Abstract
Melanoma is the most serious type of skin cancer and one of the most common cancers in the world. Advanced melanoma is often resistant to conventional therapies and has high potential for metastasis and low survival rates. Vemurafenib is a small molecule inhibitor of the BRAF serine-threonine kinase recently approved by the United States Food and Drug Administration to treat patients with metastatic and unresectable melanomas that carry an activating BRAF (V600E) mutation. Many clinical trials evaluating other therapeutic uses of vemurafenib are still ongoing. The ATP-binding cassette (ABC) transporters are membrane proteins with important physiological and pharmacological roles. Collectively, they transport and regulate levels of physiological substrates such as lipids, porphyrins and sterols. Some of them also remove xenobiotics and limit the oral bioavailability and distribution of many chemotherapeutics. The overexpression of three major ABC drug transporters is the most common mechanism for acquired resistance to anticancer drugs. In this review, we highlight some of the recent findings related to the effect of ABC drug transporters such as ABCB1 and ABCG2 on the oral bioavailability of vemurafenib, problems associated with treating melanoma brain metastases and the development of acquired resistance to vemurafenib in cancers harboring the BRAF (V600E) mutation.
KEY WORDS: ABC transporter, Drug resistance, Melanoma, P-glycoprotein, Vemurafenib
Abbreviations: ABC, ATP-binding cassette; AML, acute myeloid leukemia; BBB, blood–brain barrier; CNS, central nervous system; CSCs, cancer stem cells; GI, gastrointestinal; MAPK, mitogen-activated protein kinase; MDR, multidrug resistance; NBDs, nucleotide-binding domains; PFS, longer progression-free survival; PKIs, protein kinase inhibitors; TKIs, tyrosine kinase inhibitors; TMDs, transmembrane domains
Graphical abstract
This review highlights some of the recent findings related to the effect of ABC drug transporters such as ABCB1 and ABCG2 on the oral bioavailability of vemurafenib, problems associated with treating melanoma brain metastases and the development of acquired resistance to vemurafenib in cancers harboring the BRAF (V600E) mutation.
1. Introduction
Melanoma is the most serious type of skin cancer. It originates in pigment-producing melanocytes1. Melanoma has become one of the most common cancers in the world. Due to its high potential for metastasis, individuals with this disease have a poor prognosis and low survival rates2. Melanoma at advanced stages is often resistant to conventional radiation therapy and chemotherapy as a result of multiple mechanisms, including increased DNA repair and alterations of several key regulatory genes or proteins3,4. Therefore, therapeutic approaches directed at specific signaling pathways or mutations in melanoma have been employed5,6. One of the targets is the RAS-activated serine-threonine protein kinase B-raf (BRAF). It plays a central role in the regulation of the mitogen-activated protein kinase (MAPK) signaling pathway that regulates cell division, proliferation and differentiation in melanoma7,8. The consequence of mutations is the constitutive activation of the BRAF kinase and downstream MAPK signaling that promotes unregulated cell proliferation and cell invasion. In melanoma patients, the BRAF(V600E; valine to glutamate) substitution is the most common mutation9, which is associated with poor clinical outcome10 and brain metastases11. Since this mutation is found in approximately 40–60% of melanoma patients8, improved clinical outcome is expected for melanoma patients with inhibition of BRAF(V600E) signaling8–10.
2. Vemurafenib treatment for BRAF (V600E) mutation patients with advanced or metastatic melanoma
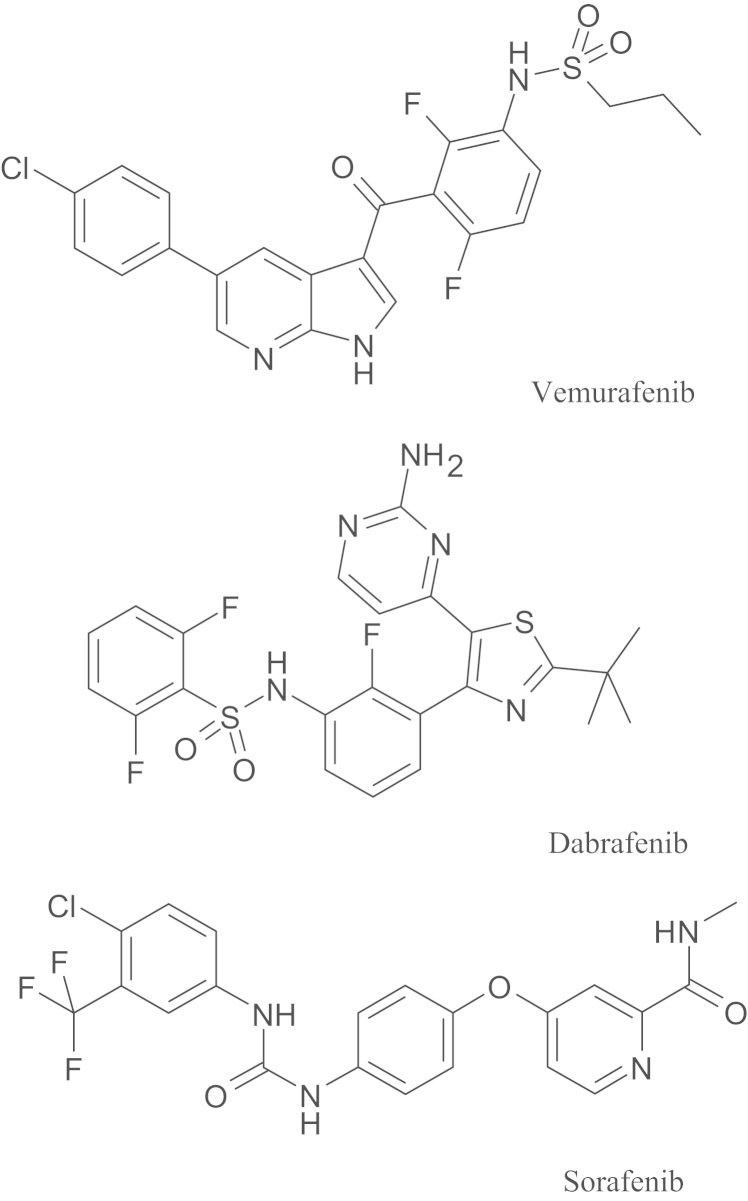
Vemurafenib (PLX4032, Zelboraf®) is a small molecule inhibitor of the cytoplasmic BRAF serine-threonine kinase (chemical structure given in Fig. 1), which in 2011 was approved by the US Food and Drug Administration (FDA) for treatment of metastatic and unresectable melanomas that carry an activating BRAF(V600E) mutation12–14. Moreover, in addition to treat unresectable BRAF(V600E) mutant melanomas12, studies on evaluating the effectiveness of vemurafenib in brain metastases of melanoma (ClinicalTrials.gov identifier NCT01378975), colorectal cancer15,16 (ClinicalTrials.gov identifier NCT00405587) and thyroid cancer17 (ClinicalTrials.gov identifier NCT01709292) are ongoing. Unfortunately, acquired drug resistance to vemurafenib and relapse among patients were reported frequently within months of therapy12,14. Identifying and overcoming mechanisms that lead to acquired clinical resistance to vemurafenib presents a significant therapeutic challenge18.
