Abstract
Possibly the best-characterized cubic membrane transition has been observed in the mitochondrial inner membranes of free-living giant amoeba (Chaos carolinense). In this ancient organism, the cells are able to survive in extreme environments such as lack of food, thermal and osmolarity fluctuations and high levels of reactive oxygen species. Their mitochondrial inner membranes undergo rapid changes in three-dimensional organization upon food depletion, providing a valuable model to study this subcellular adaptation. Our data show that cubic membrane is enriched with unique ether phospholipids, plasmalogens carrying very long-chain polyunsaturated fatty acids. Here, we propose that these phospholipids may not only facilitate cubic membrane formation but may also provide a protective shelter to RNA. The potential interaction of cubic membrane with RNA may reduce the amount of RNA oxidation and promote more efficient protein translation. Thus, recognizing the role of cubic membranes in RNA antioxidant systems might help us to understand the adaptive mechanisms that have evolved over time in eukaryotes.
Keywords: cubic membranes, oxidative stress, plasmalogens, very long-chain polyunsaturated fatty acids, structural antioxidant, RNA protection
Biomembranes are traditionally viewed as flat sheets of phospholipid bilayers dividing the cytoplasm into multiple subcellular compartments with specialized functions. However, biomembranes may also fold up into three-dimensional periodic arrangements termed ‘cubic membranes’ (figure 1) [1,2]. Cubic membranes can be observed in virtually any membrane-bound subcellular organelles [3]. Such induced membrane transition changes are frequently accompanied by alterations in cellular oxidative stress responses, such in neoplasia, inflammation and viral infection conditions [4,5]. We have suggested on the basis of these observations that cubic membrane formation may be associated with oxidative stress [6]. In living organisms, antioxidant enzymes form the first line of defence against reactive oxygen species (ROS) in the cellular environments [7]. These enzymes work in tandem to decrease the damaging effects of ROS in the cells.
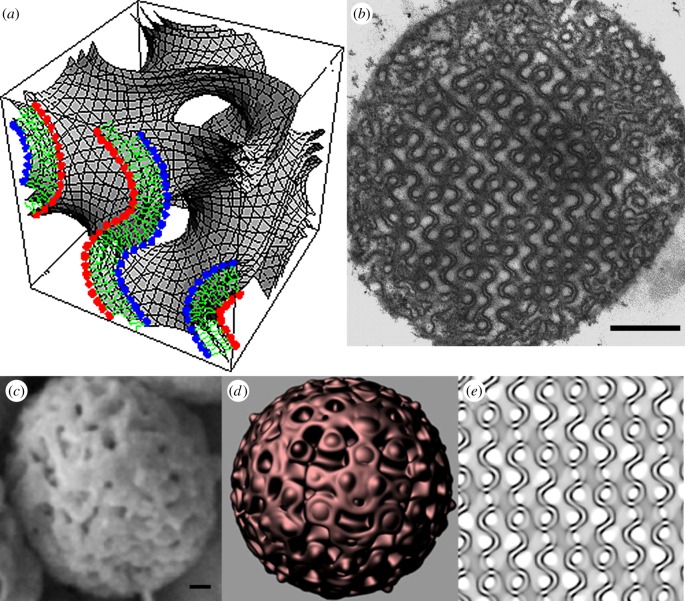
Figure 1.
Cubic membrane architecture. (a) Three-dimensional mathematical model representing the phospholipid bilayer of cubic membrane organization. (b) Two-dimensional transmission electron micrograph of the same three-dimensional model presented in (a). (c) Scanning electron micrograph and its corresponding (d) three-dimensional and (e) two-dimensional computer simulation model of cubic membranes found in the mitochondria of 10-day starved amoeba Chaos cells. Scale bars, (b) 500 nm and (c) 100 nm.
However, living organisms developed several other defence mechanisms to cope with oxidative stress. For example, in the unicellular organism Escherichia coli, levels of fumarase C, which is insensitive to superoxide anions, increase during oxidative stress, probably to replace fumarases A and B which are susceptible to damage by superoxide anions [8]. In other organisms, ‘sacrificial agents' are oxidized preferentially in oxidative stress conditions to protect important biomolecules [9]. These observations suggest that living organisms may use a wide range of biomolecules and mechanisms other than antioxidant enzymes to ameliorate the damaging effects of ROS.
