Abstract
Background
It has been reported that collagenases (matrix metalloproteinase 2 [MMP-2] and matrix metalloproteinase 9 [MMP-9]) are associated with hair cycle, whereas the mechanism of the association is largely unknown.
Methods
The mice were randomly allocated into four groups: saline, and 5, 10, and 15 nM SB-3CT. Immunohistochemical analysis was employed to examine MMP-2 and MMP-9 protein. Real-time polymerase chain reaction and enzyme-linked immunosorbent assay were performed to determine mRNA and protein levels of VEGF, IGF-1, TGF-β, and GAPDH. Growing hair follicles from anagen phase III–IV were scored based on hematoxylin and eosin staining. Hair regrowth was also evaluated.
Results
Results showed that mRNA expressions of enzymes changed with a peak at late anagen and a trough at telogen after depilation. Immunostaining showed that the highest expression of MMP-2 was more than that of MMP-9, and the highest expression of enzymes changed during anagen. The localizations of MMP-2 changed from dermal papilla, keratinocyte strand, out of root sheath, and basal plate at early anagen, to hair bulb, inner root sheath, and outer root sheath at late anagen. The localization of MMP-9 changed from partial keratinocyte to dermal papilla at early anagen and to outer root sheath at late anagen. VEGF, IGF-1, and TGF-β have been shown to regulate hair growth. We found mRNA and protein expressions of VEGF and IGF-1 fluctuated with a peak at anagen and a decrease at catagen to telogen. In contrast, mRNA and protein expressions of TGF-β changed with highest and lowest levels at anagen and telogen, respectively. With selective inhibitor of collagenase IV, SB-3CT, mice showed significant suppressed hair growth and decreased expression of VEGF, IGF-1, and TGF-β. The MMPs agonist also significantly increased expression of VEGF, IGF-1, and TGF-β. Meanwhile, SB-3CT treatment significantly suppressed hair growth.
Conclusion
All these data suggest that the type IV collagenases, MMP-2 and MMP-9, play important roles in hair cycle, and this could be mediated by induced expression of VEGF, IGF-1, and TGF-β.
Keywords: MMP-2, MMP-9, SB-3CT, telogen
Introduction
Hair loss is a very common and distressing problem. In the United States, hair loss affects about 40 million men. It is well known that hair loss can result from the dysregulation of hair cycle, which consists of three identified stages: anagen (active growth phase), catagen (the intermediate phase), and telogen (resting phase). In most mammals, each hair follicle is a mammalian skin organ that produces hair and controls the hair growth cycle. Previous studies have shown that various growth factors are expressed in the hair follicle, such as VEGF, IGF-1, TGF-β, EGF, and keratinocyte growth factor (KGF/FGF-7).1 Among these, VEGF, IGF-1, and KGF have been reported to stimulate hair growth, whereas overexpressions of EGF and TGF-β have been shown to suppress hair growth.2–4 In addition, some inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1α (IL-1α), can induce significant morphologic changes and growth arrest of the hair follicle in vivo as well as in vitro.5,6
Dermal papillae are small mesenchymally derived zones at the bases of hair follicles. The papilla cells are enmeshed in a dense extracellular matrix (ECM) has been suggested to play a structural role, and contains an abundance of basement membrane components composed of type IV collagen, laminin, and other structural proteins.7 It has been demonstrated that the volume and composition of the ECM changes during hair cycle.8 Matrix metalloproteinases (MMPs) are a family of endopeptidases involved in the degradation of ECM. Among these members, MMP-2 and MMP-9 (type IV collagenases), are responsible for the cleavage of collagen IV, V, VII, and X. In the skin, the type IV collagenases are detectable and play an important role in the remodeling of ECM.9 A previous study has shown that human hair follicles in culture in vitro spontaneously secreted collagenolytic MMP-2 and MMP-9.6 For MMP-9, an important role in promoting hair growth was shown based on the characterization of decreased hair follicle number and hair canal width in MMP-9 knockout mice.10 Unexpectedly, increased activity of MMP-9 was suggested to contribute to the hair bulb involution and remodeling in vitro and/or in vivo.6 For MMP-2, the expression levels are much higher than MMP-9, and this was suggested to be associated with hair cycle.7–11 However, the exact role of MMP-2 in the hair cycle needs further elucidation.
It has been shown that MMP-2 and MMP-9 can be induced by a number of growth factors, such as VEGF, TGF-β, TNF-α, and IL-1α, which play different roles in hair growth.12–15 In addition, the increased expression of MMP-2 and MMP-9 has been demonstrated to be pathogenic in some diseases, such as cancer and lung injury.16,17
This study aims to examine the effect of co-inhibition of MMP-2 and MMP-9 on hair cycle after depilation. SB-3CT is a selective inhibitor of MMP-2 and MMP-9, and is widely used to investigate the role of the enzymes.17,18 With this inhibitor, we provide the first evidence that collagenase IV plays an important role in hair cycle, which is mediated by VEGF, IGF-1, and TGF-β.