Figure 1.
Chemical structures of vemurafenib, dabrafenib and sorafenib.
3. The impact of ATP-binding cassette transporter-mediated drug transport on cancer chemotherapy
Generally, the success of cancer chemotherapy depends on several key factors. For an anticancer agent to be effective, a sufficient amount of the drug must be distributed to the target site(s), which is dependent on the chemical and biological properties of the therapeutic agent, as well as the location of the target site(s). Cancer cells can often acquire resistance through adaptation or spontaneous induction of certain key regulatory genes during the course of chemotherapy, which is dependent on the patient, cancer type, stage of the disease and treatment strategy4,19. Collectively, drug absorption, distribution and acquired resistance may result in poor response to chemotherapy and unfavorable patient outcome. Among various adverse factors in cancer chemotherapy, energy dependent drug efflux and drug compartmentalization are the most common ways that cancer cells evade drug absorption and drug penetration20,21.
Normally, the first line of cellular defense against xenobiotics is to rapidly reduce the intracellular concentration of xenobiotics by means of a transporter-mediated efflux system. Unfortunately, cancer cells can utilize the same protective mechanism by up-regulating some of the drug transporters that reduce drug sensitivity in patients, many of whom eventually relapse with multidrug-resistant forms of cancer19. One of the most common causes of acquired drug resistance in cancer is energy-dependent drug efflux by members of the human ATP-Binding Cassette (ABC) protein superfamily. Human ABC proteins are subdivided into seven families (ABCA-ABCG), based on structural and sequence similarities20. Several ABC proteins are transporters that can utilize energy derived from ATP to mediate direct drug efflux. These ABC transporters are membrane proteins, consisting of transmembrane domains (TMDs) and distinctive nucleotide-binding domains (NBDs). The TMDs form substrate-binding pockets, while the NBDs generate energy from ATP hydrolysis to actively transport a wide range of substrates, including anticancer agents, across biological membranes, reducing intracellular drug concentration and eventually resulting in multidrug resistance (MDR)22. ABCA9, ABCB1, ABCB5, ABCB8, ABCC2, ABCD1 and ABCG2 are some of the ABC proteins that have been identified in melanoma cells23–28. In this review, we focus mainly on the potential roles of ABCB1, ABCG2 and ABCB5 in limiting the absorption, distribution and penetration of vemurafenib, as well as in the development of resistance to this drug in cancer cells expressing a BRAF(V600E) mutation.
3.1. ABCB1
The 170 kDa cell membrane ABCB1 (also known as P-glycoprotein, P-gp) was the first member of the mammalian ABC protein family to be identified29. ABCB1 consists of two transmembrane domains, each containing six α-helices, both linked to ATP-binding domains that provide energy by hydrolyzing ATP to transport drug substrate across cell membranes. A large number of classical anticancer agents including taxanes, Vinca alkaloids, etoposide, teniposide, camptothecins, methotrexate, colchicines, actinomycin D, anthracyclines and mitoxantrone are well-known drug substrates of ABCB1. More importantly, many of the newly developed targeted therapy drugs such as tyrosine kinase inhibitors (TKIs), have been identified as substrates of ABCB1 as well30. ABCB1 is expressed in endothelial cells at the blood–brain barrier (BBB) sites in normal brain tissue and also in primary brain tumors, and it functions to limit penetration of the brain by many chemotherapeutics31,32. In addition, ABCB1 is highly expressed in many normal tissues, including those of the liver and intestinal walls, signifying the physiological and pharmacological importance of ABCB120. Moreover, ABCB1 is known to be overexpressed in many types of cancer and is linked to the MDR phenotype33. Considering the wide tissue distribution and substrate specificity of ABCB1, it is not surprising that ABCB1 plays a key role in limiting the oral bioavailability of anticancer drugs, preventing drug distribution and penetration through the blood–brain barrier and affecting therapeutic outcome in patients19. In terms of melanomas, endogenous ABCB1 mRNA has been detected in the melanoma cell lines SK-MEL-28, SK-MEL-5 and M1623,34, as well as non-cutaneous melanomas35,36. ABCB1 was also detected in a subpopulation of human melanoma cells that co-express ABCB5, hTERT, and Nanog, and has high self-renewal capacity, representing characteristics of melanoma stem cells37. Interestingly, though the MDR phenotype has been shown in human BRO melanoma cells transfected with human ABCB138, the relevance of endogenous ABCB1 in conferring drug resistance in melanomas has not been demonstrated yet.
