Abstract
Background and Aims Shoot-borne roots contribute to most of the nutrient uptake throughout the life cycle of maize (Zea mays). Compared with numerous studies with embryonic roots, detailed information on the phenotypic plasticity of shoot-borne roots in response to a heterogeneous nitrogen supply is scarce. The present study therefore provides a comprehensive profile of fine-scale plastic responses of distinct root types to localized high nitrate supply.
Methods Seedlings of the maize inbred line B73 were grown in split-root systems. The anatomy and morphological plasticity of the primary root and the roots initiated from the 2nd, 5th and 7th shoot nodes, and their lateral roots, were studied in response to local high nitrate supply to one side of the root system.
Key Results In contrast to the insensitivity of axial roots, local high nitrate supply increased the length of 1st-order lateral roots on the primary root and the three whorls of shoot-borne roots at different growth stages, and increased the density of 1st-order lateral roots on the 7th shoot-borne root after silking. The length and density of 2nd-order lateral roots on the three whorls of shoot-borne roots displayed a more flexible response to local high nitrate than 1st-order lateral roots. Root diameter and number, and total area and diameter of metaxylem vessels increased from the primary root to early and then later developed shoot-borne roots, which showed a positive relationship with shoot growth and N accumulation.
Conclusions Maize axial roots and lateral roots responded differently to local high nitrate, and this was related to their function. The extent of morphological plasticity of lateral roots in response to local high nitrate depended on the initiation time of the shoot-borne roots on which the lateral roots developed. Morphological plasticity was higher on 2nd-order than on 1st-order lateral roots. The results suggest that higher order lateral root branching might be a potential target for genetic improvement in future maize breeding.
Keywords: Embryonic root, lateral root, localized high nitrate supply, maize, morphological plasticity, shoot-borne root, split-root system, Zea mays
INTRODUCTION
Morphological variations of root systems have been proposed as a major strategy to cope with the naturally occurring heterogeneous distribution of soil resources (Hodge, 2004, 2006). The availability and distribution of highly soluble nitrate in aerobic soil influence root architecture and the extension of morphological responses (Marschner, 2012). In spite of sufficient application of chemical fertilizers in intensive agricultural systems, temporally and spatially heterogeneous distribution of nutrient resources in the soil profile is very common (Hodge, 2004, 2006; Marschner, 2012). Numerous studies have demonstrated root plastic responses in different plant species at the seedling stage to localized high nitrate supply (Drew et al., 1973; Jackson and Caldwell, 1989; Farley and Fitter, 1999; Fransen et al., 1999; Hodge et al., 1999, 2000).
Maize (Zea mays) plays a key role in global food, animal feed and biofuel production. The complex root stock of maize has been an important model in root research during the last decade (Hochholdinger et al., 2004; Hochholdinger and Zimmermann, 2008; Nibau et al., 2008; Hochholdinger and Tuberosa, 2009; Smith and De Smet, 2012). The embryonic root system of maize consists of a single primary root and a variable number of seminal roots, which is instrumental for early vigour and establishes a framework to explore the soil resources. The temporary functions of embryonic roots in young grass plants for nutrient and water uptake are rapidly replaced by the post-embryonic shoot-borne roots, which initiate between the elongation and silking stage, when maize requires more water and nutrients and anchorage for adult plants (Wang et al., 1994; Shane and McCully, 1999; Hochholdinger et al., 2004). Primary, seminal and shoot-borne roots are defined as axial roots and share the common feature of lateral root formation via the division of pericycle and endodermis cells adjacent to phloem poles of the root (Fahn, 1990; Feldman, 1994). The primordia of shoot-borne roots develop from dedifferentiated cells of the stem parenchyma just behind the stem cortex and below the intercalary meristem of the overlying internodes. The mean diameter of the roots developed at successively higher nodes increases, as does the mean number of metaxylem elements seen in transversal sections (Hoppe et al., 1986).
Lateral roots, initiated from embryonic primary and seminal roots and post-embryonic shoot-borne roots, constitute an extensive underground branching network, including secondary, tertiary and higher orders of branching that increase the interacting surface (Malamy, 2005). Systematic surveys of morphological and histological traits of seedling roots in maize hybrids and their parental inbred lines have shown that lateral root density could display the highest degree of heterosis (Hoecker et al., 2006; Paschold et al., 2010). An increased primary root length, lateral root density and seminal root number might significantly contribute to early seedling vigour and thus provide hybrids with an advantage over less vigorous homozygous inbred lines (Paschold et al., 2010). Thus, genotypic variation of shoot-borne roots of adult maize could be a far-reaching breeding target (Hammer et al., 2009; Singh et al., 2010), because of the key impact of shoot-borne roots on yield and adult fitness.
Nitrate, as a critical nutrient and a signalling molecule, stimulates lateral root growth in nitrate-rich patches (Crawford, 1995; Zhang and Forde, 1998; Walch-Liu et al., 2006). A series of experiments in arabidopsis has unravelled key components of the AFB3 regulatory network and auxin-mediated nitrate signalling by NRT1.1 leading to changes in lateral root growth in response to nitrate (Gifford et al., 2008; Krouk et al., 2010; Vidal et al., 2010, 2013; Mounier et al., 2014). Proliferation of lateral roots enhances the capacity of roots to capture nitrate (Hodge et al., 1999; Robinson et al., 1999). In contrast to arabidopsis, the knowledge of the molecular basis of the phenotypic plasticity of cereal root types in response to localized nitrate patches is limited. Although root responses of maize seedlings to heterogeneous nitrate supply have been studied extensively (Granato and Raper, 1989; Schortemeyer et al., 1993), the mechanisms of root branching have not been fully explored (Lynch and Brown, 2012; Orman-Ligeza et al., 2013). We recently demonstrated in the field and under hydroponic conditions that maize plants at the reproductive stage could respond to heterogeneous nitrate distribution by altering their root morphology, and the responses of adult roots were more dramatic than those of seedlings (Peng et al., 2012; Yu et al., 2014a, b). However, the mechanisms controlling root architecture might be different between embryonic and post-embryonic roots (Hochholdinger and Tuberosa, 2009; Zhu et al., 2011). In the present study, we adopted split-root systems and tracked morphological responses of the primary root, different whorls of shoot-borne roots and their lateral roots to local high nitrate in the maize inbred line B73. The aim of the study was to provide a comprehensive overview on the plasticity of distinct root types in response to local high nitrate during development.
MATERIALS AND METHODS
Seed germination and plant culture
Seeds of the Zea mays L. inbred line B73 were surface-sterilized in 10 % H2O2 for 30 min, washed with deionized water then germinated between layers of moist filter paper in saturated CaSO4 solution in the dark. At the two-leaf stage (9 d after germination), seedlings displayed one single primary root and on average four seminal roots. The endosperm of each seedling was excised, and uniform seedlings with two visible leaves were transferred into full-strength nutrient solution with the following composition (mm): K2SO4 0·75, MgSO4 0·65, KCl 0·1, KH2PO4 0·25, H3BO3 10–3, MnSO4 10–3, CuSO4 10–4, ZnSO4 10–3, (NH4)6Mo7O24 5·0 × 10–6 and Fe-EDTA 0·2. Ca(NO3)2 was supplied at 0·5 mm (LN) and 4·0 mm (HN) as the N source. CaCl2 was added as an additional calcium source under LN treatment. The initial pH of the nutrient solution was adjusted to 6·0.