Materials and methods
Materials
The antibodies against MMP-2 and MMP-9 were purchased from Abcam (Cambridge, MA, USA). The enzyme-linked immunosorbent assay (ELISA) kit was purchased from RayBiotech (Norcross, GA, USA), and other reagents or materials were obtained from Sigma-Aldrich Co. (St Louis, MO, USA).
Animals
Seven-week old female C57BL/6 mice were purchased from Guangzhou University of Chinese Medicine Laboratory Animal Center, Guangzhou, People’s Republic of China. All procedures performed in this study were strictly in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. This study was approved by the ethics committee of Southern Medical University, Guangzhou, China. The mice received 1 week acclimatization, and were provided with food and water ad libitum under controlled conditions as follows: 23°C±2°C temperature, 55%±10% relative humidity, and a light/dark cycle of 12 hours. After a week of acclimatization, the dorsal area (2 cm ×4 cm) of the animals was shaved with an animal clipper in the telogen phase (pink color), and then was exposed to a hair removal cream (Kanebo Cosmetics Ltd., Tokyo, Japan). Then, they were randomly allocated into four groups according to the different topical applications: saline, and 5, 10, and 15 nM SB-3CT. Each kind of solution (100 μL) was topically applied to the shaved dorsal area daily for 5, 15, or 25 days to observe the effect of SB-3CT on mice.
After identification of the dose of SB-3CT, some other mice (additional control mice) were depilated and then topically treated with vehicle or 10 nM SB-3CT. After a certain duration of treatment (duration of 5, 15, or 25 days), the skin samples were collected and stored until use.
Immunohistochemical examination
After 5, 15, or 25 days of treatment, the mice were euthanized with CO2 gas. The dorsal skin sample of each mouse was excised from the line connecting both scapula, and fixed in 10% formaldehyde overnight. Embedded in paraffin, the samples were sectioned at about 5 μm. The tissue sections were blocked with 10% horse serum for 2 hours, followed by incubation overnight at 4°C with the primary antibody against MMP-2 and MMP-9. Subsequently, horseradish peroxidase-conjugated secondary antibody was pipetted on the sections and incubated for 30 minutes at 37°C. Negative control was obtained by utilizing 10% bovine serum substituted with the primary antibody. With Diaminobenzidine (DAB), the reaction product was visualized as brown staining. Then, the examined sections were counterstained by hematoxylin.
Real-time polymerase chain reaction
Total RNA was extracted from the skin samples with TRIzol (Thermo Fisher Scientific, Waltham, MA, USA). Then, DNase I was used to remove genomic DNA. Two hundred nanograms of total RNA of each sample was reversely transcribed into complementary DNA. Then, real-time polymerase chain reaction was performed to determine the mRNA levels of VEGF, IGF-1, TGF-β, and GAPDH, based on the published primers which are shown in Table 1. Thermal cycling performed was 10 minutes at 95°C for enzyme activation, denaturation for 15 seconds at 95°C, annealing for 60 seconds at 60°C, and extension for 60 seconds at 72°C. The real-time polymerase chain reaction was performed for at least 30 cycles. A dissociation curve was generated to assure the absence of nonspecific products or primer dimers. Relative quantification was conducted with the ∆∆Ct method. Expression data of the genes of interest were normalized with the housekeeping gene GAPDH.
Table 1.
Primer pairs used for the Real-time Polymerase Chain Reaction
| Gene | Forward primers | Reverse primers |
|---|---|---|
| GAPDH | 5′-GGCCTCCAAGGAGTAAGAAA-3′ | 5′-GCCCCTCCTGTTATTATGG-3′ |
| MMP-2 | 5′-ATCGCAGACTCCTGGAATG-3′ | 5′-CCAGCCAGTCTGATTTGATG-3′ |
| MMP-9 | 5′-TCCAGTACCAAGACAAAGC-3′ | 5′-GAGCCCTAGTTCAAGGGCAC-3′ |
| VEGF | 5′-CCTCGCAGTCCGAGCCGGA-3′ | 5′-GACCCAAAGTGCTCCTCGAAG-3′ |
| IGF-1 | 5′-GTGTGGACCGAGGGGCTTTTACTTC-3′ | 5′-GCTTCAGTGGGGCACAGTACATCTC-3′ |
| TGF-β | 5′-ATCCTGTCCAAACTAAGGCTCG-3′ | 5′-ACCTCTTTAGCATAGTAGTCCGC-3′ |
ELISA
The skin samples were isolated, snap-frozen in liquid nitrogen, and immediately homogenized in ice-cold Radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich Co.) containing a cocktail of protease inhibitors (Hoffman-La Roche Ltd., Basel, Switzerland). Denatured total cell and tissue lysates were added to the ELISA analysis. VEGF, IGF-1, and TGF-β were measured with specific ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). In brief, polystyrene 96-well plates coated with the specific antibody were incubated with the prepared standard or sample at 4°C for 2 hours. The plates were washed and incubated at 4°C for 2 hours with primary biotinylate-labeled antibody. Subsequently, the plates were washed again and incubated with streptavidin-conjugated horseradish peroxidase at room temperature for 45 minutes. After washing, tetramethylbenzidine was added to each well and incubated at room temperature for 30 minutes. Finally, sulfuric acid was used to terminate the reaction and the absorbance at 450 nm was determined with an automated microplate reader.