3.2. ABCG2
ABCG2 (also known as breast cancer resistance protein, BCRP; or placenta-specific ABC transporter, ABCP; or mitoxantrone resistance protein, MXR) was identified in 199839,40. In contrast to ABCB1, ABCG2 consists of a single ATP-binding domain followed by a transmembrane domain with six α-helices in a reverse orientation41. A functional unit of ABCG2 is a dimer or a multimer. Similar to ABCB1, ABCG2 is overexpressed in many cancers, and is linked to reduced drug accumulation and to the development of MDR in patients with advanced non-small cell lung cancer or acute myeloid leukemia (AML)42,43. ABCG2 is capable of transporting a large variety of anticancer agents such as etoposide, docetaxel, topotecan, CPT-11, SN-38, methotrexate, flavopiridol, anthracyclines, mitoxantrone, and similar to ABCB1, many tyrosine kinase inhibitors including imatinib, nilotinib, saracatinib and ponatinib30,44,45. ABCG2 also has a physiological and pharmacological impact on drug bioavailability, drug distribution, protection of cells or tissues from xenobiotics and the transport of porphyrins and sterols33. Similar to ABCB1, ABCG2 has been detected at the luminal membrane of brain capillaries and the BBB, protecting the brain from xenobiotics and chemotherapeutics46,47. Studies have shown that both the protein expression and function of ABCG2 are up-regulated in neuro-epithelial tumors, restricting penetration of chemotherapeutics and leading to the development of MDR48,49. ABCG2 is believed to play a protective role in cancer stem cells (CSCs) or “side population” cells, with self-renewal properties and critical roles in tumorigenesis, metastasis and relapse50. Since ABCG2 is expressed in a wide range of human stem cells, it is considered as a biomarker for stem cells. ABCG2, along with CD133 and nestin, have been detected in melanomas25,51–53, but the potential contribution of ABCG2 to chemoresistance in melanomas remains to be determined. Recently, ABCG2 has been linked to the disease gout, as mutations (for example Q141K) in this transporter result in decreased efflux of urate from kidney epithelial cells54,55.
3.3. ABCB5
ABCB5 is predominantly expressed in pigment-producing (melanogenic) melanoma cells23,24. The melanogenesis-related vesicles, called “melanosomes” are derived from lysosomes and represent a unique feature of melanomas56,57. Structurally, ABCB5 has 73% sequence homology with ABCB1 protein24,26. In contrast to ABCB1, which mediates drug efflux from cells, ABCB5 is thought to confer chemoresistance to cisplatin, doxorubicin and daunorubicin by intracellular drug sequestration16,24,57,58. Furthermore, studies have reported that ABCB5 protein expression is up-regulated upon exposure to the chemotherapeutic drugs dacarbazine (DTIC) and doxorubicin28,59. Both ABCB1 and ABCG2 are known to be present in cancer stem cells, and hence are used as stem cell markers60. Similar cancer stem cell properties were discovered in metastatic melanoma cells, in which ABCB5 was present61. These ABCB5-positive melanoma stem cells are not only drug-resistant, but also possess self-renewal, differentiation and tumorgenic capabilities58,62. Interestingly, a recent study showed that ABCB5-expressing cells are resistant to temozolomide, dacarbazine and vemurafenib, suggesting that ABCB5 may contribute to the drug resistance mechanism, and thus is a potential therapeutic target for melanoma chemotherapy28. However, ABCB5-mediated transport of these drugs in melanoma patient samples has not yet been demonstrated.
4. The pharmacological impact of ABC drug transporters on the bioavailability and distribution of vemurafenib
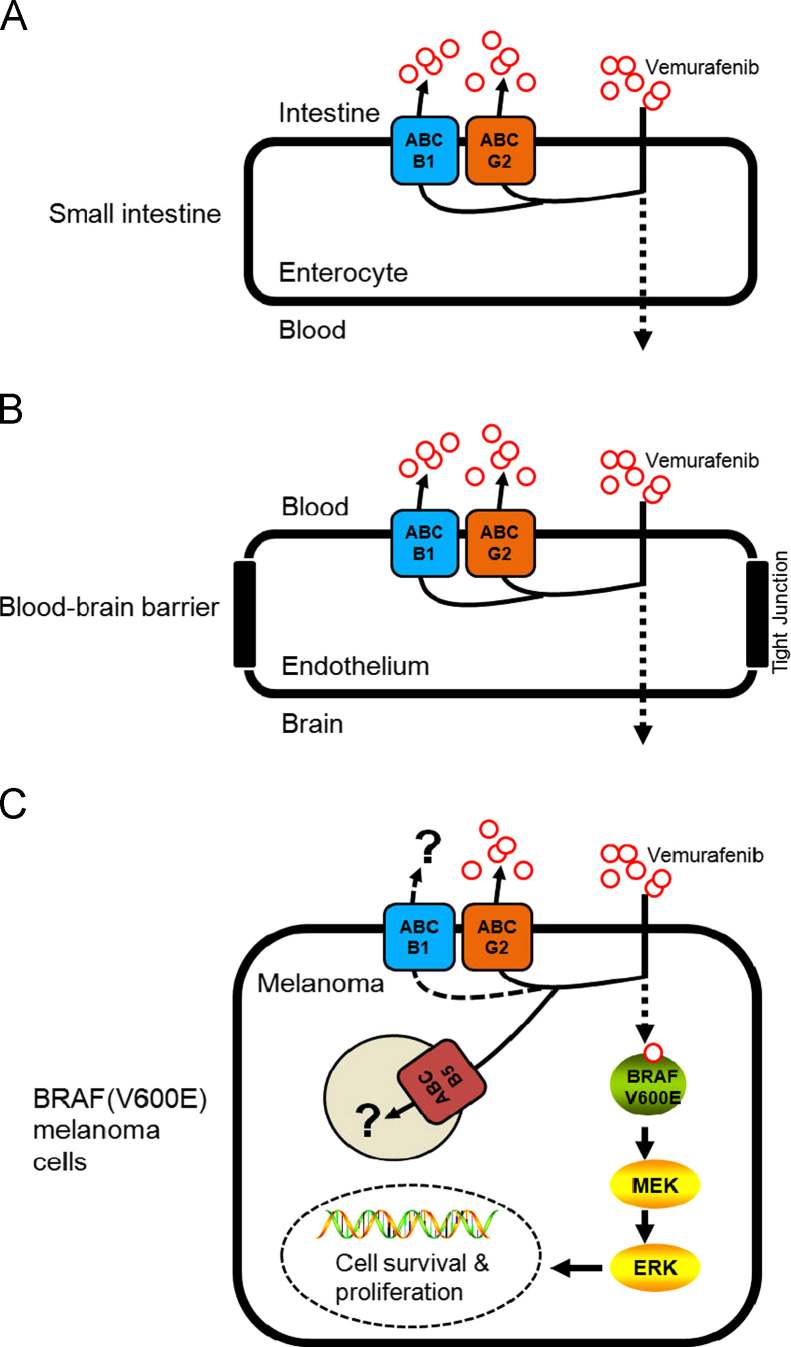