Split-root experiment of embryonic roots
Ten uniform seedlings with one primary root and four seminal roots were transferred into a black polyethylene two-compartment container (30 cm long, 20 cm wide and 20 cm high) as described by Yu et al. (2014a) (Fig. 1A). The primary roots of all ten seedlings were placed in one compartment with all 40 seminal roots in the other compartment. Each compartment contained 6 L of nutrient solution. The nutrient solution was continuously aerated and renewed every 3 d. The plants were grown in the greenhouse on the campus of China Agricultural University, Beijing, from 20 March to 2 April 2013, under natural light with an approx. 25 °C/approx. 15 °C day/night temperature regime.
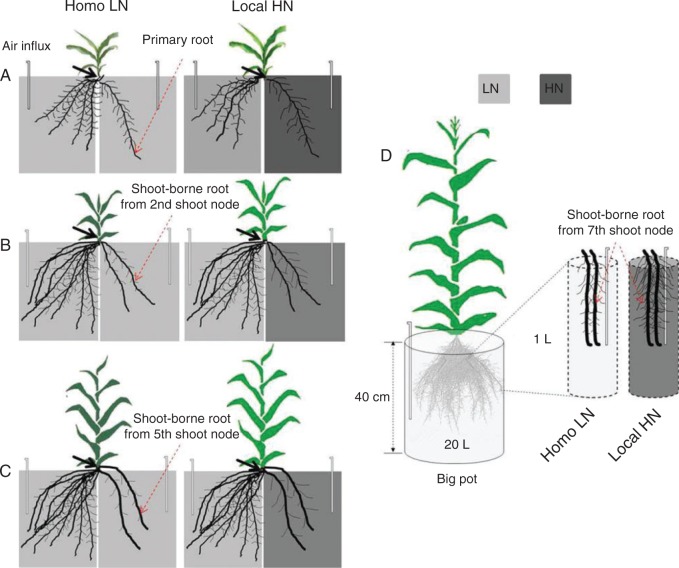
Fig. 1.
Set-up of split nitrate supply [0·5 mm for low nitrate (LN) and 4·0 mm for high nitrate (HN)] to roots of the maize inbred line B73. (A) Two-compartment container for studying the morphology of embryonic roots. There were ten plants in each container. The primary roots of all ten seedlings were placed in one compartment and all seminal roots in the other compartment. (B and C) Two-compartment container for studying the morphology of the 2nd (B) and 5th (C) shoot-borne roots (SBRs), respectively. There were two (B) plants and one plant (C) in each container, respectively. The two randomly selected 2nd SBRs or 5th SBRs of each plant were placed in one compartment with the rest of the whole root system in the other compartment. Maize plants were fixed with sponge strips into each slot evenly distributed along the middle wall of the compartments. Both compartments were continuously aerated. Homo LN, both sides of the two-compartment container were supplied with low nitrate (0·5 mm); Local HN, the primary roots of seedling (indicated by arrows in A) and the selected two SBRs (indicated by arrows in B–D) of each plant were split-supplied with high nitrate (4·0 mm). (D) Split-root system in a 20 L black plastic pot with two plastic bags, which were fixed on the pot cover and floated in nutrient solution. Two of the 7th SBRs were placed in each of the plastic bags, and treated by low or high nitrate. The air was distributed into the big pots and plastic bags, respectively, from the same pump. From the jointing stage onward, plants were anchored properly using overhead wires to prevent lodging.
The following two N treatments were applied: (1) LN (0·5 mm) in both compartments (homogeneous LN supply, Homo LN); and (2) LN (0·5 mm) in the compartment with seminal roots and HN (4 mm) in the compartment with primary roots (local HN supply, Local HN) (Fig. 1A). There were four biological replicates for each treatment. Plants were harvested 13 d after transfer as follows: (1) primary roots of two plants in each two-compartment container were kept at 4 °C for root length analysis; and (2) primary roots, seminal roots and shoots of the remaining plants were separated and dried for dry weight and N content measurement.
Split-root experiments of shoot-borne roots
Uniform seedlings were transferred into either 6 or 20 L black plastic pots containing full-strength solution on 27 March 2013. One plant was grown per pot; the plant was fixed at the shoot base with sponge strips in the central hole of the cover. Nutrient solution (see composition above) was continuously aerated, supplied with LN (0·5 mm), and replaced every 3 d for the 6 L pots and every 10 d for the 20 L pots.
One month after the transfer of the seedlings, the concentrations of the nutrient components in the 20 L pots were adjusted in order to meet the increased demand of plant growth: KH2PO4 was increased from 0·25 to 0·5 mm; MgSO4 from 0·65 to 1·2 mm; MnSO4 from 10–3 to 6·0 × 10–3 mm; ZnSO4 from 10–3 to 1·5 ×10–3 mm; and CuSO4 from 10–4 to 1·5 × 10–4 mn. The N concentrations were not altered. From the jointing stage onwards, plants grown in the 20 L pots were properly anchored using overhead wires to prevent lodging. The experiment was conducted in the same greenhouse as the experiment studying embryonic roots.
Treatments of shoot-borne roots initiated from the 2nd and 5th shoot node
When shoot-borne roots initiated from the 2nd or 5th shoot node of maize cultured in 6 L pots grew to about 3 cm length after 21 and 35 d, respectively, the plants were transferred into the same two-compartment container as described above for the embryonic root study (Fig. 1B, C). In each two-compartment container, two plants were grown for the 2nd node shoot-borne root experiment and a single plant for the 5th node shoot-borne root experiment. Two of the 2nd node shoot-borne roots or 5th node shoot-borne roots (Fig. 1B, C) of each plant were transferred into one compartment and the rest of the root system was in the other compartment. There were two N treatments, Homo LN and Local HN, as described for the embryonic root study. In the Local HN treatment, HN was applied in the compartment which contained two shoot-borne roots of each plant. Four biological replicates were analysed for each treatment. The nutrient solution was replaced every 2 d. Plants were harvested 13 d after the N treatments with the same procedure as described for the embryonic experiment above.
Treatments of shoot-borne roots initiated from the 7th node after silking
When plants began to silk after 63 d of cultivation in 20 L of nutrient solution (29 July 2013), each plant had about 34 shoot-borne roots. Four of the 7th node shoot-borne roots of each plant were treated as follows: each two neighbouring shoot-borne roots were put into one plastic bag (8 cm wide × 50 cm long) containing nutrient solution. The plastic bags were fixed on the pot cover and floated in nutrient solution. There were two N treatments: (1) LN (0·5 mm) in both the pot and two plastic bags (homogeneous LN supply, Homo LN); and (2) LN in the pot and HN (4 mm) in two plastic bags (Local HN) (Fig. 1D). The nutrient solution in the plastic bags was continuously aerated and replaced every 3 d. The plants were harvested 13 d after the treatments. Each treatment was performed in four biological replicates. At harvest, two 7th shoot-borne roots in one of the two plastic bags in each pot were excised separately. Roots in one bag were kept at 4 °C for root length measurements, and roots in the second bag were dried for dry weight and N content measurements. The remaining root system outside the two plastic bags was harvested for measurement of the axial root length of different whorls of shoot-borne roots and dry weight. The shoot of each plant was harvested and dried for dry weight and N content measurements.
Dynamic measurements of growth and senescence of leaves, and initiation time of different whorls of shoot-borne roots
Leaves of maize grown in 20 L pots were numbered in ascending order, starting with the least mature leaf, which was designated as leaf 1. The maximal area of each leaf was determined at the silking stage. The length and width of each expanded leaf was measured with a hand ruler and calculated using the formula: green leaf area = leaf length × leaf width × 0·75 (Gallais et al., 2006). Green leaf longevity (number of days from full expansion to 50 % senescence) of each leaf was recorded for all biological replicates and traced by hand until harvest (76 d of cultivation). The initiation time and the number of each whorl of shoot-borne roots was recorded when the next whorl of roots began to be initiated.