Histological analysis
After euthanasia, the skin tissues were collected from the sacrificed mice. The samples were fixed in 10% neutral buffered formalin for about 24 hours, dehydrated with increasing concentrations of ethanol (70%–100%), and embedded in paraffin wax. Then, sections of 5 μm thickness were prepared. In the hematoxylin and eosin stained skin sections, growing hair follicles from anagen phase III–IV were scored based on the accepted morphological guidelines with a microscope,19 and the length of hair follicle was measured with ImageJ software.
Hair regrowth evaluation
The evaluation of hair regrowth was conducted as previously described.6,11,19 In brief, photographs of the mice were regularly taken during the test. Then, the hair growth effect was assessed by image analysis according to the following formula:
| (1) |
Statistical analysis
Data was expressed as mean ± standard deviation. With SPSS software (19.0 IBM Corporation, Armonk, NY, USA), the statistical analysis was performed. Student’s t-test was applied for pairwise comparisons. Analysis of variance was used for pairwise comparisons. P,0.05 was considered to be statistically significant.
Results
Expression of MMP-2 and MMP-9 during the hair cycle
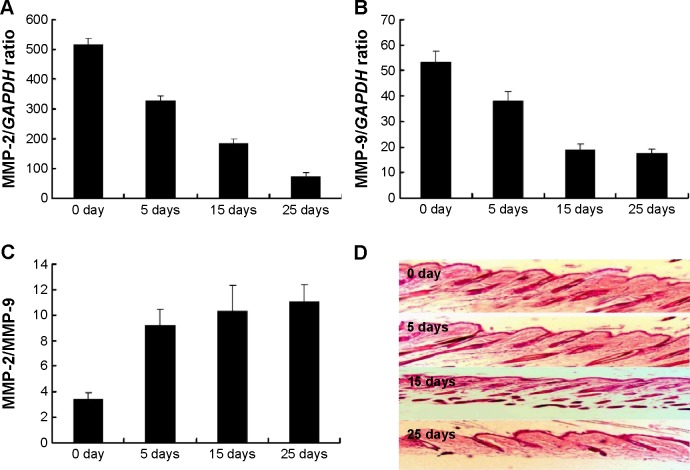
The activities of MMP-2 and MMP-9 fluctuated during hair cycle with a peak at early anagen and then decreasing during catagen to telogen. In this study, the mRNA expressions of MMP-2 and MMP-9 in the skin including the hair follicle were examined at 5, 15, and 25 days after depilation. As shown in Figure 1A, the enzymes were present at each time point with the highest level at 5 days after depilation and the lowest level at 25 days after depilation. Histologic observation showed that 5, 15, and 25 days after depilation represented early anagen, late anagen, and telogen, respectively (Figure 1B). All these indicated that the expressions of the enzymes elevated at early anagen to late anagen and then decreased to the trough at telogen. Moreover, MMP-2 mRNA expression levels were markedly higher than MMP-9 at each time-point mentioned above (Figure 1B). The MMP-2 comparison to MMP-9 at 5, 15, and 25 days after depilation was also evaluated. Figure 1C indicates that the level of MMP-2/MMP-9 was increased following with the increased treatment days. Furthermore, we also examined MMP-2 and MMP-9 by using immunohistochemical staining analysis. The results indicated that the typical histologic sections from the mice at 5 days with the significant higher expression of MMP-2 and MMP-9 after depilation (Figure 1D).
Figure 1.
The mRNA expressions of MMP-2 and MMP-9 after depilation.
Notes: Dorsal skin of the mice at 5, 15, and 25 days after depilation was homogenized and measured for MMP-2 and MMP-9. (A) MMP-2 mRNA expression; (B) MMP-9 mRNA expression; (C) MMP-2 compared to MMP-9 at 5, 15, and 25 days after depilation; (D) typical histologic sections from the mice at 5, 15, and 25 days after depilation.