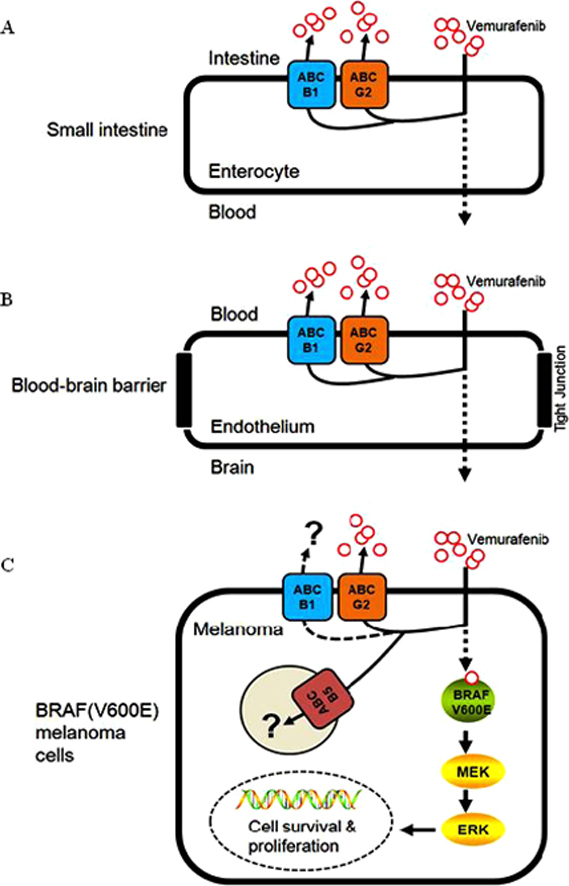
Reports have shown a high incidence of melanoma metastases in the brain63,64. Prior to the discovery of vemurafenib, a patient's response to the standard therapy of interleukin-2 and dacarbazine was extremely poor14,65. However, in order for vemurafenib to be effective against brain metastases of melanoma, sufficient amounts of vemurafenib must first be absorbed in the gastrointestinal (GI) tract (Fig. 2A), be distributed, and also penetrate the BBB and accumulate in the brain (Fig. 2B). The vasculature structure of the BBB consists of tightly sealed tight-junction protein complexes combined with overexpression of several ABC transporters that actively transport chemotherapeutics back into the bloodstream (Fig. 2B), making drug penetration of the brain a major obstacle in chemotherapy66.
Figure 2.
The potential role of multidrug resistance-associated ABC drug transporters in the oral bioavailability, brain penetration and therapeutic efficacy of vemurafenib in melanoma and other cancer cells harboring V600E mutation in BRAF kinase. (A) Highly active ABCB1 and ABCG2 transporters in intestinal epithelial cells can significantly limit the absorption of vemurafenib into the blood stream, reducing its bioavailability. (B) The presence of both ABCB1 and ABCG2 at the blood–brain barrier restricts vemurafenib penetration of the brain, reducing its effectiveness in patients with brain metastatic melanoma. (C) The presence of ABCG2 confers resistance to vemurafenib in BRAF(V600E) mutant A375 melanoma cells. The role of the ABCB5 transporter in melanoma remains to be evaluated.
A recent study by Mohammed et al.67 reported that the delivery of vemurafenib to the brain is restricted due to its direct transport by human ABCB1 and mouse Abcg2 at the blood–brain barrier. In their in vitro experiments, the intracellular accumulation of vemurafenib was reduced in MDCKII cells transfected with ABCB1 or ABCG2, as a direct result of ABCB1 and ABCG2-mediated transport of vemurafenib. Moreover, the ABCB1 and ABCG2-mediated transport of vemurafenib can be inhibited by zosuquidar and Ko143, respectively. Furthermore, in their knockout mouse model, the brain-to-plasma ratios of vemurafenib were increased significantly when Abc1a/1b and Abcg2 were both absent. The authors concluded that vemurafenib is a substrate of both ABCB1 and ABCG2, and both transporters play a significant role in limiting the central nervous system (CNS) distribution of vemurafenib. The findings by Mohammed et al. were later supported by an independent group. Durmus et al.68 reported that inhibition of both ABCB1 and ABCG2 could significantly improve the bioavailability (Fig. 2A) and brain penetration (Fig. 2B) of vemurafenib. In their in vitro experiments, vemurafenib transport mediated by either ABCB1 or ABCG2 was demonstrated by using MDCK II cells transduced with either human ABCB1 or ABCG2. The ABCB1- and ABCG2-mediated transport of vemurafenib was inhibited completely by the ABCB1 inhibitor zosuquidar and the ABCG2 inhibitor Ko14368. In vivo, the dual Abcb1a/1b and Abcg2 inhibitor elacridar significantly elevated the plasma levels of vemurafenib and brain accumulation in WT mice to the same levels as in Abc1a/1b−/−; Abcg2−/− mice. Interestingly, Durmus et al.68 found that Abcg2 is responsible for reducing the intestinal uptake of vemurafenib, but limited to a lower oral dose. In contrast, Abcb1a/1b is accountable for reducing plasma levels of vemurafenib at later stages. This particular observation is in accordance with findings by Chapman et al.14, that in BRAF(V600E) mutant A375 melanoma cells, ABCG2 behaves as a high-affinity but low capacity transporter of vemurafenib.