Root anatomical structure and vascular tissue measurements
Fresh roots (2 cm root length from the root tip ofthe primary root and shoot-borne roots) of maize cultured under Homo LN were fixed in 4 % (w/v) paraformaldehyde in phosphate buffer for at least 1 h at 4 °C. Root fragments of 5 mm were embedded in 8 % agarose with 0·5 % gelatine, and transverse sections of 100 µm were prepared with a vibratome (Leica VT1200S, Nussloch, Germany), mounted with distilled water and immediately observed under bright-field with a Axio-Imager epifluorescence microscope (Carl Zeiss, Germany). The number of the cortex cell layers and metaxylem vessels was counted. The diameter and total area of the metaxylem vessels were determined using the software ImageJ (version 1·40, NIH, Bethesda, MD, USA). When the sections were imaged, a micrometer and a scale bar were used. The ratio of pixels to the scale bar length was then calculated during image analysis. Four randomly selected plants were measured for each treatment.
Measurements of root morphological and branching traits
Primary roots and all shoot-borne roots were harvested 13 d after Homo LN and Local HN treatment. The length of the treated root samples of embryonic roots and shoot-borne roots initiated from the 2th, 5th and 7th node was analysed as described by Peng et al. (2010). Three root parameters were determined: (1) length of root axes of the treated primary root and shoot-borne roots initiated from different nodes; (2) length of the 1st-order lateral roots formed on the above root axes; and (3) length of the 2nd-order lateral roots emerged from the 1st-order lateral roots. Lateral roots of ≥3rd order were never observed in any plant in our experiments.
Root axis length was measured using a ruler before scanning lateral roots. Total lateral root length was scanned at a resolution of 400 dpi during picture acquisition and then analysed using the the WinRhizo Pro v. 4.0 software package (Regent Instruments Inc.). Since the 1st- and the 2nd-order lateral roots could not be distinguished by the software, the 1st-order lateral roots were excised after scanning and the length of the 1st-order lateral roots was measured after excision by using a hand ruler. The length of the 2nd-order lateral roots was obtained by total lateral root length minus the length of 1st-order lateral roots. Lateral root density of the 1st-order lateral roots indicated the number of the 1st-order lateral roots per unit length of an axial root. Similarly, lateral root density of the 2nd-order lateral roots was the number of the 2nd-order lateral roots per unit length of the 1st-order lateral roots. The number of the 1st- and the 2nd-order lateral roots was obtained by counting. The specific root length of distinct root orders was the ratio of total lateral root length to root dry weight of the same order of roots.
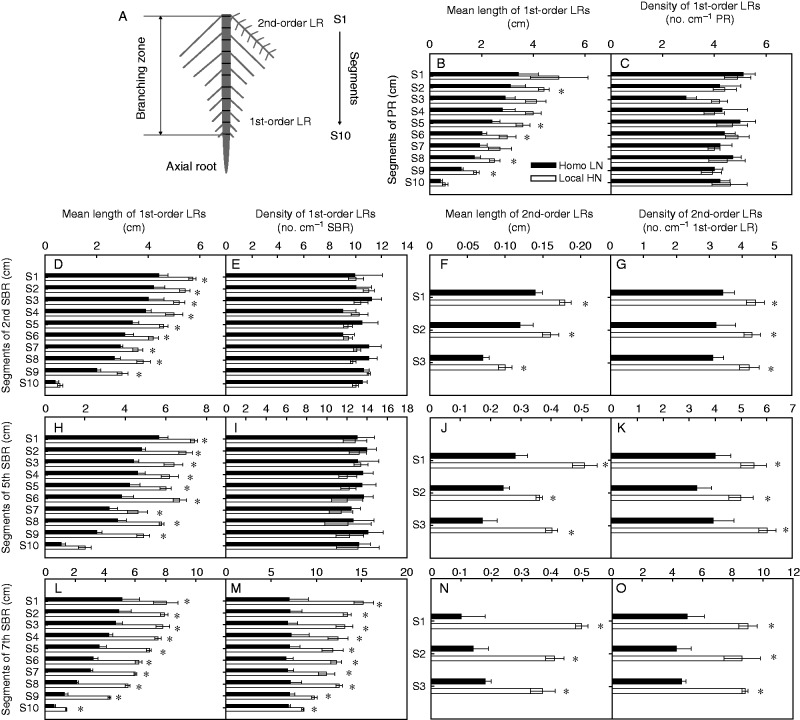
Primary roots and all shoot-borne roots of plants grown in 20 L pots were harvested 13 d after Homo LN and Local HN treatment. The branching zone (Fig. 6A) of the root axes of the treated primary or shoot-borne roots was divided into ten segments of 3 cm each, starting with the oldest part in the root base, which was designated as S1. The length and density of the 1st- and the 2nd-order LRs initiated on all treated roots were quantified by hand-tracing roots after root scanning.
Fig. 6.
Comparison of the length and density of 1st- and the 2nd-order lateral roots branched on primary roots (PRs) or shoot-borne roots (SBRs) initiated from the 2nd/5th/7th node with split-supply of LN (0·5 mm, Homo LN) or HN (4·0 mm, Local HN) in the maize inbred line B73. All plants were harvested 13 d after Homo LN or Local HN treatment. (A) The branching zone of the root axes of the treated primary root or shoot-borne roots was divided equally into ten segments of 3 cm each, starting with the oldest part in the root base, which was designated as S1. (B and C) Mean length and density of the 1st-order lateral roots branched on S1–S10 of both Homo LN- and Homo LN-treated primary root. No 2nd-order lateral roots were observed on the primary root. (D and E) Length and density of 1st-order lateral roots branched on S1– S10 of the 2nd shoot-borne root. (F and G) Length and density of 2nd-order lateral roots branched on 1st-order lateral roots initiated from S1 to S3 of the 2nd node shoot-borne root. (H–K) Branching of 1st- and 2nd-order lateral roots on the 5th node shoot-borne root. (L–O) Branching of 1st- and 2nd-order lateral roots on the 7th node shoot-borne root. Bars indicate the s.e.m., n = 4. An asterisk above each pair of columns on the same segment represents a significant difference between treatments (Homo LN vs. Local HN) (P < 0·05).
Determination of dry weight and N content
All harvested root and shoot samples were dried in an oven at 105 °C for 30 min, subsequently dried at 70 °C until the weight was constant, and ground into powder after the dry weight was determined. Appropriate amounts of ground plant materials were used to determine total N content by a modified Kjeldahl digestion method (Nelson and Somers, 1973).
Statistical analyses
Data were analysed using the one-way PROC ANOVA of SAS (SAS Institute Inc., Cary, NC, USA) program. Means of different treatments were compared using the least significant difference (LSD) at a 0.05 level of probability. Correlation between anatomical traits and shoot dry weight was processed at the 0·05 significant level using the Sigmaplot 12.0 (Systat Software Inc., Chicago, IL, USA).
RESULTS
Shoot and root growth
The leaf expansion rate, total green leaf area and developmental rate of different whorls of shoot-borne roots of maize plants changed synchronistically until silking (Supplementary Data Fig. S1A–C). Because the plants were harvested only 13 d after silking, the growth of the shoot-borne roots developed from the higher order nodes did not reach their maximum (Fig. S1D–F).