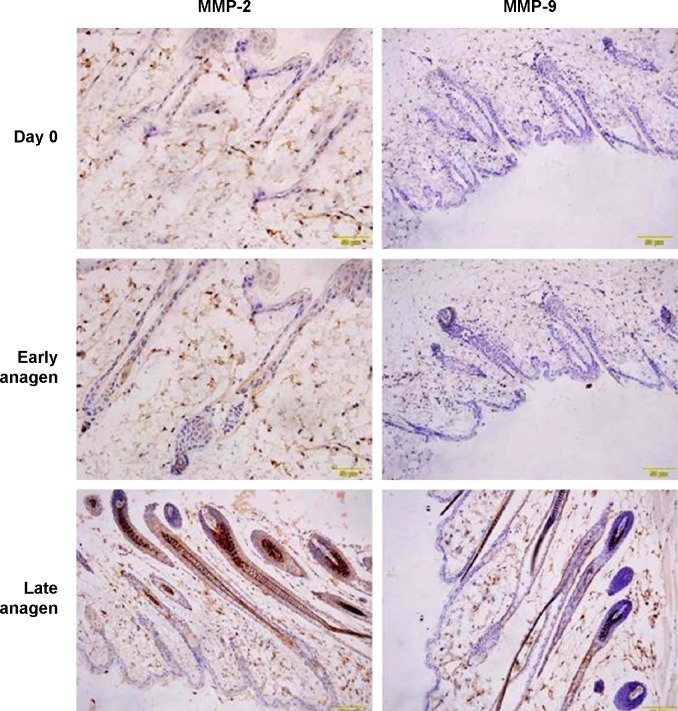
Localization of MMP-2 and MMP-9 in the anagen
With immunohistochemistry, the localizations of MMP-2 and MMP-9 at day 0 and at 5 and 15 days after depilation were characterized. As shown in Figure 2, immunoreactivity of MMP-2 was observed in dermal papilla, keratinocyte strand, outer root sheath, and basal plate at early anagen (5 days after depilation), and in hair bulb, inner root sheath, and outer root sheath at late anagen (15 days after depilation). There were no significant differences between day 0 and 5 days after depilation. For MMP-9, immunoreactivity was observed in partial keratinocyte strand at early anagen, and in outer root sheath at late anagen.
Figure 2.
Localization of MMP-2 and MMP-9 at day 0 and during anagen after depilation.
Note: Bar =100 μm.
Effect of the collagenase IV inhibitor on regulation of hair cycle in mice
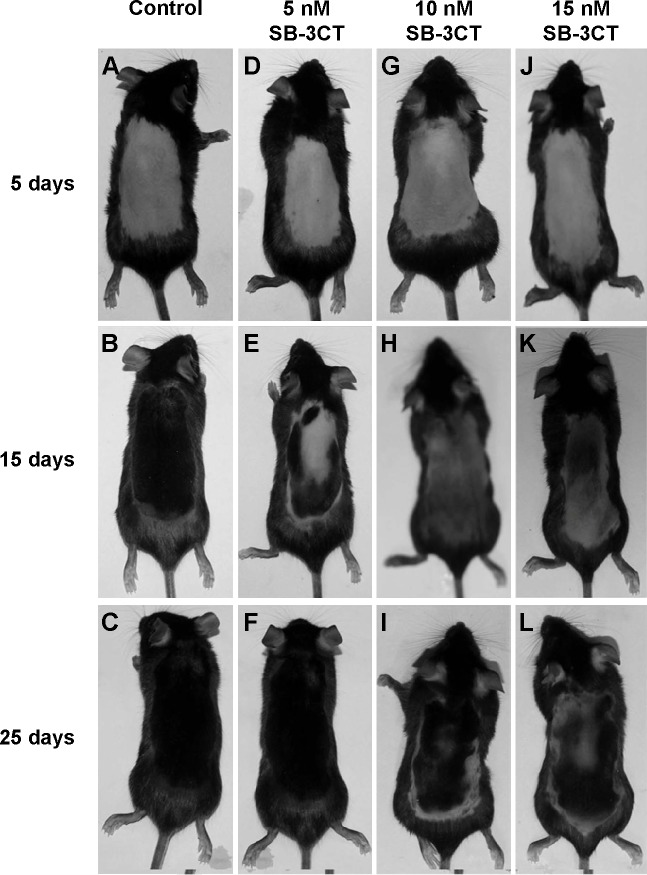
To evaluate the effect of SB-3CT on hair growth, typical photos of the dorsal skin in mice at 0, 5, 15, and 25 days after depilation were taken. As seen in Figure 3, the mice treated with 5, 10, and 15 nM SB-3CT showed dose-dependent inhibition of hair recovery compared to the control group. At 25 days after depilation, SB-3CT-treated mice showed hair regrowth of about 90%, 80%, and 52% at 5, 10, and 15 nM, respectively, whereas vehicle treated mice showed about 93% (Figure 3). Although the inhibitory effect on hair regrowth was most significant at 15 nM (Figures 3 and 4), the mice showed slightly decreased activity during the early phase of treatment. Therefore, 10 nM SB-3CT was selected for the following study.
Figure 3.
Effect of topical application of the collagenase IV inhibitor, SB-3CT, on hair regrowth.
Notes: After 5, 15, and 25 days treatment, typical photos of dorsal skin treated with vehicle (A–C), and SB-3CT at 5 nM (D–F), 10 nM (G–I) and 20 nM (J–L). Upper row is from the mice 5 days after application, middle row 15 days, and bottom row 25 days.
Figure 4.
