug-loaded liposomes are targeted to tissues/organs by active targeting, based on the attachment of specific ligands to the liposomal surface to bind certain antigen on the targeted cells, while antibody-targeted liposomes have demonstrated high potential for application34,35.
In addition to the issues addressed above, computational methods could be used as supplementary, based on crystal structures and/or homology models, including antibody–antigen docking and energy calculations, which might provide guidance for experimental studies to improve affinities and physicochemical properties as to accelerate the process of obtaining new protein-based drugs36.
4. Implications of R&D in drug innovation for the growth of biomedicine industry in china
4.1. R&D innovation is supported by interdisciplinary cooperation between government–academia and –industry
Development of innovative drugs (also known as new chemical/molecular entity) requires heavy investment, and is always accompanied with long timelines and high potential of failure; thus, it is not wise for domestic company to follow this approach as even top pharmaceutical companies cannot afford the cost. While generic products can fulfill the need in China, successful cases have already demonstrated that products with non-specific structure and uncertain mechanism of action can also benefit the patients. Furthermore, the best-in-class products are determined by marketing and sales, rather than the R&D process.
According to the current situation of science and technology, and developmental status of pharmaceutical industry in China, innovation is required to be supported by interdisciplinary collaboration by all participants. There are now several issues to be dealt with, such as: (1) How to make proper judgment on project quality, (2) appropriate risk evaluation and control, and (3) set up benchmarks and threshold for innovation.
4.2. Innovation is driven by national strategy and policy
In the opening ceremony of Bioeconomy conference 2013, the secretary of the department of science and technology mentioned that the portion of research papers in biology and medicine published during the period of 2003–2013 was over 50% of the growth rate of global bio/medicine industry and twice as that of GDP. In China, the impact of innovation in biotechnology and economic development, the society and people׳s daily lives has already emerged. Rapidly developed bioinformatics, life sciences, stem cells and bioprocesses have promoted the application of biotechnology in agricultural, medicine, energy and the environment. Special consideration has been given by central authority to develop biotechnology and relevant industries. Measures are taken to strength the study of life sciences and innovation in biotechnology. Plans, policy and regulations have been proposed: (1) under the guidance of <Planning of Development of Biology Industry> and <12th 5-Year-Plan on Development of Biotechnology, biotechnology is supported by resource integration, (2) establish internationally recognized mechanism of drug review to promote industrialization of novel medicine, as well as public service platform for technology incubation and transfer, and quality inspection in order to reduce the cost for small companies, (3) share of public/medical resources, for instance, set up effective mechanisms of public service of state key laboratory and national centers of engineering technology, as well as regulations of national resources in biology information, and (4) set up a team of highly skilled professionals, for instance establish joint training centers between industry and universities/research institutes, introduce internationally recognized specialists, identifying and pursuing opportunities for collaboration, and providing support for first-line scientists, particularly of young generation. It is anticipated that the increased presence of China Pharma/Biotech R&D will meet both local and global medical and market needs.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Changxiao Liu, Email: liuchangxiao@vip.163.com.
Panayiotis P. Constantinides, Email: ppconstantinides@bpddc.com.
References
- 1.Liu CX. Biomedical development strategy inventory: strategy and tactics looking back and forward [I]. China Science Daily, Biology Week, Jan 30, 2013.
- 2.Liu CX. Biomedical development strategy inventory: strategy and tactics looking back and forward [II]. China Science Daily, Biology Week, Feb 6. 2013.
- 3.Sang GW. Keynote lectures: the new drug R&D and the safety of biological drugs in China. Abstract: 2013 International bioeconomy conference; Jan 2, 2013.
- 4.Wang C. Key project of drug innovation and progress. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation. Tianjin, China; 2013
- 5.Szumski R. Sino-Canada vaccine program. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; 2013.
- 6.Chen ZN. Development of molecular medicine and future of translational research. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation. Tianjin, China; Jun 25, 2013. p. 41–4.
- 7.Zhang SH. Advance the abbvie pipeline. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation. Tianjin, China; Jun 25, 2013. p. 47–8.
- 8.Constantinides PP. Integrated drug discovery and development strategy and models. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation. Tianjin, China: Jun 25, 2013. p. 39–40.
- 9.Zhang MQ. Importance of translational medicine in drug discovery. Abstract: 2013 International bioeconomy conference, Session 2: R&D in drug innovation. Tianjin, China; Jun 25, 2013. p. 45–6.
- 10.Mahato R. Emerging trends in development of small molecules, siRNA and miRNA based therapeutics. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation. Tianjin, China; June 25, 2013. p. 49–51.
