Abstract
The in vivo mitogenicity of basic fibroblast growth factor (bFGF) for arterial smooth muscle cells relies on the removal of endothelium, raising the question of whether the endothelium serves as a mechanical barrier preventing contact of circulating bFGF with underlying smooth muscle cells or as a biochemical barrier that produces a local inhibitor of bFGF activity. To better define the role of the intact endothelium in modulating the vascular and tissue deposition of bFGF, we compared the fate of intravenous injections of 125I-labeled bFGF with perivascular controlled growth factor release. Peak serum bFGF levels were detected within 1 min of injection, and the growth factor was cleared thereafter with a serum half-life of almost 3 min. Polymeric controlled release devices delivered bFGF to the extravascular space without transendothelial transport. Deposition within the blood vessel wall was rapidly distributed circumferentially and was substantially greater than that observed following intravenous injection. The amount of bFGF deposited in arteries adjacent to the release devices was 40 times that deposited in similar arteries in animals who received a single intravenous bolus of bFGF. Endothelial denudation had a minimal effect on deposition following perivascular release, and it increased deposition following intravenous delivery 2-fold. The presence of intimal hyperplasia increased deposition of perivascularly released bFGF 2.4-fold but decreased the deposition of intravenously injected bFGF by 67%. In contrast, bFGF was 5- to 30-fold more abundant in solid organs after intravenous injection than it was following perivascular release. Deposition was greatest in the kidney, liver, and spleen and was substantially lower in the heart and lung. Thus, bFGF is rapidly cleared following intravenous injection and is deposited within both solid organs and the walls of blood vessels. Unlike the mitogenic potential of bFGF within blood vessels, vascular deposition is virtually independent of the presence of endothelium. Perivascular delivery is far more efficient than intravenous delivery at depositing bFGF within the arterial wall, and an increased neointima may provide added substrate for potential bFGF deposition but has limited contact with intravascular growth factor as a result of dilutional and flow-mediated effects.
Full text
PDF