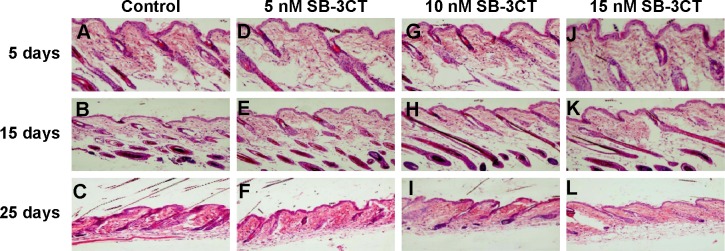
Effect of topical application of the collagenase IV inhibitor on hair follicles.
Notes: Typical histology of dorsal skin in mice after 5, 15, and 25 days of treatment with saline (A–C) or SB-3CT (D–F). Typical hematoxylin and eosin stained skin sections of mice treated with 10 nM SB-3CT (G–I) or 15 nM SB-3CT (J–L). Upper row is from the mice 5 days after application, middle row 15 days, and bottom row 25 days.
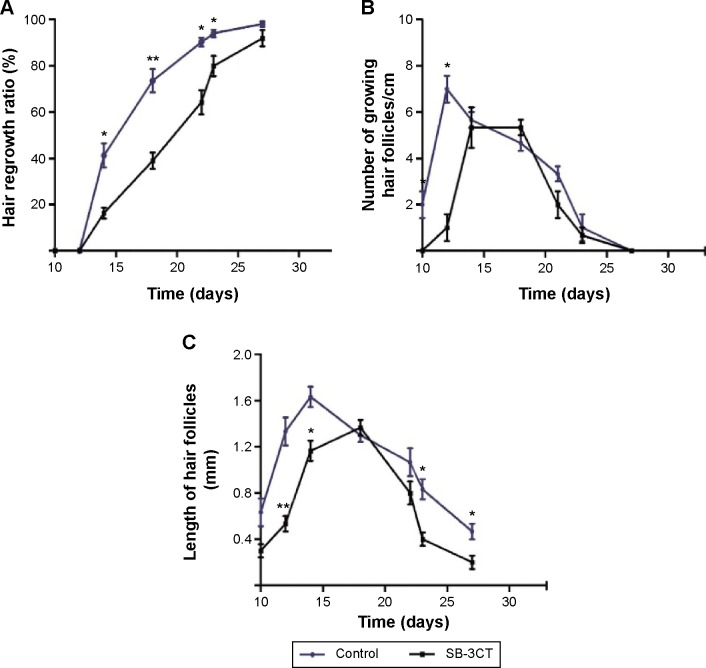
To observe hair cycle, the skin samples were extracted from the mice treated with 10 nM SB-3CT at 10, 12, 14, 17, 21, 24, and 28 days after depilation. The hematoxylin and eosin stained sections were prepared to evaluate the growth of hair follicles. As shown in Figure 5A, SB-3CT significantly inhibited hair regrowth rate at 14, 17, 21, and 24 days after depilation compared with control group. In both groups, the number of growing hair follicles increased rapidly and then decreased slowly. At 12 days after depilation, the maximum number of growing hair follicles in vehicle-treated mice was observed, whereas that was delayed about 2 days in SB-3CT-treated mice (Figure 5B). For the length of hair follicles, the maximum length was observed at 14 days after depilation in control group, whereas it was at 17 days after depilation in the SB-3CT-treated group (Figure 5C). Moreover, the number of growing hair follicles and the length of hair follicles indicated a shortened catagen duration.
Figure 5.
Image analysis of mice hair.
Notes: After depilation, the mice were treated with SB-3CT. The parameters shown were assessed from typical photographs taken at 10, 12, 17, 21, 24, and 28 days after treatment. (A) Rate of hair regrowth; (B) growing hair follicles; and (C) lengths of hair follicles. *P<0.05, **P<0.01 versus vehicle control group at the same time-point.
Furthermore, we also evaluate the effect of the MMP-2 inhibitor (TIMP-2) and MMP-9 inhibitor (TIMP-1) on hair growth of the dorsal skin in mice at 0, 5, 15, and 25 days after depilation. For the TIMP-1-treated group, the results indicated that the TIMP-2 could significantly decrease the hair regrowth ratio (Figure S1A), number of growing hair follicles (Figure S1B), and length of hair follicles (Figure S1C). For the TIMP-1-treated group, TIMP-1 could also significantly decrease the hair regrowth ratio (Figure S1D), number of growing hair follicles (Figure S1E), and length of hair follicles (Figure S1F).