- 11.Varney M. Introduction to genetech research and early development and the translation of biological insights into useful cancer therapies. Abstract: 2013 International bioeconomy conference, Session 2: R&D in drug innovation. Tianjin, China; Jun 25, 2013. p. 52–3.
- 12.Cooper M. Asian century-entrepreneurs & science. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 61–2.
- 13.Smith M. Therapeutic innovation Australia: Queensland node – an Australian government initiative for accelerating life sciences research translation. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 63–4.
- 14.Tang LD. Drug discovery & development: strategies and management. Abstract: 2013 International Bioeconomy Conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 59–60.
- 15.He W. Small molecular drug innovation & development. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 67–9.
- 16.Xu T. Large-scale animal genetic screens for identifying therapeutic targets and drugs: a solution for the R&D problem of the pharmaceutical Industry. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 70–2.
- 17.Shi B. Rodent cancer model for drug development: regulation, consideration and selection. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 73–5.
- 18.Zhang ZY. Target therapy and personalized medicine. Abstract: 2013 International bioeconomy conference, session 2: R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 55–6.
- 19.Zhu DM. Nano medicine: the stars in the evening sky. Abstract: 2013 International bioeconomy conference, session 2:R&D in drug innovation, Tianjin, China; Jun 26, 2013. p. 76–9.
- 20.Liu CX. Rethinking on research and development in innovation of biomedicines. Drugs Clinic. 2013;28:469–475. [Google Scholar]
- 21.Schuhmacher A., Germann P.G., Trill H., Gassmann O. Models for open innovation in the pharmaceutical industry. Drug Discov Today. 2013;18:1133–1137. doi: 10.1016/j.drudis.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Munos Bernard. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- 23.Herper M. The cost of creating a new drug now $5 billion, pushing big pharma to change. Forbes pharma healthcare; August 11, 2013. Available From: 〈http://www.forbes.com/sites/matthewherper/2013/08/11/how-the-staggering-cost-of-inventing-new-drugs-is-shaping-the-future-of-medicine/〉.
- 24.Williams M. Productivity shortfalls in drug discovery: contributions from the preclinical sciences? J Pharmacol Exp Ther. 2011;336:3–8. doi: 10.1124/jpet.110.171751. [DOI] [PubMed] [Google Scholar]
- 25.Orloff J.J., Stanski D. Innovative approaches to clinical development and trial design. Ann Ist Super Sanita. 2011;47:8–13. doi: 10.4415/ANN_11_01_03. [DOI] [PubMed] [Google Scholar]
- 26.Agarwa P., Searls D.B. Can literature analysis identify innovation drivers in drug discovery? Nat Rev Drug Discov. 2009;8:865–878. doi: 10.1038/nrd2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantinides P.P. Advances in nanotechnology and commercialization perspectives. In: PillaiGopal K., TranHieu T,, editors. vol. 3. Center for Nanotechnology, Education, Research and Applications, Sullivan University, College of Pharmacy; Louisville, (KY, US): 2011. (Advances in nanotechnology and applications). [Google Scholar]
- 28.Haskell R., Constantinides P.P., Sun D. Perspectives in pharmaceutical nanotechnology. AAPS News Mag. 2012 [Google Scholar]
- 29.Mattheolabakis G., Rigas G., Constantinides P.P. Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomedicine. 2012;7:1577–1590. doi: 10.2217/nnm.12.128. [DOI] [PubMed] [Google Scholar]
- 30.Steinbery F.M., Raso J. Biotech pharmaceuticals and biotherapy: an overview. J Pharm Pharm Sci. 1998;1:48–59. [PubMed] [Google Scholar]
- 31.Adler M.J., Dimitrov D.S. Therapeutic antibodies against cancer. Hematol Oncol Clin North Am. 2012;26:447–481. doi: 10.1016/j.hoc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firer M.A., Gellerman G. Targeted drug delivery for cancer therapy: the other side of antibodies. J Hematol Oncol. 2012;9:70. doi: 10.1186/1756-8722-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson H.K., Lambert J.M. ADME of antibody-maytansinoid conjugates. AAPS J. 2012;14:799–805. doi: 10.1208/s12248-012-9386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zawilska J.B., Wojcieszak J., Olejniczak A.B. Prodrugs: a challenge for the drug development. Pharmcol Rep. 2013;65:1–14. doi: 10.1016/s1734-1140(13)70959-9. [DOI] [PubMed] [Google Scholar]
- 35.Sawant R.R., Torchilin V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14:303–315. doi: 10.1208/s12248-012-9330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuroda D., Shirai H., Jacobson M.P., Nakamura H. Computer-aided antibody design. Protein Eng Des Sel. 2012;25:507–521. doi: 10.1093/protein/gzs024. [DOI] [PMC free article] [PubMed] [Google Scholar]