It had been established that starved amoebae (Chaos carolinense) contain greater levels of free radicals than fed amoebae [6]. Starvation induces cubic membrane formation in amoeba Chaos mitochondria [10]. Similarly, in the higher plants, ‘light starvation’ (absence of the light) also induces cubic membrane formation in the photosynthetic thylakoid membranes, prolamellar bodies [11]. The transformed inner mitochondrial membranes into cubic organization in the starved amoeba Chaos exhibit a high content of very long-chain polyunsaturated fatty acids (VLC-PUFAs), specifically the C22 : 5n-6 modified phosphatidylcholine plasmalogen, phosphatidylethanolamine plasmalogen and phosphatidylinositol species, that appear to be critical for development and maintaining highly ordered yet curved interwoven cubic membrane structures [12].
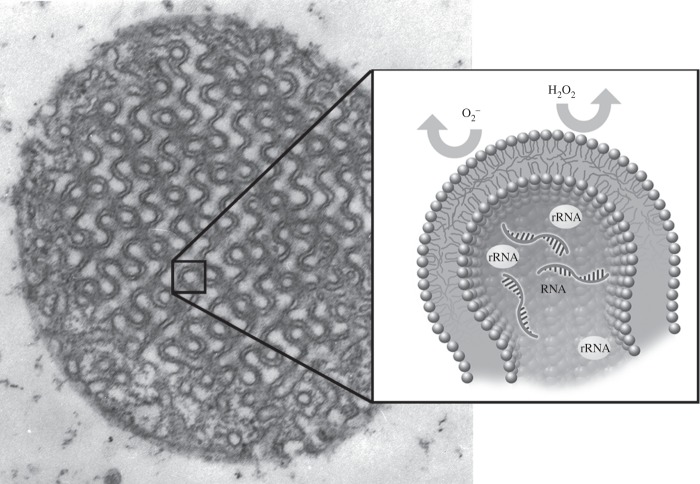
We note that the mitochondria with cubic membrane organization isolated from starved amoeba Chaos interact sufficiently with short segments of phosphorothioate oligonucleotides (PS-ODNs, resemble RNA in biological systems). We also study the ability to provide ODN uptake via cubic membranes [13]. Specifically, we have observed ODNs condensed within the convoluted channels (most likely within the mitochondrial intermembrane space rather than the matrix) of cubic membranes by an unknown passive targeting mechanism [13]. Moreover, the interaction between ODNs and cubic membranes is sufficient to retard ODN oxidation by free radicals in vitro (figure 2). Hence, the close similarity between the ODNs used experimentally and the RNAs. Cubic membranes, therefore, may act as a ‘protective’ shelter minimizing or preventing the oxidation of biologically essential macromolecules such as RNAs.
Figure 2.
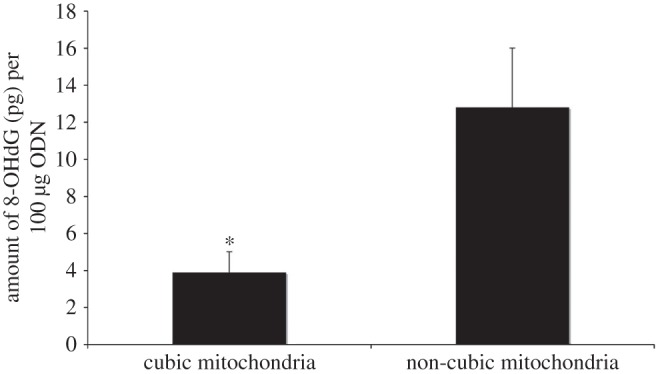
Bar graph depicts the difference in the amount of 8-OHdG (pg) per 100 μg of ODN in a mixture containing cubic mitochondria and that containing non-cubic mitochondria. The mixture containing non-cubic mitochondria has approximately four times as much 8-OHdG as that containing cubic mitochondria. In this experiment, mitochondria with cubic membrane organization were isolated from 7-day starved amoeba Chaos and the mitochondria without cubic membrane organization were isolated from mouse liver. Same amount of mitochondria protein was incubated with the same amount of ODN in separate tubes before the mixture was exposed to superoxide anions generated by the Fenton reaction. After exposure to the Fenton reaction, ODNs were isolated and assessed for oxidative damage. *p-value < 0.05.