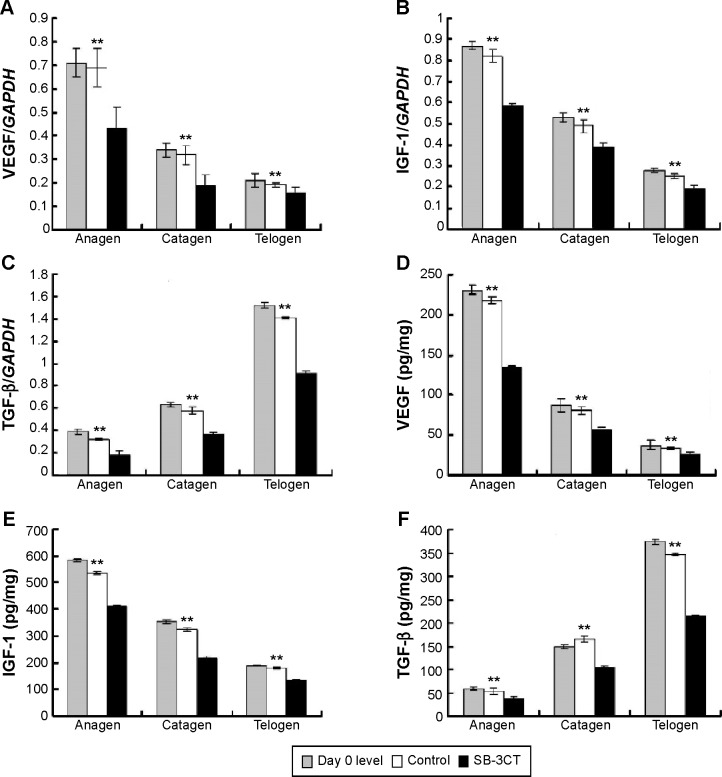
Effect of collagenase IV inhibitor on growth factor expression within hair cycle
To observe the effect of collagenase IV inhibition on the expression of growth factors after depilation, the mRNA and protein expression of VEGF, IGF-1, and TGF-β were determined. The expression levels of the growth factors changed differently during hair cycle (Figure 6). For VEGF and IGF-1, the highest expression levels were observed at anagen, and the lowest at telogen. In contrast, the lowest expression levels of TGF-β were noted at anagen, while the highest were at telogen. Following daily treatment with 10 nM SB-3CT, a significant decrease of VEGF, IGF-1, and TGF-β in the mRNA and protein expression were noted at each stage of hair cycle. Furthermore, the results also indicated that there were no significant differences between the data at day 0 and the control group (Figure 6).
Figure 6.
Effect of collagenase IV inhibitor on the expression of VEGF, IGF-1, and TGF-β.
Notes: The VEGF, IGF-1, and TGF-β were measured in homogenates of dorsal skin at each stage. (A) VEGF, (B) IGF-1, and (C) TGF-β mRNA by real-time polymerase chain reaction analysis are shown. Enzyme-linked immunosorbent assay analysis of (D) VEGF, (E) IGF-1, and (F) TGF-β. **P<0.01 versus control group at the same stage of hair cycle.
Abbreviation: VEGF, vascular endothelial growth factor; TGF, transforming growth factor; IGF-1, insulin-like growth factor 1.
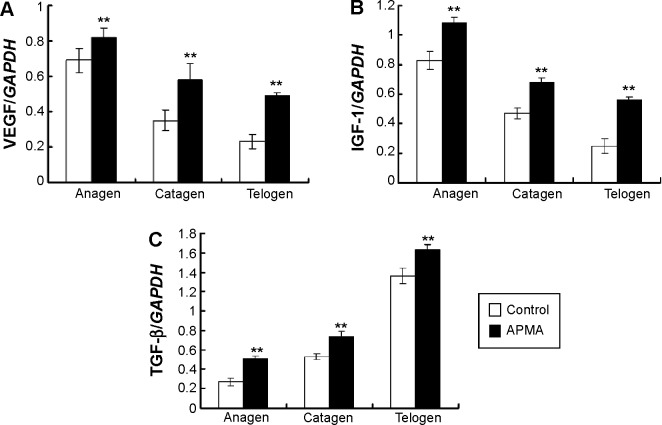
Effect of MMPs agonist on growth factor expression within hair cycle
To observe the effect of MMPs agonist on the expression of growth factors after depilation, the mRNA expression of VEGF, IGF-1, and TGF-β were examined. The results indicated that the mRNA expression of the growth factors in MMPs agonist-treated group significantly increased during hair cycle compared to the control group (Figure 7).
Figure 7.
Effect of MMPs agonist on the expression of VEGF, IGF-1, and TGF-β.
Notes: The VEGF, IGF-1, and TGF-β were measured in homogenates of dorsal skin at each stage. (A) VEGF, (B) IGF-1, and (C) TGF-β mRNA by real-time polymerase chain reaction analysis are shown. **P<0.01 versus control group at the same stage of hair cycle.
Abbreviation: VEGF, vascular endothelial growth factor; TGF, transforming growth factor; IGF-1, insulin-like growth factor 1; APMA, radioimmunoprecipitation assay.
Discussion
ECM provides an important environment in which hair follicles can maintain morphology and physiological function. The degradation and remodeling of ECM is necessary for the hair cycle progression and hair follicle development. In the present study, we confirmed that MMP-2 and MMP-9 play an important role in the hair cycle progression and hair growth. Furthermore, we demonstrated here, for the first time, that MMP-2 and MMP-9 promote hair growth by inducing the expression of some growth factors, such as VEGF, IGF-1, and TGF-β.