5. The potential impact of ABC drug transporters on vemurafenib-based treatment of advanced or metastatic melanoma
Initial success at using vemurafenib to treat patients with metastatic and unresectable melanomas or other cancers that carry an activating BRAF(V600E) mutation was short lived. The rapid development of acquired resistance to vemurafenib is now becoming a major obstacle in the treatment of patients diagnosed with BRAF(V600E)-positive cancer12,14. Multiple mechanisms involving the reactivation of the mitogen-activated protein kinase (MAPK) pathway have been reported in vemurafenib-resistant BRAF(V600E) mutant cancer cells. Up-regulation of CRAF69,70 and overexpression of Tpl2/COT69, RAS activation38,71, enhanced activation of the FGFR3/RAS pathway72, pathways that lead to reactivation of ERK signaling73 and activation of RTK signaling pathways such as IGF-1R or PDGFRβ25,71,74 have all been shown to contribute to acquired resistance to vemurafenib, depending on the cancer type17,26.
Recently, we have discovered that in addition to a RAF isoform switch and activation of various compensatory survival pathways25,38,69–74, the overexpression of ABCG2 could also contribute to the development of acquired resistance to vemurafenib in BRAF(V600E) mutant cancer cells (Fig. 2C)6. This is not surprising since the overexpression of ABC transporters is one of the most common mechanisms of acquired resistance to anticancer agents33. In our study, the interactions of vemurafenib with three major MDR-associated ABC drug transporters, ABCB1, ABCC1 and ABCG2 were investigated. Results showed that vemurafenib binds directly to the substrate binding pockets of ABCG2, inhibits its function and stimulates ATP hydrolysis. Similar interactions between vemurafenib and ABCB1 were observed, but the binding affinity and the stimulation of ATP hydrolysis were significantly lower. We found that since vemurafenib binds to the drug binding site of human ABCG2 with relatively high affinity, it effectively inhibited ABCG2-mediated transport of other drug substrates. Moreover, at non-toxic concentrations, vemurafenib was able to restore chemosensitivity of ABCG2-overexpressing HEK293 cells to anticancer agents such as mitoxantrone and topotecan. Similarly, vemurafenib also restored the sensitivity of drug-resistant ABCG2-overexpressing and also expressing (V600E) mutant BRAF A375 melanoma cells to mitoxantrone6. In contrast, no interaction was detected between vemurafenib and ABCC1 protein. Moreover, 72 h of vemurafenib treatment had no significant effect on the expression of ABCB1, ABCC1 or ABCG2 protein in cancer cells expressing wild-type BRAF. Surprisingly, while overexpression of human ABCG2 had no effect on the chemosensitivity of wild-type BRAF cancer cells to vemurafenib, the ectopic expression of human ABCG2 led to vemurafenib resistance in A375 melanoma cells harboring the BRAF(V600E) mutation. We found that in A375 melanoma cells, BRAF kinase inhibition by vemurafenib was significantly reduced in the presence of functional ABCG2, implicating ABCG2-mediated efflux as a mechanism of resistance for vemurafenib6. Unfortunately, it is still unknown whether prolonged treatment with vemurafenib leads to overexpression of ABC drug transporters in BRAF(V600E) melanoma, thyroid or colorectal cancers. Furthermore, the potential impact of ABCB1 or ABCB5 or other MDR-associated ABC drug transporters on the therapeutic outcome using vemurafenib in melanomas or other cancers harboring the BRAF(V600E) mutation needs to be determined.
6. Impact of ABC drug transporters on treatment with other BRAF inhibitors (dabrafenib and sorafenib)
Dabrafenib (GSK2118436) is a new BRAF inhibitor (Fig. 1) designed to target melanomas expressing V600E and V600K mutant BRAF. Good clinical response rates have been observed in metastatic melanoma (including brain metastases) patients receiving dabrafenib75,76, but cases of acquired resistance to dabrafenib have also been reported77–79. Although the link between ABC drug transporters and acquired resistance to dabrafenib is still lacking, a recent study using MDCKII cells indicated that dabrafenib is a substrate of both ABCB1 and ABCG280. Moreover, Mittapalli et al.80 showed that in both in vivo and intact BBB models, the dabrafenib brain distribution is limited by the function of both ABCB1 and ABCG2. In contrast to vemurafenib and dabrafenib, sorafenib is a nonselective BRAF inhibitor (Fig. 1) that targets both BRAF and CRAF, and inhibits other multiple kinases81. A phase I/II clinical trial reported that in metastatic melanoma patients, combination therapy of sorafenib, carboplatin and paclitaxel demonstrated a better response rate and longer progression-free survival than with standard chemotherapy82. Like vemurafenib and dabrafenib, the interactions between sorafenib, ABCB1 and ABCG2 have been demonstrated by several independent groups. Other studies have reported that sorafenib is transported by both ABCB183,84 and ABCG2, but more efficiently by ABCG284, and consistent with these findings the penetration of the brain by sorafenib was significantly higher in Abcg2−/− mice than in WT84,85.