It is believed that the alterations of gene expression and faithful translation of RNAs are the key factors of the metabolic changes essential for cell survival and the rapid organismal adaptation to new environmental stimuli. Oxidative damage to both coding and non-coding RNAs may therefore affect the regulation of gene expression and, potentially, result in the failure of protein synthesis. This failure may impair the organismal capacity of flexibly adapting to a novel or unusual internal or external environmental stimulus [14]. Furthermore, experimental data suggest that oxidative damage of RNAs may be involved in the cellular patho-mechanisms of several diseases and cell survivability [14]. In a eukaryotic cell, nearly all the DNA in the cell is sequestered within the nucleus. The nuclear envelope delimits the compartment and physically limits the interaction between the nucleus and the cytoplasm. Thus, RNAs rather than DNAs are ‘vulnerable’ targets of oxidation because of their biochemical structure, relatively abundant in the cell and they are mostly located in the vicinity of ROS producing organelles such as mitochondria and peroxisomes [15–21]. As a consequence, oxidative damage to RNA rather than DNA may be the more proximate cause of impairment in cellular performance and adaptability. Specific protective mechanisms of RNAs and the control of their degradation would be therefore expected to be present in cells [22].
Plasmalogens are a unique class of ether phospholipids with a vinyl ether bond at sn-1 position and enriched in PUFAs at sn-2 position of the glycerol backbone [23]. They form the major components of cubic membrane phospholipids and play a critical role in membrane plasticity and transformation into cubic structure [12]. We are interested whether the unusually high amounts of VLC-PUFAs and plasmalogens in cubic membranes and their ability to interact with ODNs play a role in the defence system of RNAs and, therefore, of the gene expression regulatory system in living organisms. Although the functions of VLC-PUFAs and plasmalogens in cubic membranes are still far from being fully understood, recent studies have shown that VLC-PUFAs may affect the expression of many genes and that these effects appear to be independent of any changes in membrane composition [16,24,25].
A number of studies have shown that plasmalogens are protective in lipid peroxidation [26,27]. Sindelar et al. [27] demonstrated that brain phospholipids with and without plasmalogens in separate liposomal systems were subjected to oxidative stress. The results revealed that in the presence of plasmalogens, markers for lipid peroxidation were significantly decreased. This implies that plasmalogens protect PUFAs from damage. Although the mechanism for this phenomenon has not been elucidated, it may involve vinyl ether bonds in plasmalogens which are more susceptible to oxidative attack than via ester bonds in phospholipids [28].
The free radical species formed during the peroxidation of the vinyl ether bond may either be more stable or be less efficient to abstract hydrogen than the alkyl radicals produced during the peroxidation of PUFAs [27]. Also, it is likely that the oxygenated vinyl ether radicals are broken down into water-soluble radical compounds which are unable to further propagate the oxidation cascade [27].
In addition to the mitochondrial antioxidant enzymes, plasmalogens can limit the diffusion of ROS within the different compartments of cubic membrane because of their proneness to be peroxidized. This mechanism would limit the intercellular transmission of ROS and that the oxidative cascade might spread to RNAs segregated into the inner compartments of cubic membranes. Some support for this proposition comes from experimental observations where cubic membranes strikingly correlate with viral infections; notably, RNA viruses [5]. Viral entry, proliferation and release are processes closely linked to cubic membrane formation [5]. Generating cubic membrane during viral genome proliferation may provide a protective membrane environment to protect the viral RNA from oxidative damage and facilitate faithful genomic transcription and translation.
Plasmalogen oxidation and VLC-PUFA peroxidation in phospholipids of cubic membranes have a number of negative downstream effects on the physical properties and structure of the membranes, such as the decrease in their fluidity and increases in lamellar membrane formation rather than cubic organization. However, an increasing body of evidence suggests that peroxidized phospholipids can be repaired by the enzymes phospholipase A2 and acyl-transferase [29]. While unesterified VLC-PUFAs present in the cytoplasm can be incorporated into the cellular membranes to replace the oxidized molecules.