MMP-2 and MMP-9 are typical ECM-degrading enzymes. Consistent with the fluctuation of the activities of MMP-2 and MMP-9 in the unshaved mice, we observed that the mRNA expressions of the enzymes changed, with the highest level at late anagen after depilation. As observed in our study, MMP-2 and MMP-9 localization in hair follicle changed during anagen and the inhibition of MMP-2 and MMP-9 suppressed hair follicle development and decreased duration of catagen. These findings indicated that the enzymes play an important role in hair growth. In our previous study, it appeared that the activity of MMP-2 was much higher than that of MMP-9, which is consistent with a previous in vitro study.6 In addition to the same staining places as MMP-9, more immunoreactivity localizations were observed for MMP-2, such as dermal papilla, basal plate at early anagen, and hair bulb at late anagen. However, the immunoreactivity of MMP-2 and MMP-9 is not comparable since the primary antibody is different. Therefore, in future studies we will investigate the relationship between MMP-2 and MMP-9 immunoreactivity. All of the previous descriptions indicated that MMP-2 plays a more important role in the hair growth, but this needs to be further demonstrated. It has been demonstrated that MMP-9 functionally contributes to hair growth.10 Unexpectedly, MMP-9 expression was found to be restricted to the keratinocyte strand close to dermal papilla in early anagen, suggesting that the keratinocytes proximate to dermal papilla might play a slightly different role during hair cycle.
It has been reported that vascular network is associated with the hair growth cycle.20 Coupled to the hair cycle, the perifollicular capillary network fluctuates with a peak during anagen and then regresses during catagen to telogen.21 Active hair growth is accompanied by the induction of angiogenesis, which provides increased blood, other factors for the rapid proliferation of follicular keratinocytes, and the consequent elongation and thickening of the hair shaft.22 MMP-2 and MMP-9 are the important inducible factors to angiogenesis, indicating that inhibition of MMP-2 and MMP-9 might inhibit hair growth by direct suppression of angiogenesis.
A list of growth factors known to show direct effects on stimulating hair growth includes VEGF, IGF-1, HGF, and KGF. It has been reported that MMP-2 and MMP-9 may play a role by releasing growth factors from ECM and can regulate the activation of growth factors, such as VEGF.23,24 Therefore, inhibition of MMP-2 and MMP-9 might suppress the effect of these growth factors on stimulating hair growth by blocking them from reaching the receptors and reducing the activity of VEGF.
In the present study, we observed that the expression of VEGF, IGF-1, and TGF-β significantly decreased following MMP-2 and MMP-9 inhibition. It has been shown that VEGF and IGF-1 can stimulate hair growth, and can also inhibit TGF-β signaling which leads to a blockade in anagen reentry and a retarded hair shaft.2,3,25 These processes indicated that MMP-2 and MMP-9 functionally contribute to hair growth by inducing the expression of VEGF, IGF-1, and TGF-β. It has been shown that these growth factors are locally expressed in hair follicles. For example, VEGF and IGF-1 are primarily present in dermal papilla, while TGF-β is detectable in inner root sheath, outer root sheath, bulb matrix, and dermal papilla. The immunoreactivity in inner root sheath and dermal papilla was observed for MMP-2, but not for MMP-9.6,12 This indicated that the expression of VEGF, IGF-1, and TGF-β might be primarily mediated by MMP-2.
Our data suggest important roles for MMP-2 and MMP-9 in regulating hair growth. The proof of MMP-2’s and MMP-9’s involvement in hair growth by VEGF, IGF-1, and TGF-β may provide an insight into the therapy of hair loss.
Supplementary material
Image analysis of mouse hair treated with MMPs inhibitors.
Notes: After depilation, the mice were treated with TIMP-2 and TIMP-1. The parameters shown were assessed from typical photographs taken at 10, 12, 14, 17, 21, 24, and 28 days after treatment. (A) Rate of hair regrowth after treatment with TIMP-2; (B) growing hair follicles after treatment with TIMP-2; and (C) lengths of hair follicles after treatment with TIMP-2. (D) Rate of hair regrowth after treatment with TIMP-1; (E) growing hair follicles after treatment with TIMP-1; and (F) lengths of hair follicles after treatment with TIMP-1. *P<0.05, **P<0.01 versus vehicle control group at the same time-point.
Abbreviations: TIMP, metallopeptidase inhibitor; MMPs, matrix metalloproteinases.