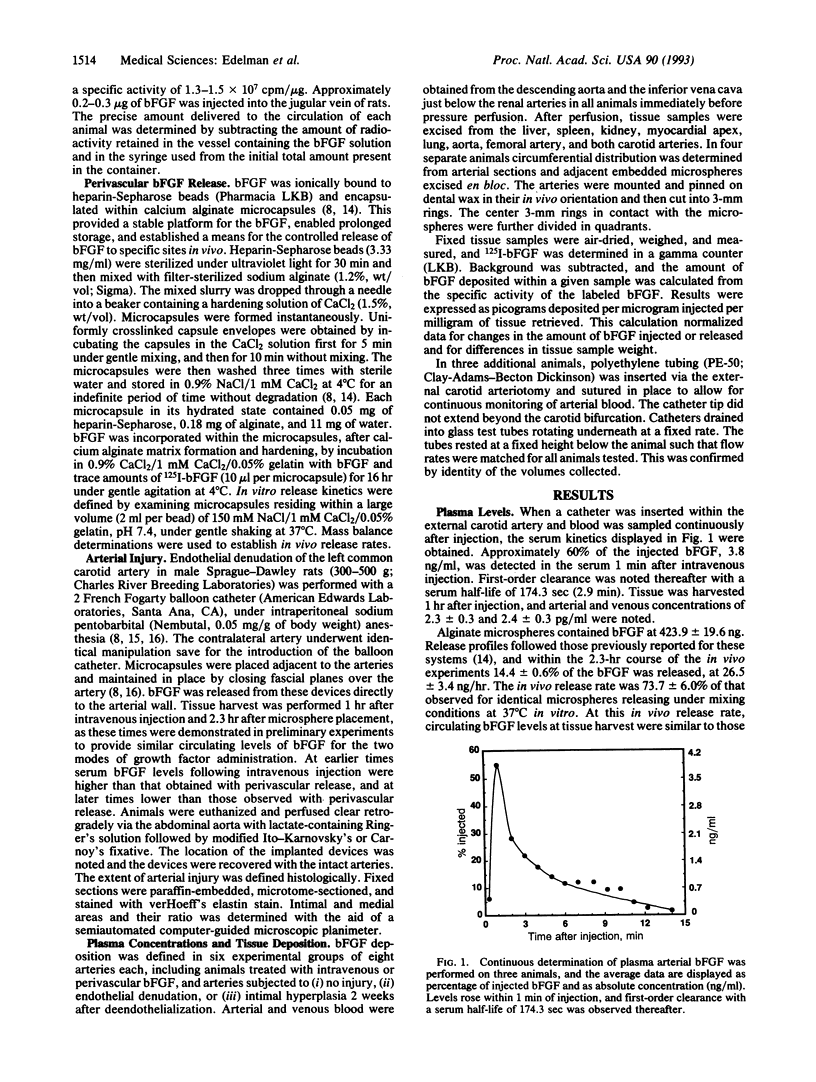
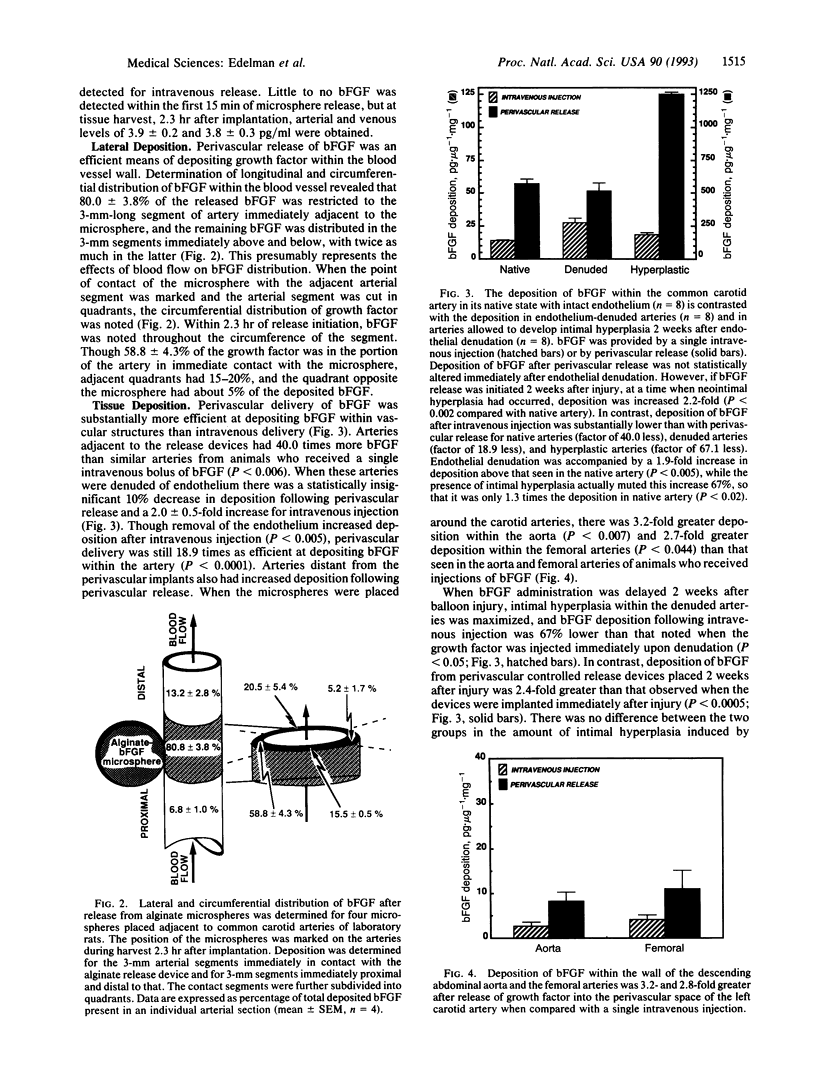
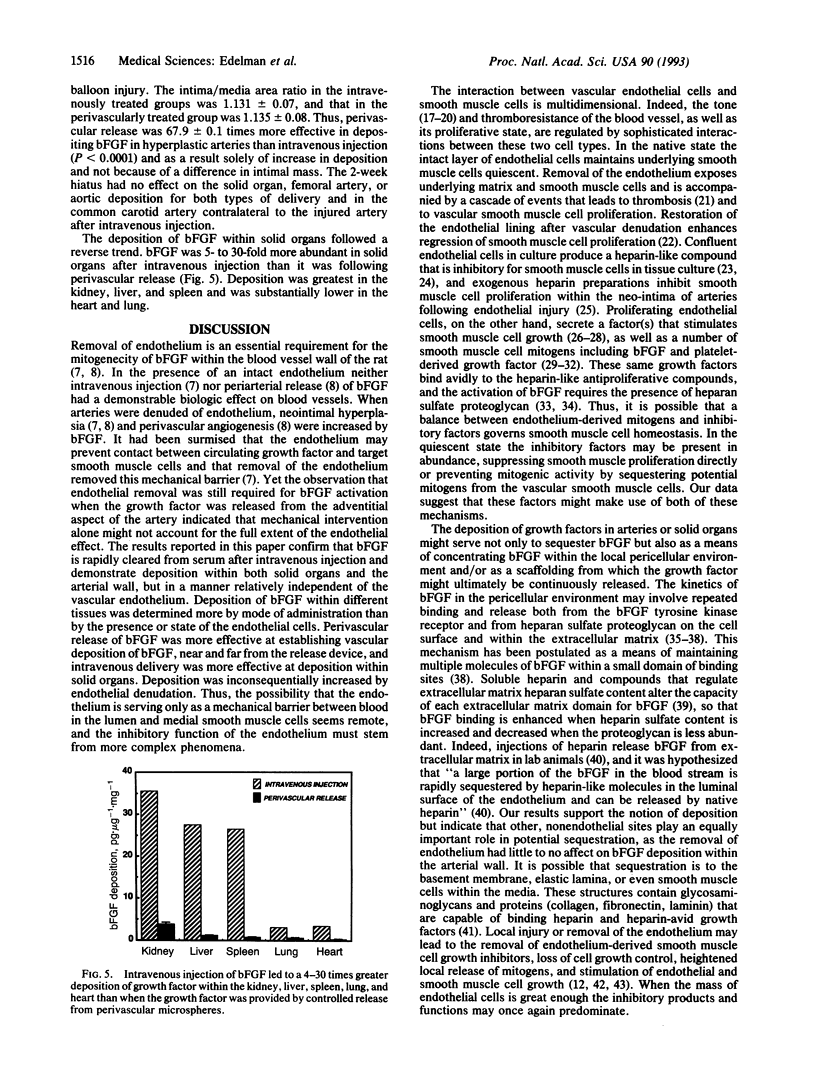

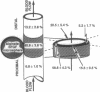
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger A. C., Beeuwkes R., 3rd, Lainey L. L., Silverman K. J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984 Jan 19;310(3):175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Bjornsson T. D., Dryjski M., Tluczek J., Mennie R., Ronan J., Mellin T. N., Thomas K. A. Acidic fibroblast growth factor promotes vascular repair. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8651–8655. doi: 10.1073/pnas.88.19.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Connolly D. T., Stoddard B. L., Harakas N. K., Feder J. Human fibroblast-derived growth factor is a mitogen and chemoattractant for endothelial cells. Biochem Biophys Res Commun. 1987 Apr 29;144(2):705–712. doi: 10.1016/s0006-291x(87)80022-0. [DOI] [PubMed] [Google Scholar]
- Cuevas P., Gonzalez A. M., Carceller F., Baird A. Vascular response to basic fibroblast growth factor when infused onto the normal adventitia or into the injured media of the rat carotid artery. Circ Res. 1991 Aug;69(2):360–369. doi: 10.1161/01.res.69.2.360. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Mathiowitz E., Langer R., Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991 Sep;12(7):619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M., Sasse J., Wadzinski M., Ingber D., Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988 Feb;130(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Fuster V., Steele P. M., Chesebro J. H. Role of platelets and thrombosis in coronary atherosclerotic disease and sudden death. J Am Coll Cardiol. 1985 Jun;5(6 Suppl):175B–184B. doi: 10.1016/s0735-1097(85)80552-0. [DOI] [PubMed] [Google Scholar]
- Hayek A., Culler F. L., Beattie G. M., Lopez A. D., Cuevas P., Baird A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun. 1987 Sep 15;147(2):876–880. doi: 10.1016/0006-291x(87)91011-4. [DOI] [PubMed] [Google Scholar]
- Ishai-Michaeli R., Svahn C. M., Weber M., Chajek-Shaul T., Korner G., Ekre H. P., Vlodavsky I. Importance of size and sulfation of heparin in release of basic fibroblast growth factor from the vascular endothelium and extracellular matrix. Biochemistry. 1992 Feb 25;31(7):2080–2088. doi: 10.1021/bi00122a027. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Edelman E. R. Biological and biochemical properties of fibroblast growth factors. Implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989 May-Jun;9(3):269–278. doi: 10.1161/01.atv.9.3.269. [DOI] [PubMed] [Google Scholar]