7. Conclusions
Collectively, the actions of ABCB1 and ABCG2 in the GI tract and at the BBB contribute significantly to reduced oral bioavailability and limit the penetration of the brain by vemurafenib (Fig. 2A and B), which is a major obstacle when treating patients with melanoma brain metastases. The clinical application of a dual ABCB1 and ABCG2 inhibitor such as elacridar could possibly provide a solution to increase the oral bioavailability and enhance brain penetration of vemurafenib in patients with brain metastatic melanoma86. At the cellular level, the presence of MDR-associated ABC drug transporters may present new therapeutic challenges when treating cancers expressing the V600E mutant version of BRAF kinase. The ability of ABC drug transporters to effectively reduce the intracellular concentration of vemurafenib in cancer cells can potentially lead to acquired resistance to this drug (Fig. 2C). Moreover, the reported high affinity of vemurafenib for binding to ABCG2 suggests the potential use of vemurafenib as a chemosensitizer that would work alongside classical anticancer agents to treat ABCG2-positive MDR cancers. Consistent with these findings, vemurafenib was found to dock in the drug-binding pocket of the homology model of human ABCB1 and ABCG2 and also modulate the function of the ABCC10 (MRP 7) transporter87. Thus, we propose that simultaneous administration of vemurafenib and protein kinase inhibitors targeting key signaling pathways that are involved in the development of acquired resistance to vemurafenib25,38,69–74, as well as inhibiting the actions of ABC drug transporters in BRAF(V600E) mutant cancers33, may offer great promise for effective treatment of melanoma patients.
Acknowledgments
CP Wu was supported by funds from National Science Council of Taiwan (Grant No. NSC102-2320-B-182-036). S.V. Ambudkar was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. National Cancer Institute, NIH, Center for Cancer Research. We thank George Leiman for editorial assistance.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Shukla S., Skoumbourdis A.P., Walsh M.J., Hartz A.M., Fung K.L., Wu C.P. Synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna (nilotinib): evidence for transport of Tasigna and its fluorescent derivative by ABC drug transporters. Mol Pharm. 2011;8:1292–1302. doi: 10.1021/mp2001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch C.M., Gershenwald J.E., Soong S.J., Thompson J.F., Atkins M.B., Byrd D.R. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin L., Garraway L.A., Fisher D.E. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 4.Soengas M.S., Lowe S.W. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 5.Tsao H., Atkins M.B., Sober A.J. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf A., Bauer B., Hartz A.M. ABC transporters and the Alzheimer's disease enigma. Front Psychiatry. 2012;3:54. doi: 10.3389/fpsyt.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan P.T., Garnett M.J., Roe S.M., Lee S., Niculescu-Duvaz D., Good V.M. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Hartz A.M., Miller D.S., Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arkenau H.T., Kefford R., Long G.V. Targeting BRAF for patients with melanoma. Br J Cancer. 2011;104:392–398. doi: 10.1038/sj.bjc.6606030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berghoff A.S., Capper D., Preusser M. Lack of BRAF V600E protein expression in primary central nervous system lymphoma. Appl Immunohistochem Mol Morphol. 2013;21:351–353. doi: 10.1097/PAI.0b013e3182688e59. [DOI] [PubMed] [Google Scholar]
- 12.Bollag G., Hirth P., Tsai J., Zhang J., Ibrahim P.N., Cho H. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facchetti F., Monzani E., La Porta C.A. New perspectives in the treatment of melanoma: anti-angiogenic and anti-lymphangiogenic strategies. Recent Pat Anticancer Drug Discov. 2007;2:73–78. doi: 10.2174/157489207779561390. [DOI] [PubMed] [Google Scholar]
- 14.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartz A.M., Bauer B. ABC transporters in the CNS–an inventory. Curr Pharm Biotechnol. 2011;12:656–673. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- 16.Cheung P.F., Cheng C.K., Wong N.C., Ho J.C., Yip C.W., Lui V.C. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PLoS One. 2011;6:e28246. doi: 10.1371/journal.pone.0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouzaud F., Costin G.E., Yamaguchi Y., Valencia J.C., Berens W.F., Chen K.G. Regulation of constitutive and UVR-induced skin pigmentation by melanocortin 1 receptor isoforms. FASEB J. 2006;20:1927–1929. doi: 10.1096/fj.06-5922fje. [DOI] [PubMed] [Google Scholar]
- 18.Das Thakur M., Salangsang F., Landman A.S., Sellers W.R., Pryer N.K., Levesque M.P. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esiobu N., Green M., Echeverry A., Bonilla T.D., Stinson C.M., Hartz A. High numbers of Staphylococcus aureus at three bathing beaches in South Florida. Int J Environ Health Res. 2013;23:46–57. doi: 10.1080/09603123.2012.699027. [DOI] [PubMed] [Google Scholar]
- 20.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Naї Rev. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 21.Calcagno A.M., Salcido C.D., Gillet J.P., Wu C.P., Fostel J.M., Mumau M.D. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Nat Cancer Inst. 2010;102:1637–1652. doi: 10.1093/jnci/djq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins C.F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 23.Szakacs G., Annereau J.P., Lababidi S., Shankavaram U., Arciello A., Bussey K.J. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–137. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Chen K.G., Szakacs G., Annereau J.P., Rouzaud F., Liang X.J., Valencia J.C. Principal expression of two mRNA isoforms (ABCB 5alpha and ABCB 5beta ) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res. 2005;18:102–112. doi: 10.1111/j.1600-0749.2005.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facchetti F., Monzani E., Cavallini G., Bergamini E., La Porta C.A. Effect of a caloric restriction regimen on the angiogenic capacity of aorta and on the expression of endothelin-1 during ageing. Exp Gerontol. 2007;42:662–667. doi: 10.1016/j.exger.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Frank N.Y., Pendse S.S., Lapchak P.H., Margaryan A., Shlain D., Doeing C. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 27.Elliott A.M., Al-Hajj M.A. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol Cancer Res. 2009;7:79–87. doi: 10.1158/1541-7786.MCR-08-0235. [DOI] [PubMed] [Google Scholar]