Local HN supply to primary roots and shoot-borne roots initiated from the 2nd, 5th and 7th node resulted in a significant increase in shoot N concentration and content of maize plants, while the treatments did not influence shoot and root dry weight (Table 1).
Table 1.
Shoot and total root dry weight (SDW, RDW), shoot N concentration (SNconc.) and content (SNcont.) of whole plants, and total root length (TRL) and specific root length (SRL) of the treated single primary or shoot-borne root initiated from the 2nd/5th/7th shoot node of the maize inbred line B73 under split-supply with LN (0·5 mm) or HN (4·0 mm)
| Variable | Primary root |
2nd shoot-borne root |
5th shoot-borne root |
7th shoot-borne root |
||||
|---|---|---|---|---|---|---|---|---|
| Homo LN | Local HN | Homo LN | Local HN | Homo LN | Local HN | Homo LN | Local HN | |
| SDW (g) | 0·17 ± 0·01 | 0·20 ± 0·02 | 2·6 ± 0·2 | 2·8 ± 0·3 | 22·2 ± 0·5 | 23·9 ± 1·1 | 58·3 ± 2·4 | 54·6 ± 4·7 |
| RDW (g) | 0·05 ± 0·009 | 0·05 ± 0·00·7 | 0·77 ± 0·1 | 0·81 ± 0·2 | 3·21 ± 0·6 | 4·32 ± 0·8 | 8·2 ± 1·1 | 9·3 ± 0·9 |
| SNconc. (g kg–1) | 27·2 ± 2·1 | 38·6 ± 2·5* | 14·0 ± 1·2 | 18·2 ± 0·9* | 11·0 ± 0·2 | 14·8 ± 1·2* | 9·9 ± 0·1 | 11·1 ± 0·3* |
| SNcont. (mg) | 4·7 ± 0·9 | 7·8 ± 1·1* | 36·4 ± 3·4 | 51·0 ± 6·6* | 244·2 ± 12·4 | 353·7 ± 11·2* | 578·7 ± 12·3 | 606·3 ± 8·9* |
| TRL (m) | 3·32 ± 0·2 | 4·58 ± 0·4* | 10·1 ± 0·6 | 13·2 ± 1·1* | 16·0 ± 2·7 | 28·3 ± 4·2* | 6·2 ± 3·3 | 28·1 ± 5·8* |
| SRL (m g–1) | 151·6 ± 9·6 | 217·1 ± 22·4* | 80·4 ± 9·3 | 100·8 ± 7·5* | 57·1 ± 12·6 | 85·8 ± 21·9* | 29·2 ± 16·5 | 77·7 ± 15·3* |
For the experiment with embryonic roots, seminal and primary roots of seedlings were split and treated with either homogeneous LN (Homo LN) or HN to primary roots (Local HN). For the experiment with shoot-borne roots, the whole root system was supplied with homogeneous LN, and two of the 2nd or 5th whorl of shoot-borne roots were split-supplied with LN or HN on the 21st and 35th day after transfer, respectively. Similarly, four of the 7th whorl of shoot-borne roots were split-supplied with LN or HN at the silking stage.
Data are means of six individual primary roots or four individual roots from six individual maize plants each grown under Homo LN or Local HN conditions. Each pair of values on the same root type in a row was compared and an asterisk represents a significant difference between treatments Homo LN vs. Local HN (P < 0·05).
Each treatment was performed in four biological replicates.
Anatomical structures of primary and shoot-borne roots
The metaxylem vessels in the mature zone of the apical part of the root axis (2 cm from the root tip) are arranged centrally for the primary root (Fig. 2A) and peripherically for shoot-borne roots (Fig. 2B–D). Shoot-borne roots that develop from higher order nodes display more pith tissue in the central part of the root than such roots formed on lower order nodes (Fig. 2B–D). The number of cortical cell layers in the apical part was relatively stable for different whorls of shoot-borne roots, whereas shoot-borne roots displayed significantly more cortical cell layers than the primary root (Table 2). Root diameter, number and total area of metaxylem vessels in the root apical part increased from the primary root to the 2nd node shoot-borne roots until the 7th shoot-borne roots. The average diameter of metaxylem vessels is larger in shoot-borne roots than in the primary root (Table 1). Both the number and total area of metaxylem vessels in roots showed a positive relationship with shoot dry weight (Fig. 3).
Fig. 2.
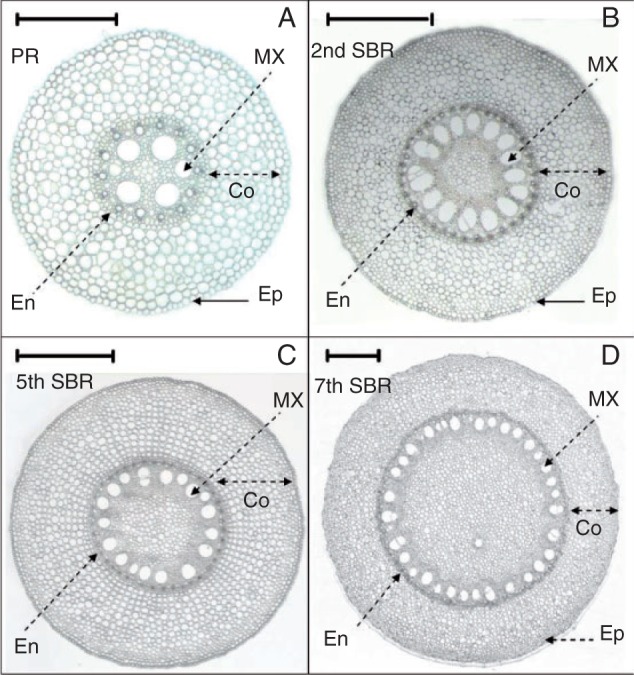
Anatomical structure of the primary root (A) and shoot-borne roots initiated from the 2nd (B), 5th (C) and 7th (D) node of the maize inbred line B73 cultured with 0·5 mm nitrate. The epidermis (Ep), cortex (Co), endodermis (En) and metaxylem (MX) are indicated by the solid arrowheads. Scale bars: (A) 300 µm, (B) 500 µm, (C) 1 mm, (D) 1·5 mm. The major anatomical differences are summarized in Table 1.
Table 2.
Comparison of the anatomical traits in the 2 cm apical part of the primary root tip 18 d after germination, and the 2nd node shoot-borne roots 34 d, 5th node shoot-borne roots 52 d and 7th node shoot-borne roots 68 d after germination in the maize inbred line B73 grown in the greenhouse
| Root type | Root diameter (mm) | Cortical cell layers | No. of metaxylem vessels | Average diameter of metaxylem vessels (µm) | Total area of metaxylem vessels (mm–2) |
|---|---|---|---|---|---|
| Primary root | 0·82 ± 0·06b | 6 ± 1·2b | 6 ± 0·8c | 56·2 ± 8·4c | 0·0149 ± 0·0012c |
| 2nd shoot-borne root | 1·43 ± 0·08a | 13 ± 2·4a | 15 ± 2·2b | 108·5 ± 17·2b | 0·14 ± 0·009b |
| 5th shoot-borne root | 2·9 ± 0·07a | 15 ± 2·1a | 18 ± 3·8b | 162·8 ± 22·1a | 0·374 ± 0·0081b |
| 7th shoot-borne root | 4·5 ± 0·11a | 16 ± 3·1a | 32 ± 3·6a | 157·1 ± 8·9a | 0·62 ± 0·012a |
Data are the means of six individual primary roots or four individual roots from six individual maize plants each grown under Homo LN conditions.