- Koo E. W., Gotlieb A. I. Endothelial stimulation of intimal cell proliferation in a porcine aortic organ culture. Am J Pathol. 1989 Mar;134(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Koo E. W., Gotlieb A. I. Neointimal formation in the porcine aortic organ culture. I. Cellular dynamics over 1 month. Lab Invest. 1991 Jun;64(6):743–753. [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Devesly P. Turnover of functional basic fibroblast growth factor receptors on the surface of BHK and NIH 3T3 cells. Growth Factors. 1990;3(1):25–33. doi: 10.3109/08977199009037499. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Metabolism of receptor-bound and matrix-bound basic fibroblast growth factor by bovine capillary endothelial cells. J Cell Biol. 1988 Aug;107(2):753–759. doi: 10.1083/jcb.107.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. A., Edelman E. R. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry. 1992 Sep 22;31(37):8876–8883. doi: 10.1021/bi00152a026. [DOI] [PubMed] [Google Scholar]
- Nugent M. A., Edelman E. R. Transforming growth factor beta 1 stimulates the production of basic fibroblast growth factor binding proteoglycans in Balb/c3T3 cells. J Biol Chem. 1992 Oct 15;267(29):21256–21264. [PubMed] [Google Scholar]
- Presta M., Maier J. A., Rusnati M., Ragnotti G. Basic fibroblast growth factor is released from endothelial extracellular matrix in a biologically active form. J Cell Physiol. 1989 Jul;140(1):68–74. doi: 10.1002/jcp.1041400109. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Anderson K. D., DiPietro J. M., Zwiebel J. A., Zametta M., Anderson W. F., Maciag T. Site-directed neovessel formation in vivo. Science. 1988 Sep 9;241(4871):1349–1352. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Whalen G. F., Shing Y., Folkman J. The fate of intravenously administered bFGF and the effect of heparin. Growth Factors. 1989;1(2):157–164. doi: 10.3109/08977198909029125. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Williams S. K. Regulation of intimal hyperplasia: do endothelial cells participate? Lab Invest. 1991 Jun;64(6):721–723. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]