- 28.Chartrain M., Riond J., Stennevin A., Vandenberghe I., Gomes B., Lamant L. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012;7:e36762. doi: 10.1371/journal.pone.0036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 30.Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat Rev. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 31.Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989;37:159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 32.Albino A.P., Nanus D.M., Mentle I.R., Cordon-Cardo C., McNutt N.S., Bressler J. Analysis of ras oncogenes in malignant melanoma and precursor lesions: correlation of point mutations with differentiation phenotype. Oncogene. 1989;4:1363–1374. [PubMed] [Google Scholar]
- 33.Rouzaud F., Hearing V.J. Regulatory elements of the melanocortin 1 receptor. Peptides. 2005;26:1858–1870. doi: 10.1016/j.peptides.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 34.Maellaro E., Pacenti L., Del Bello B., Valentini M.A., Mangiavacchi P., de Felice C. Different effects of interferon-alpha on melanoma cell lines: a study on telomerase reverse transcriptase, telomerase activity and apoptosis. Br J Dermatol. 2003;148:1115–1124. doi: 10.1046/j.1365-2133.2003.05301.x. [DOI] [PubMed] [Google Scholar]
- 35.Dunne B.M., McNamara M., Clynes M., Shering S.G., Larkin A.M., Moran E. MDR1 expression is associated with adverse survival in melanoma of the uveal tract. Hum Pathol. 1998;29:594–598. doi: 10.1016/s0046-8177(98)80008-7. [DOI] [PubMed] [Google Scholar]
- 36.McNamara M., Clynes M., Dunne B., NicAmhlaoibh R., Lee W.R., Barnes C. Multidrug resistance in ocular melanoma. Br J Ophthalmol. 1996;80:1009–1012. doi: 10.1136/bjo.80.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keshet G.I., Goldstein I., Itzhaki O., Cesarkas K., Shenhav L., Yakirevitch A. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368:930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Lincke C.R., van der Bliek A.M., Schuurhuis G.J., van der Velde-Koerts T., Smit J.J., Borst P. Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res. 1990;50:1779–1785. [PubMed] [Google Scholar]
- 39.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allikmets R., Schriml L.M., Hutchinson A., Romano-Spica V., Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 41.McDevitt C.A., Collins R.F., Conway M., Modok S., Storm J., Kerr I.D. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Yoh K., Ishii G., Yokose T., Minegishi Y., Tsuta K., Goto K. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 43.Wu C.P., Hsieh C.H., Wu Y.S. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 44.Dohse M., Scharenberg C., Shukla S., Robey R.W., Volkmann T., Deeken J.F. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38:1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai C.L., Tiwari A.K., Wu C.P., Su X.D., Wang S.R., Liu D.G. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hori S., Ohtsuki S., Hosoya K., Nakashima E., Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 47.Cooray H.C., Blackmore C.G., Maskell L., Barrand M.A. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 48.Bleau A.M., Hambardzumyan D., Ozawa T., Fomchenko E.I., Huse J.T., Brennan C.W. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ginguene C., Champier J., Maallem S., Strazielle N., Jouvet A., Fevre-Montange M. P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) localize in the microvessels forming the blood-tumor barrier in ependymomas. Brain Pathol. 2010;20:926–935. doi: 10.1111/j.1750-3639.2010.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 51.Dou J., Pan M., Wen P., Li Y., Tang Q., Chu L. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–472. [PubMed] [Google Scholar]
- 52.Grichnik J.M., Burch J.A., Schulteis R.D., Shan S., Liu J., Darrow T.L. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142–153. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- 53.Klein W.M., Wu B.P., Zhao S., Wu H., Klein-Szanto A.J., Tahan S.R. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 54.Woodward O.M., Tukaye D.N., Cui J., Greenwell P., Constantoulakis L.M., Parker B.S. Gout-causing Q141K mutation in ABCG2 leads to instability of the nucleotide-binding domain and can be corrected with small molecules. Proc Natl Acad Sci USA. 2013;110:5223–5228. doi: 10.1073/pnas.1214530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuo H., Takada T., Ichida K., Nakamura T., Nakayama A., Ikebuchi Y. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 56.Berens W., van den Bossche K., Yoon T.J., Westbroek W., Valencia J.C., Out C.J. Different approaches for assaying melanosome transfer. Pigment Cell Res. 2005;18:370–381. doi: 10.1111/j.1600-0749.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen K.G., Valencia J.C., Lai B., Zhang G., Paterson J.K., Rouzaud F. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA. 2006;103:9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank N.Y., Margaryan A., Huang Y., Schatton T., Waaga-Gasser A.M., Gasser M. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 59.Johannessen C.M., Boehm J.S., Kim S.Y., Thomas S.R., Wardwell L., Johnson L.A. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Grouw E.P., Raaijmakers M.H., Boezeman J.B., van der Reijden B.A., van de Locht L.T., de Witte T.J. Preferential expression of a high number of ATP binding cassette transporters in both normal and leukemic CD34+CD38- cells. Leukemia. 2006;20:750–754. doi: 10.1038/sj.leu.2404131. [DOI] [PubMed] [Google Scholar]