Different letters after values in columns indicate a significant difference (P < 0·05).
Fig. 3.
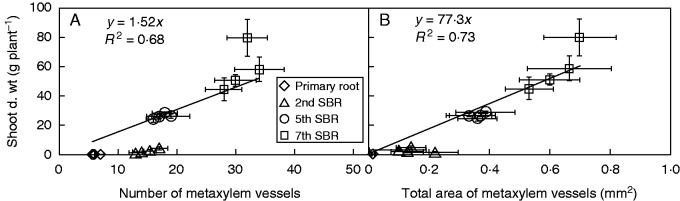
Relationship between shoot dry weight (d. wt) and the number of metaxylem vessels (A, R2 = 0·68, P < 0·05) and the total area of metaxylem vessels (B, R2 = 0·73, P < 0·05) in the apical part of the primary root, and the 2nd, 5th and 7th shoot-borne root (SBR) in the maize inbred line B73 cultured with 0·5 mm nitrate. Each data point represents a biological replicate, and error bars indicate the s.e.m., n = 6 for primary roots and n = 4 for shoot-borne roots. The correlation between anatomical traits and shoot dry weight was fitted as a polynomial linear model at the significant level of 0·05 using the Shapiro–Wilk test in Sigmaplot 12·0 (Systat Software Inc.).
Responses of biomass and length of root axes and lateral roots of primary and shoot-borne roots to Local HN supply
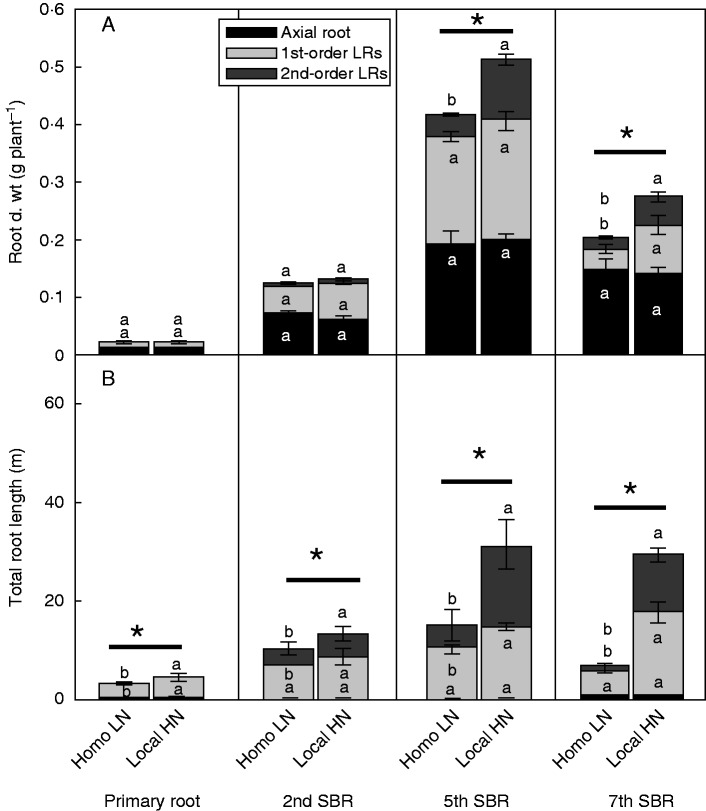
No 2nd-order lateral roots were observed in Homo LN- and Local HN-treated primary roots. In primary roots and different whorls of shoot-borne roots supplied with Homo LN, around 50 % of the root biomass was allocated to root axes, 30–40 % to 1st-order lateral roots and the rest to 2nd-order lateral roots (Fig. 4A). Local HN treatment increased the total biomass of the treated 5th and 7th node shoot-borne roots. This increase was not caused by the changes in dry weight of root axes, but rather by the increase in dry weight of the 2nd-order lateral roots on 5th node shoot-borne roots and of both the 1st- and the 2nd-order lateral root on the 7th shoot-borne roots (Fig. 4A).
Fig. 4.
Distribution of dry weight (d. wt, A) and length (B) among the axial root, 1st- and 2nd-order lateral roots (LRs) of Homo LN- and Local HN-treated primary root and different whorls of shoot-borne roots (SBRs) in the maize inbred line B73. Bars indicate the s.e.m., n = 4. Different letters above each pair of sub-columns represent significant differences of the respective root type between treatments (Homo LN v. Local HN). An asterisk above each pair of integral columns represents a significant difference between treatments (Homo LN vs. Local HN) (P < 0·05).
The total root length of the Local HN-treated primary root and shoot-borne root initiated from the 2nd, 5th and 7th node increased by 38, 31, 77 and 353 % compared with the respective Homo LN-treated roots. Similarly, specific root length increased by 43, 25, 50 and 166 %, respectively (Table 1). Similar to root dry weight, the increased total root length was contributed by the lateral roots. The contribution of lateral roots to total root length was much more than that to total root dry weight in all root types (Fig. 4). In comparison with the Homo LN-treated roots, Local HN treatment increased total root length of all types of the treated roots, especially of the 5th and 7th shoot-borne roots. Furthermore, the increase in length of the 2nd-order lateral roots was much more than that of the 1st-order lateral roots (Fig. 4B).
The specific root length of lateral roots was much higher than that of root axes, especially in different whorls of shoot-borne roots (Supplementary Data Fig. S2). Since both biomass and length of the root axes of different types of the Local HN-treated roots were not changed (Fig. 4), their specific root length also remained unchanged, compared with those supplied with Homo LN (Fig. S2). However, the specific root length of the 1st-order lateral roots of the Local HN-treated primary roots and 7th node shoot-borne roots, and of the 2nd-order lateral roots of all Local HN-treated shoot-borne roots increased significantly compared with the respective lateral roots supplied with Homo LN (Fig. S2).
Response of lateral roots branched on the treated primary and shoot-borne roots to Local HN supply
The root axis with lateral roots is designated the branching zone. The branching zone of the treated and untreated primary root and different whorls of shoot-borne roots were divided into ten segments of 3 cm each (Fig. 6A). The length and density of the 1st- and the 2nd-order lateral roots per unit of the primary root and shoot-borne roots were comprehensively compared in the present study.
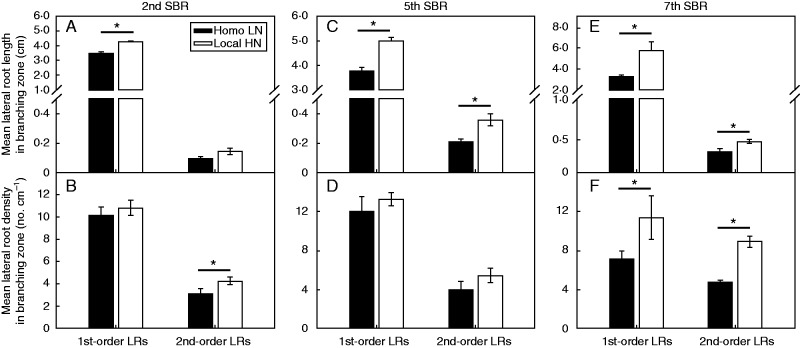
The increase in mean length of the 1st-order lateral roots in the branching zone was observed in all of the Local HN-treated shoot-borne roots, compared with those with Homo LN supply; whereas the increase in lateral root density of the 1st-order lateral roots in the branching zone was only found in the treated 7th node shoot-borne root (Fig. 5). The length and density of the 2nd-order lateral roots also increased in different whorls of Local HN-treated shoot-borne roots, compared with those with Homo LN supply (Fig. 5).