In view of these observations, we propose here an integrative model (figure 3) where natural selection has operated in order to optimize a molecular composition to have a three-dimensional membrane shape that is enriched in plasmalogens containing VLC-PUFAs. Plasmalogens that carry VLC-PUFAs would facilitate cellular membrane transformation from the lamellar into the cubic arrangement. In this model, cubic membranes interact with short segments of ODNs such as RNAs to segregate RNAs and possibly other translationary systems into the inner compartments of cubic membranes. The high susceptibility of VLC-PUFAs and plasmalogens in cubic membranes to oxidation further retards RNAs oxidation. Cubic membranes may indirectly play a role in the defence system of RNAs. Our proposal implies biochemical pathways and highly ordered three-dimensional membranes in shape that are functionally integrated, possibly resulting in an evolutionary module such as cubic membranes for cell survival. The understanding of the biological relevance of RNA protection by cubic membranes and of its evolutionary underpinnings may explain fundamental aspects of selective pressures modulating the awareness of evolution in eukaryotic cells.
Figure 3.
The proposed mechanism of antioxidant defence system of cubic membranes in oxidative stress conditions. The high content of plasmalogens in cubic membranes may preferentially interact with superoxide anions protecting the biomolecules in cubic membrane channels. This may provide a safe environment for RNA and other protein synthesis machinery molecules (e.g. ribosomal RNA) within the internal compartments of the cubic membranes.
Acknowledgements
We thank Craig McLachlan for the critical reading of the manuscript. We also thank Mark Mieczkowski for the cubic membrane simulation projection program (QMSP), Felix Margadant for computer simulation of scanning electron microscopic image and Aikkia Khaw for his artwork presented in figure 3.
References
- 1.Landh T. 1995. From entangled membranes to eclectic morphologies: cubic membranes as subcellular space organizers. FEBS Lett. 369, 13–17. ( 10.1016/0014-5793(95)00660-2) [DOI] [PubMed] [Google Scholar]
- 2.Almsherqi ZA, Kohlwein SD, Deng Y. 2006. Cubic membranes: a legend beyond the Flatland of cell membrane organization. J. Cell Biol. 173, 839–844. ( 10.1083/jcb.200603055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almsherqi ZA, Landh T, Kohlwein SD, Deng Y. 2009. Cubic membranes: the missing dimension of cell membrane organization. Int. Rev. Cell Mol. Biol. 274, 275–341. ( 10.1016/S1937-6448(08)02006-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almsherqi ZA, McLachlan CS, Mossop P, Knoops K, Deng Y. 2005. Direct template matching reveals a host subcellular membrane gyroid cubic structure that is associated with SARS virus. Redox Rep. 10, 167–171. ( 10.1179/135100005X57373) [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Almsherqi ZA, Ng MM, Kohlwein SD. 2010. Do viruses subvert cholesterol homeostasis to induce host cubic membranes? Trends Cell Biol. 20, 371–379. ( 10.1016/j.tcb.2010.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, Kohlwein SD, Mannella CA. 2002. Fasting induces cyanide-resistant respiration and oxidative stress in the amoeba Chaos carolinensis: implications for the cubic structural transition in mitochondrial membranes. Protoplasma 219, 160–167. ( 10.1007/s007090200017) [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martin V, Reiter RJ. 2004. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36, 1–9. ( 10.1046/j.1600-079X.2003.00092.x) [DOI] [PubMed] [Google Scholar]
- 8.Payá M, Halliwell B, Hoult JR. 1992. Peroxyl radical scavenging by a series of coumarins. Free Radic. Res. Commun. 17, 293–298. ( 10.3109/10715769209079522) [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B, Gutteridge J. 2007. Free radicals in biology and medicine, 4th edn. New York, NY: Oxford University Press.