- 61.Sigalotti L., Covre A., Zabierowski S., Himes B., Colizzi F., Natali P.G. Cancer testis antigens in human melanoma stem cells: expression, distribution, and methylation status. J Cell Physiol. 2008;215:287–291. doi: 10.1002/jcp.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schatton T., Murphy G.F., Frank N.Y., Yamaura K., Waaga-Gasser A.M., Gasser M. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson J.D., Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344. [PubMed] [Google Scholar]
- 64.Fife K.M., Colman M.H., Stevens G.N., Firth I.C., Moon D., Shannon K.F. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 65.Atkins M.B., Lotze M.T., Dutcher J.P., Fisher R.I., Weiss G., Margolin K. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 66.Chanda P., Yuhki N., Li M., Bader J.S., Hartz A., Boerwinkle E. Comprehensive evaluation of imputation performance in African Americans. J Hum Genet. 2012;57:411–421. doi: 10.1038/jhg.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammed R.L., Echeverry A., Stinson C.M., Green M., Bonilla T.D., Hartz A. Survival trends of Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium perfringens in a sandy South Florida beach. Mar Pollut Bull. 2012;64:1201–1209. doi: 10.1016/j.marpolbul.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Durmus S., Sparidans R.W., Wagenaar E., Beijnen J.H., Schinkel A.H. Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm. 2012;9:3236–3245. doi: 10.1021/mp3003144. [DOI] [PubMed] [Google Scholar]
- 69.Hartz A.J., Sherr B.F., Sherr E.B. Photoresponse in the heterotrophic marine dinoflagellate Oxyrrhis marina. J Eukaryot Microbiol. 2011;58:171–177. doi: 10.1111/j.1550-7408.2011.00529.x. [DOI] [PubMed] [Google Scholar]
- 70.O'Neill L., Hartz A.J. Lower mortality rates at cardiac specialty hospitals traceable to healthier patients and to doctors' performing more procedures. Health Aff (Millwood) 2012;31:806–815. doi: 10.1377/hlthaff.2011.0624. [DOI] [PubMed] [Google Scholar]
- 71.Nazarian R., Shi H., Wang Q., Kong X., Koya R.C., Lee H. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith F.O., Klapper J.A., Wunderlich J.R., Rosenberg S.A., Dudley M.E. Impact of a recombinant fowlpox vaccine on the efficacy of adoptive cell therapy with tumor infiltrating lymphocytes in a patient with metastatic melanoma. J Immunother. 2009;32:870–874. doi: 10.1097/CJI.0b013e3181b36b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valencia J.C., Watabe H., Chi A., Rouzaud F., Chen K.G., Vieira W.D. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J Cell Sci. 2006;119:1080–1091. doi: 10.1242/jcs.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartz A.M., Bauer B., Soldner E.L., Wolf A., Boy S., Backhaus R. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–523. doi: 10.1161/STROKEAHA.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falchook G.S., Long G.V., Kurzrock R., Kim K.B., Arkenau T.H., Brown M.P. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ascierto P.A., Gogas H.J., Grob J.J., Algarra S.M., Mohr P., Hansson J. Adjuvant interferon alfa in malignant melanoma: an interdisciplinary and multinational expert review. Crit Rev Oncol Hematol. 2013;85:149–161. doi: 10.1016/j.critrevonc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Flach E.H., Rebecca V.W., Herlyn M., Smalley K.S., Anderson A.R. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol Pharm. 2011;8:2039–2049. doi: 10.1021/mp200421k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlino M.S., Saunders C.A., Haydu L.E., Menzies A.M., Martin C.C., Jr., Lebowitz P.F. (18)F-labelled fluorodeoxyglucose-positron emission tomography (FDG-PET) heterogeneity of response is prognostic in dabrafenib treated BRAF mutant metastatic melanoma. Eur J Cancer. 2013;49:395–402. doi: 10.1016/j.ejca.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 79.Wilmott J.S., Tembe V., Howle J.R., Sharma R., Thompson J.F., Rizos H. Intratumoral molecular heterogeneity in a BRAF-mutant, BRAF inhibitor-resistant melanoma: a case illustrating the challenges for personalized medicine. Mol Cancer Ther. 2012;11:2704–2708. doi: 10.1158/1535-7163.MCT-12-0530. [DOI] [PubMed] [Google Scholar]
- 80.Mittapalli R.K., Vaidhyanathan S., Dudek A.Z., Elmquist W.F. Mechanisms limiting distribution of the threonine-protein kinase B-RaFV600E inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344:655–664. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 82.Flaherty K.T., Schiller J., Schuchter L.M., Liu G., Tuveson D.A., Redlinger M. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res. 2008;14:4836–4842. doi: 10.1158/1078-0432.CCR-07-4123. [DOI] [PubMed] [Google Scholar]
- 83.Abraham J., Edgerly M., Wilson R., Chen C., Rutt A., Bakke S. A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clin Cancer Res. 2009;15:3574–3582. doi: 10.1158/1078-0432.CCR-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lagas J.S., Fan L., Wagenaar E., Vlaming M.L., van Tellingen O., Beijnen J.H. P-glycoprotein (P-gp/Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin Cancer Res. 2010;16:130–140. doi: 10.1158/1078-0432.CCR-09-1321. [DOI] [PubMed] [Google Scholar]
- 85.Agarwal S., Hartz A.M., Elmquist W.F., Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17:2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hyafil F., Vergely C., Vignaud D.P., Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–4602. [PubMed] [Google Scholar]
- 87.Vispute S.G., Chen J.J., Sun Y.L., Sodani K.S., Singh S., Pan Y. Vemurafenib (PL4032, Zelboraf®), a BRAF inhibitor, modulates ABCB1-, ABCG2-, and ABCC10-mediated multidrug resistance. J Cancer Res Updates. 2013;2:306–317. [Google Scholar]