Fig. 5.
Dissection of the length and density of 1st- and the 2nd-order lateral roots (LRs) branched on the treated 2nd shoot-borne root (SBR) (A, B), 5th SBR (C, D) and 7th SBR (E, F) of the maize inbred line B73 with split-supply of localized high nitrate (Local HN, 4 mm), compared with those supplied with homogeneous low nitrate (Homo LN, 0·5 mm). Bars indicate the s.e.m., n = 4. An asterisk above each pair of columns represents a significant difference between treatments (Homo LN vs. Local HN) (P < 0·05).
In primary roots, Homo LN treatment caused a significant increase in length of the 1st-order lateral root on S2–S9 (Fig. 6B), while no changes in lateral root density of the 1st-order lateral roots were observed throughout the whole branching zone (Fig. 6C). The significant increase in length of the 1st-order lateral roots could be observed on almost all of the segments of the treated 2nd/5th/7th node shoot-borne roots from basal to apical parts of the branching zone (Fig. 6D, H, L). However, a significant increase in lateral root density of 1st-order lateral roots was only found on the 7th node shoot-borne root (Fig. 6M). On the basal parts of different whorls of the shoot-borne roots, e.g. branching zone S1–S3, the 2nd-order lateral roots branched on the 1st-order lateral root were observed. The length (Fig. 6F, J, N) and the density (Fig. 6G, K, O) of the 2nd-order lateral roots branched on the Local HN-treated shoot-borne roots increased significantly, especially on the later initiated 7th whorl of shoot-borne roots, compared with the respective Homo LN-treated shoot-borne roots.
DISCUSSION
The current study comprehensively investigated the morphological plastic responses of diverse root types of maize to heterogeneous nitrate supply. In particular, the employed split-root systems make it possible to model the heterogeneous environments, although these cultural conditions differ dramatically from the real soil substrates in the natural system. In addition, these pure nutritional environments exclude the influences of environmental stresses, such as nutrients, water, mechanical resistance and soil microbes, on root growth and development (Eissenstat and Yanai, 1997; Eissenstat et al., 2000; Niu et al., 2010; Marschner, 2012).
Morphological plasticity of maize roots in response to Local HN supply: stability in axial root growth
Root architecture determines the ability of roots stocks to take up heterogeneously distributed resources (Fitter et al., 2002; Dunbabin et al., 2003; Lynch, 2011). The proliferation of a root system is governed by the resource levels and distribution in soil (Fitter, 1994; Hodge et al., 1999). Systemic N deficiency or oversupply can reshape root architecture at both seedling and adult stages of maize plants, especially with respect to the number and angle of axial roots (Gaudin et al., 2011; Gao et al., 2014; Saengwilai et al., 2014a). A series of reports on barley (Hordeum vulgare) has demonstrated that lateral roots are initiated and elongate in response to patchy nutrient availability (Drew et al., 1973; Drew, 1975; Drew and Saker, 1975). However, the difference of diverse root types in response to patchy nutrient availability was not analysed. In the present study, the morphological responses of different root types of maize to Local HN supply were comprehensively studied during development. The length, dry weight (Fig. 4) and specific root length of root axes of different root types did not respond to Local HN supply (Supplementary Data Fig. S2). Axial roots of both embryonic and post-embryonic origin determine the growth direction of the root system and provide anchorage for the shoot in maize plants. Axial root growth is relatively stable and is controlled genetically (Fitter, 1994; Hochholdinger et al., 2004; Hochholdinger, 2009). However, root diameter, number and diameter of metaxylem vessels, and total area of metaxylem vessels of axial roots from the primary root to different whorls of shoot-borne roots increased during development (Fig. 2; Table 2), which is obviously related to the increased shoot growth and demand for nutrients (Fig. 3). The results indicate the importance of axial roots for shoot anchorage and solute translocation from roots to the shoot. The size of metaxylem vessels has a profound effect on their relative capacities to deliver water and nutrients to the shoot (McCully and Canny, 1988; McCully, 1995). An increased number of meta- or protoxylem vessels implies a larger number of potential founder cells and an increased possibility for the induction of branched lateral roots (Dubrovsky et al., 2001).
Morphological plasticity of maize roots in response to Local HN supply: flexibility in lateral root growth
In contrast to the stability of axial root growth, lateral root growth displays more flexibility in response to Local HN supply. The morphological plasticity of the total root length increased during development, especially after silking (Figs 4–6; Yu et al., 2014a). Again, this could reflect the increased shoot demand and the transition from the vegetative to the reproductive stage (Hodge, 2004). In contrast to root axes, lateral roots contribute mainly to water and nutrient uptake. Because of the morphological response of the total length of the Local HN-treated roots, shoot N content and concentration increased significantly, compared with the plants treated with Homo LN (Table 1).
A dramatic increase in the density of the 1st-order lateral roots was found exclusively on the 7th node shoot-borne roots after local HN treatment (Figs 5 and 6). It appears that the different responses of the 1st-order lateral roots to Local HN treatment were not due to the root types they branched, in spite of the similar anatomical organization among the primary root and different whorls of shoot-borne roots (Fig. 2), but rather to the time at which the treatment commenced. The mechanisms controlling the responses of shoot-borne roots before and after the reproductive stage appear to differ and merit further investigation. The 7th whorl shoot-borne roots often develop slowly and only grow into soil at the silking stage (Hoppe et al., 1986). Below each leaf insertion, some vascular bundles from this leaf can be traced down the stem in two peripheral rings of axial bundles (Ruffel et al., 2011; Kumpf et al., 2013). Our recent study demonstrates that the auxin maxima in phloem pole cells and transport in the 7th node shoot-borne roots of maize plants after silking can be restored by local high nitrate (unpublished data), which might be responsible for the increased density of the 1st-order lateral roots.
In comparison with the 1st-order lateral roots, the 2nd-order lateral roots were more flexible in response to Local HN supply, as illustrated by increases in lateral root length and density (Figs 4–6). In Citrus species, a large number of passage cells of 2nd-order lateral roots are important for resource uptake compared with lower order roots (Peterson and Enstone, 1996; Eissenstat and Achor, 1999). Maize plants optimize root architecture and modify 2nd-order lateral roots in order to capture resources, thus increasing the efficiency of adapting to heterogeneous environments. The developmental instability of lateral root formation can be traced back to the earliest stochastic events at the molecular and cellular level, such as uncertain numbers of founder cells that contribute to lateral root initiation (Dubrovsky et al., 2001), stochastic distribution of auxin sensitivities in the pericycle cell population (Gilroy and Trewavas, 2001) and variable numbers of cortical and endodermal cell files in mature lateral roots (Dolan et al., 1993).
Root morphological responses to local HN supply increased N accumulation in seedlings and adult maize plants, but did not influence shoot and root dry weight, compared with local HN supply (Table 1; Yu et al., 2014a). The results indicated that the concentration of 0·5 mm nitrate in the Homo LN treatment was not an N-limiting condition for vegetative growth of maize in the present study, but was low enough to induce root morphological responses.