- 10.Deng Y, Mieczkowski M. 1998. Three-dimensional periodic cubic membrane structure in mitochondria of amoeba Chaos carolinensis. Protoplasma 203, 16–25. ( 10.1007/BF01280583) [DOI] [Google Scholar]
- 11.Selstam E, Schelin J, Williams WP, Brain AP. 2007. Structural organisation of prolamellar bodies (PLB) isolated from Zea mays. Parallel TEM, SAXS and absorption spectra measurements on samples subjected to freeze-thaw, reduced pH and high-salt perturbation. Biochim. Biophys. Acta 1768, 2235–2245. ( 10.1016/j.bbamem.2007.05.005) [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Almsherqi ZA, Shui GH, Wenk MR, Kohlwein SD. 2009. Docosapentaenoic acid (DPA) is a critical determinant of cubic membrane formation in amoeba Chaos mitochondria. FASEB J. 23, 2866–2871. ( 10.1096/fj.09-130435) [DOI] [PubMed] [Google Scholar]
- 13.Almsherqi Z, Hyde S, Ramachandran M, Deng Y. 2008. Cubic membranes: a structure-based design for DNA uptake. J. R. Soc. Interface 5, 1023–1029. ( 10.1098/rsif.2007.1351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. 2009. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 118, 151–166. ( 10.1007/s00401-009-0508-1) [DOI] [PubMed] [Google Scholar]
- 15.Poulsen HE, et al. 2012. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 52, 1353–1361. ( 10.1016/j.freeradbiomed.2012.01.009) [DOI] [PubMed] [Google Scholar]
- 16.de Urquiza AM, Liu SY, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perimann T. 2000. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290, 2140–2144. ( 10.1126/science.290.5499.2140) [DOI] [PubMed] [Google Scholar]
- 17.Jenner P. 2003. Oxidative stress in Parkinson's disease. Ann. Neurol. 53(Suppl. 3), S26–S36: discussion S36–S38 ( 10.1002/ana.10483) [DOI] [PubMed] [Google Scholar]
- 18.Bŕegeon D, Sarasin A. 2005. Hypothetical role of RNA damage avoidance in preventing human disease. Mutat. Res. 577, 293–302. ( 10.1016/j.mrfmmm.2005.04.002) [DOI] [PubMed] [Google Scholar]
- 19.Fiala ES, Conaway CC, Mathis JE. 1989. Oxidative DNA and RNA damage in the livers of Sprague-Dawley rats treated with the hepatocarcinogen 2-nitropropane. Cancer Res. 49, 5518–5522. [PubMed] [Google Scholar]
- 20.Li Z, Wu J, DeLeo CJ. 2006. RNA damage and surveillance under oxidative stress. IUBMB Life 58, 581–588. ( 10.1080/15216540600946456) [DOI] [PubMed] [Google Scholar]
- 21.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. 1999. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 19, 1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houseley J, Tollervey D. 2009. The many pathways of RNA degradation. Cell 136, 763–776. ( 10.1016/j.cell.2009.01.019) [DOI] [PubMed] [Google Scholar]
- 23.Braverman NE, Moser AB. 2012. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 1822, 1442–1452. ( 10.1016/j.bbadis.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 24.Kitajka K, Puskás LG, Zvara A, Hackler L, Barcelo-Coblijn G, Yeo YK, Farkas T. 2002. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc. Natl Acad. Sci. USA 99, 2619–2624. ( 10.1073/pnas.042698699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitajka K, Sinclair AJ, Weisinger RS, Weisinger HS, Mathai M, Jayasooriya AP, Halver JE, Puskás LG. 2004. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl Acad. Sci. USA 101, 10 931–10 936. ( 10.1073/pnas.0402342101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. 1999. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 338, 769–776. ( 10.1042/0264-6021:3380769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sindelar PJ, Guan Z, Dallner G, Ernster L. 1999. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 26, 318–324. ( 10.1016/S0891-5849(98)00221-4) [DOI] [PubMed] [Google Scholar]
- 28.Brites P, Waterham HR, Wanders RJA. 2004. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta 1636, 219–231. ( 10.1016/j.bbalip.2003.12.010) [DOI] [PubMed] [Google Scholar]
- 29.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. 2001. The essentiality of long-chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 40, 1–94. ( 10.1016/S0163-7827(00)00017-5) [DOI] [PubMed] [Google Scholar]