Acknowledgments
This study was supported by funds from the Natural Science Foundation of China (numbers 31170949 and 81471900), the Key Clinical Specialty Discipline Construction Program, the Specialized Research Fund for the Doctoral Program of Higher Education (number 20124433110012), the Natural Science Foundation of the Guangdong (number S2012010009454), and the Dean Fund (number 2013Z013).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Danilenko DM, Ring BD, Pierce GF. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 1996;2:460–467. doi: 10.1016/1357-4310(96)10045-9. [DOI] [PubMed] [Google Scholar]
- 2.Lachgar S, Moukadiri H, Jonca F, et al. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 3.Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102:857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- 4.Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- 5.Lu D, Chen L, Shi X, et al. A functional polymorphism in interleukin-1 alpha (IL1A) gene is associated with risk of alopecia areata in Chinese populations. Gene. 2013;521:282–286. doi: 10.1016/j.gene.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Jarrousse F, Boisnic S, Branchet MC, et al. Identification of clustered cells in human hair follicle responsible for MMP-9 gelatinolytic activity: consequences for the regulation of hair growth. Int J Dermatol. 2001;40:385–392. doi: 10.1046/j.1365-4362.2001.01239.x. [DOI] [PubMed] [Google Scholar]
- 7.Couchman JR. Rat hair follicle dermal papillae have an extracellular matrix containing basement membrane components. J Invest Dermatol. 1986;87:762–767. doi: 10.1111/1523-1747.ep12456955. [DOI] [PubMed] [Google Scholar]
- 8.Messenger AG. Extracellular matrix and the hair growth cycle. J Investigat Dermatol. 1991;96:75S. doi: 10.1111/1523-1747.ep12471914. [DOI] [PubMed] [Google Scholar]
- 9.Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharov AA, Schroeder M, Sharova TY, et al. Matrix metalloproteinase-9 is involved in the regulation of hair canal formation. J Invest Dermatol. 2011;131:257–260. doi: 10.1038/jid.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisaki N, Ohuchi A, Moriwaki S. The role of neprilysin in regulating the hair cycle. PloS One. 2013;8:e55947. doi: 10.1371/journal.pone.0055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poyer F, Coquerel B, Pegahi R, et al. Secretion of MMP-2 and MMP-9 induced by VEGF autocrine loop correlates with clinical features in childhood acute lymphoblastic leukemia. Leuk Res. 2009;33:407–417. doi: 10.1016/j.leukres.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012;12:26. doi: 10.1186/1471-2407-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuyama A, Hosokawa T, Mochitate K. Interleukin-1beta and tumor necrosis factor-alpha have opposite effects on fibroblasts and epithelial cells during basement membrane formation. Matrix Biol. 2008;27:429–440. doi: 10.1016/j.matbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Pazzaglia L, Ponticelli F, Magagnoli G, et al. Activation of metalloproteinases-2 and -9 by interleukin-1alpha in S100A4-positive liposarcoma cell line: correlation with cell invasiveness. Anticancer Res. 2004;24:967–972. [PubMed] [Google Scholar]
- 16.Zheng H, Takahashi H, Murai Y, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]
- 17.Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L652–L659. doi: 10.1152/ajplung.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Bresee J, Cheney PP, et al. Evaluation of a triple-helical peptide with quenched FluorSophores for optical imaging of MMP-2 and MMP-9 proteolytic activity. Molecules. 2014;19:8571–8588. doi: 10.3390/molecules19068571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller-Röver S, Handjiski B, van der Veen C, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou N, Fan W, Li M. Angiogenin is expressed in human dermal papilla cells and stimulates hair growth. Arch Dermatol Res. 2009;301:139–149. doi: 10.1007/s00403-008-0907-5. [DOI] [PubMed] [Google Scholar]
- 22.Mecklenburg L, Tobin DJ, Müller-Röver S, et al. Active hair growth (anagen) is associated with angiogenesis. J Invest Dermatol. 2000;114:909–916. doi: 10.1046/j.1523-1747.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki M, Tsuboi R, Lee YR, Ishidoh K, Mitsui S, Ogawa H. Hair cycle-dependent expression of hepatocyte growth factor (HGF) activator, other proteinases, and proteinase inhibitors correlates with the expression of HGF in rat hair follicles. J Investig Dermatol Symp Proc. 1999;4:312–315. doi: 10.1038/sj.jidsp.5640236. [DOI] [PubMed] [Google Scholar]
- 24.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Inves Ophthalmol Visual Sci. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 25.Lin HY, Yang LT. Differential response of epithelial stem cell populations in hair follicles to TGF-β signaling. Devel Biol. 2013;373:394–406. doi: 10.1016/j.ydbio.2012.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Image analysis of mouse hair treated with MMPs inhibitors.
Notes: After depilation, the mice were treated with TIMP-2 and TIMP-1. The parameters shown were assessed from typical photographs taken at 10, 12, 14, 17, 21, 24, and 28 days after treatment. (A) Rate of hair regrowth after treatment with TIMP-2; (B) growing hair follicles after treatment with TIMP-2; and (C) lengths of hair follicles after treatment with TIMP-2. (D) Rate of hair regrowth after treatment with TIMP-1; (E) growing hair follicles after treatment with TIMP-1; and (F) lengths of hair follicles after treatment with TIMP-1. *P<0.05, **P<0.01 versus vehicle control group at the same time-point.
Abbreviations: TIMP, metallopeptidase inhibitor; MMPs, matrix metalloproteinases.