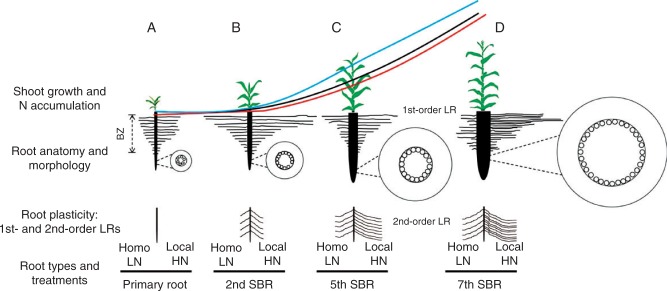
A schematic illustration of anatomical and morphological variations of distinct root types, their relationships with shoot growth and N demand at different growth stages, and root plasticity in response to Local HN supply is provided in Fig. 7.
Fig. 7.
Schematic illustration of the relationship among shoot growth (red line, expressed as shoot dry weight accumulation), shoot N accumulation [black line: homogeneous low nitrate (Homo LN, 0·5 mm); blue line: split-supply of local high nitrate (Local HN, 4·0 mm)] and variations of anatomical and morphological traits of the maize inbred line B73. The responses of length and density of lateral roots (LRs) branched on different root types (A, primary root; B, 2nd shoot-borne root, SBR; C, 5th SBR; D, 7th SBR) to Local HN supply is also illustrated. The illustration was generated based on the results of Figs 2, 3 and 5.
Carbon allocation within distinct root orders and response to Local HN supply
Root systems are a substantial metabolic sink, costing up to 50 % of daily photosynthesis by the plant (Lynch, 2007). Under the Homo LN condition, around 50 % of the root dry weight was attributed to root axes, which provided only a minimal contribution to the total root length. In contrast, lateral roots including the 1st- and the 2nd-order lateral roots consumed the rest of the assimilates translocated from the shoot but contributed to most of the total root length (Fig. 4; Yu et al., 2014a). Local HN supply shifted more carbon onto lateral roots, which resulted in a dramatic increase in total root length, especially in the later developed shoot-borne roots (Fig. 4). Lateral root extension requires less biomass and nutrient investment than the extension of other root orders (Lynch, 2007). The ability of proliferating roots to exploit nutrients in patches is related to specific root length (Eissenstat and Caldwell, 1988; Eissenstat, 1991). In some species, the capacity to transport nutrients is determined by the specific root length (Comas et al., 2002; Comas and Eissenstat, 2004). A marked increase in specific root length of lateral roots, especially of 2nd-order lateral root was observed (Supplementary Data Fig. S2).
Future prospects
Shoot-borne roots have been studied in less detail than embryonic roots in their response to local high nitrate. In the present study, higher phenotypic plasticity of lateral root length and density may reflect the high level of reactiveness of these post-embryonic roots to environmental cues (Fig. 7). To date, evidence that lateral root traits have been used successfully in cereal breeding to enhance resource capture remains scanty (Rose et al., 2012). Recent assessments of the contribution to root breeding suggest that lateral root-related traits might be promising targets in maize (Lynch, 2013; Postma et al., 2014; Saengwilai et al., 2014b). The present results contribute to the better understanding of the complex root stock of cereals and might help to identify ideal root targets for breeding. Higher order lateral root branching might be such a potential target for genetic improvement of water and nutrient capture efficiency for future maize breeding.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: dynamics in growth and development of leaves and different whorls of shoot-borne roots grown under 0·5 mm nitrate. Figure S2: specific root length of axial root, the 1st- and the 2nd-order lateral roots initiated from primary root and different whorls of shoot-borne roots with split-supply of localized high nitrate compared with homogeneous low nitrate.
ACKNOWLEDGEMENTS
We thank the National Natural Science Foundation of China (No. 31272232), the State Key Basic Research and Development Plan of China (No. 2013CB127402), the Innovative Group Grant of the National Natural Science Foundation of China (No. 31121062) and the Chinese Universities Scientific Fund (No. 2012YJ039) for financial support.
LITERATURE CITED
- Comas L, Bouma T, Eissenstat D. 2002. Linking root traits to potential growth rate in six temperate tree species. Oecologia 132: 34–43. [DOI] [PubMed] [Google Scholar]
- Comas LH, Eissenstat DM. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18: 388–397. [Google Scholar]
- Crawford NM. 1995. Nitrate: nutrient and signal for plant growth. The Plant Cell 7: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, et al. 1993. Cellular organization of the Arabidopsis thaliana root. Development 119: 71–84. [DOI] [PubMed] [Google Scholar]
- Drew MC. 1975. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75: 479–490. [Google Scholar]
- Drew MC, Saker LR. 1975. Nutrient supply and the growth of the seminal root system in barley. II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. Journal of Experimental Botany 26: 79–90. [Google Scholar]
- Drew MC, Saker LR, Ashley TW. 1973. Nutrient supply and the growth of the seminal root system in barley. I. The effect of nitrate concentration on the growth of axes and laterals. Journal of Experimental Botany 83: 1189–1202. [Google Scholar]
- Dubrovsky JG, Rost TL, Colon-Carmona A, Doerner P. 2001. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 214: 30–36. [DOI] [PubMed] [Google Scholar]
- Dunbabin V, Diggle A, Rengel Z. 2003. Is there an optimal root architecture for nitrate capture in leaching environments? Plant, Cell and Environment 26: 835–844. [DOI] [PubMed] [Google Scholar]
- Eissenstat DM. 1991. On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytologist 118: 63–68. [Google Scholar]
- Eissenstat DM, Achor DS. 1999. Anatomical characteristics of roots of citrus rootstocks that vary in specific root length. New Phytologist 141: 309–321. [DOI] [PubMed] [Google Scholar]
- Eissenstat DM, Caldwell MM. 1988. Seasonal timing of root growth in favorable microsites. Ecology 69: 870–873. [Google Scholar]
- Eissenstat DM, Yanai RD. 1997. The ecology of root lifespan. Advanced Ecological Research 27: 1–60. [Google Scholar]
- Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL. 2000. Building roots in a changing environment: implications for root longevity. New Phytologist 147: 33–42. [Google Scholar]
- Fahn A. 1990. Plant anatomy, 4th edn. New York: Pergamon Press. [Google Scholar]
- Farley RA, Fitter AH. 1999. The response of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. Journal of Ecology 87: 849–859. [Google Scholar]
- Feldman L. 1994. The maize root. In: Freeling M, Walbot V, eds. The maize handbook. New York: Springer-Verlag, 29–37. [Google Scholar]
- Fitter AH. 1994. Architecture and biomass allocation as components of the plastic response of root systems to soil heterogeneity. In: Caldwell MM, Pearcy RW, eds. Exploitation of environmental heterogeneity of plants. New York: Academic Press, 305–323. [Google Scholar]
- Fitter A, Williamson L, Linkohr B, Leyser O. 2002. Root system architecture determines fitness in an Arabidopsis mutant in competition for immobile phosphate ions but not for nitrate ions. Proceedings of the Royal Society B: Biological Sciences 269: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen B, Blijjenberg J, de Kroon H. 1999. Root morphological and physiological plasticity of perennial grass species and the exploitation of spatial and temporal heterogeneous nutrient patches. Plant and Soil 211: 179–189. [Google Scholar]
- Gallais A, Coque M, Quilléré I, Prioul J, Hirel B. 2006. Modelling postsilking nitrogen fluxes in maize (Zea mays) using 15N-labelling field experiments. New Phytologist 172: 696–707. [DOI] [PubMed] [Google Scholar]
- Gao K, Chen F, Yuan L, Zhang F, Mi G. 2014. A comprehensive analysis of root morphological changes and nitrogen allocation in maize in response to low nitrogen stress. Plant, Cell and Environment 38: 740–750. [DOI] [PubMed] [Google Scholar]
- Gaudin A, McClymont SA, Holmes BM, Lyons E, Raizada MN. 2011. Novel temporal, fine scale and growth variation phenotypes in roots of adult stage maize (Zea mays L.) in response to low nitrogen stress. Plant, Cell and Environment 34: 2122–2137. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proceedings of the National Academy of Sciences, USA 105: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Trewavas A. 2001. Signal processing and transduction in plant cells: the end of the beginning? Nature Reviews Molecular Cell Biology 2: 307–314. [DOI] [PubMed] [Google Scholar]
- Granato TC, Raper CD. 1989. Proliferation of maize (Zea mays L.) roots in response to localized supply of nitrate. Journal of Experimental Botany 40: 263–275. [DOI] [PubMed] [Google Scholar]
- Hammer GL, Dong Z, McLean G, Doherty A, Messina C. 2009. Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Science 49: 299–312. [Google Scholar]
- Hochholdinger F. 2009. The maize root system: morphology, anatomy and genetics. In: Bennetzen J, Hake S, eds. Handbook of maize: its biology. New York: Springer, 145–160. [Google Scholar]
- Hochholdinger F, Tuberosa R. 2009. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology 12: 172–177. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Zimmermann R. 2008. Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology 11: 70–74. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. 2004. From weeds to crop: genetic analysis of root development in cereals. Trends in Plant Science 9: 42–48. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162: 9–24. [Google Scholar]
- Hodge A. 2006. Plastic plants and patchy soils. Journal of Experimental Botany 57: 401–411. [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Griffiths BS, Fitter AH. 1999. Nitrogen capture by plants grown in N-rich organic patches of contrasting size and strength. Journal of Experimental Botany 50: 1243–1252. [Google Scholar]
- Hodge A, Stewart J, Robinson D, Griffiths BS, Fitter AH. 2000. Spatial and physical heterogeneity of N supply from soil does not influence N capture by two grass species. Functional Ecology 14: 645–653. [Google Scholar]
- Hoecker N, Keller B, Piepho HP, Hochholdinger F. 2006. Manifestation of heterosis during early maize (Zea mays L.) root development. Theoretical and Applied Genetics 112: 421–429. [DOI] [PubMed] [Google Scholar]
- Hoppe DC, McCully ME, Wenzel CL. 1986. The nodal roots of Zea: their development in relation to structural features of the stem. Canadian Journal of Botany 64: 2524–2537. [Google Scholar]
- Jackson RB, Caldwell MM. 1989. The timing and degree of root proliferation in fertile-soil microsites for three cold-desert perennials. Oecologia 81: 149–153. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18: 927–937. [DOI] [PubMed] [Google Scholar]
- Kumpf RP, Shi CL, Larrieu A, et al. 2013. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proceedings of the National Academy of Sciences, USA 110: 5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55: 493–512. [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2012. New roots for agriculture: exploiting the root phenome. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE. 2005. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment 28: 67–77. [DOI] [PubMed] [Google Scholar]
- Marschner P. 2012. Mineral nutrition of higher plants, 3rd edn. London: Academic Press. [Google Scholar]
- McCully ME. 1995. How do real roots work? Plant Physiology 109: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME, Canny MJ. 1988. Pathways and processes of water and nutrient movements in roots. Plant and Soil 111: 159–170. [Google Scholar]
- Mounier E, Pervent M, Ljung K, Gojon A, Nacry P. 2014. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant, Cell and Environment 37: 162–174. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Somers LE. 1973. Determination of total nitrogen in plant material. Agronomy Journal 65: 109–112. [Google Scholar]
- Nibau C, Gibbs DJ, Coates JC. 2008. Branching out in new directions: the control of root architecture by lateral root formation. New Phytologist 179: 595–614. [DOI] [PubMed] [Google Scholar]
- Niu JF, Peng YF, Li CJ, Zhang FS. 2010. Changes in root length at the reproductive stage of maize plants grown in the field and quartz sand. Journal of Plant Nutrition and Soil Science 173: 306–314. [Google Scholar]
- Orman-Ligeza B, Parizot B, Gantet PP, Beeckman T, Bennett MJ, Draye X. 2013. Post-embryonic root organogenesis in cereals: branching out from model plants. Trends in Plant Science 18: 459–467. [DOI] [PubMed] [Google Scholar]
- Paschold A, Marcon C, Hoecker N, Hochholdinger F. 2010. Molecular dissection of heterosis manifestation during early maize root development. Theoretical and Applied Genetics 120: 383–388. [DOI] [PubMed] [Google Scholar]
- Peng Y, Niu J, Peng Z, Zhang F, Li C. 2010. Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Research 115: 85–93. [Google Scholar]
- Peng Y, Li X, Li C. 2012. Temporal and spatial profiling of root growth revealed novel response of maize roots under various nitrogen supplies in the field. PLoS One 7: e37726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CA, Enstone DE. 1996. Functions of passage cells in the endodermis and exodermis of roots . Physiologia Plantarum 97: 592–598. [Google Scholar]
- Postma J A, Dathe A, Lynch J. 2014. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiology 166: 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH. 1999. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society B: Biological Sciences 266: 431–435. [Google Scholar]
- Rose TJ, Impa SM, Rose MT, et al. 2012. Enhancing phosphorus and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding. Annals of Botany 112: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM. 2011. Nitrogen economics of root foraging: transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. demand. Proceedings of the National Academy of Sciences, USA 108: 18524–18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch J. 2014a. Low crown root number enhances nitrogen acquisition from low nitrogen soils in maize (Zea mays L.). Plant Physiology 166: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Nord E, Chimungu J, Brown K, Lynch J. 2014b. Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize (Zea mays L.). Plant Physiology 166: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schortmeyer M, Feil B, Stamp P. 1993. Root morphology and nitrogen uptake of maize simultaneously supplied with ammonium and nitrate in a split-root system. Annals of Botany 72: 107–115. [Google Scholar]
- Shane MW, McCully ME. 1999. Root xylem embolisms: implications for water flow to the shoot in single-rooted maize plants. Australian Journal of Plant Physiology 26: 107–114. [Google Scholar]
- Singh V, van Oost EJ, Jordan DR, Messina CD, Cooper M, Hammer GL. 2010. Morphological and architectural development of root systems in sorghum and maize. Plant and Soil 333: 287–299. [Google Scholar]
- Smith S, De Smet I. 2012. Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal E A, Araus V, Lu C, et al. 2010. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 107: 4477–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal EA, Moyano TC, Riveras E, Contreras-López O, Gutiérrez RA. 2013. Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proceedings of the National Academy of Sciences, USA 110: 12840–12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. 2006. Nitrogen regulation of root branching. Annals of Botany 97: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, McCully ME, Canny MJ. 1994. The branch roots of Zea. IV. The maturation and openness of xylem conduits in first-order branches of soil-grown roots. New Phytologist 126: 21–29. [Google Scholar]
- Yu P, Li X, Yuan L, Li C. 2014a. A novel morphological response of maize (Zea mays) adult roots to heterogeneous nitrate supply revealed by a split-root experiment. Physiologia Plantarum 150: 133–144. [DOI] [PubMed] [Google Scholar]
- Yu P, White PJ, Hochholdinger F, Li C. 2014b. Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 240: 667–678. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. 1998. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409. [DOI] [PubMed] [Google Scholar]
- Zhu J, Ingram PA, Benfey PN, Elich T. 2011. From lab to field, new approaches to phenotyping root system architecture. Current Opinion in Plant Biology 14: 310–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.