Abstract
Individual animals are adept at making decisions and have cognitive abilities, such as memory, which allow them to hone their decisions. Social animals can also share information. This allows social animals to make adaptive group-level decisions. Both individual and collective decision-making systems also have drawbacks and limitations, and while both are well studied, the interaction between them is still poorly understood. Here, we study how individual and collective decision-making interact during ant foraging. We first gathered empirical data on memory-based foraging persistence in the ant Lasius niger. We used these data to create an agent-based model where ants may use social information (trail pheromones), private information (memories) or both to make foraging decisions. The combined use of social and private information by individuals results in greater efficiency at the group level than when either information source was used alone. The modelled ants couple consensus decision-making, allowing them to quickly exploit high-quality food sources, and combined decision-making, allowing different individuals to specialize in exploiting different resource patches. Such a composite collective decision-making system reaps the benefits of both its constituent parts. Exploiting such insights into composite collective decision-making may lead to improved decision-making algorithms.
Keywords: decision-making, memory, communication, recruitment, foraging, agent-based modelling
1. Introduction
Group decision-making is key to many social behaviours in organisms ranging from eusocial insects, through shoaling fish, to occasionally social animals such as cockroaches, and possibly even slime moulds [1–6]. In some situations, groups must make a unanimous decision (consensus decision-making), while in others different group members may make different decisions (combined decision-making). Positive feedback is a key feature of most, if not all, consensus decision-making systems. It allows differences between options to be amplified until a decision is reached, a phenomenon known as symmetry breaking [5,7,8]. Such collective decisions allow groups to make decentralized decisions about which food source to exploit, which shelter to use or which path to take [1,3,9,10]. However, one danger of positive feedback-based decision-making is a lack of flexibility [5]. Decisions, once made, are difficult to revoke, making reacting to a changing environment challenging. Furthermore, due to the phenomenon of symmetry-breaking, some studies have reported groups being unable to focus on more than one food source [1,7].
The power of individuals to make adaptive decisions is well known. For example, individuals can make adaptive speed–accuracy trade-offs depending on their danger levels, their internal state and their past experience [11,12], and can memorize the location of important resources and return to them repeatedly [13,14]. Individuals are also capable of reacting to a rapidly changing environment. For example, as some individuals make one decision, thus changing the environment, others may react by making a different decision. This, for example, allows individuals to distribute themselves optimally among resources of different quality. Both individually foraging ants and mass-recruiting ants have been shown to be able to track changes in the environment by sending more workers to the current best food source [15–17] This, however, is in contrast to the classic understanding of mass-recruiting ants, which are often considered to be both unable to effectively track changes in the environment, and unable to focus on more than one resource at a time [1,7,10,18]. Thus, it is clear that something is missing from the traditional understanding of collective decision-making. We hypothesized that a key missing feature in our understanding of collective decision-making is an understanding of how the use of social and private information by individuals affects group-level decision-making. We propose that consensus and combined decision-making can function in tandem.
Differences between individuals can drive collective decision-making [8,19]. Even a single informed individual can guide a shoal of naive fish to a feeding location, and similar effects have been demonstrated in swarming bees and human crowds [2,20,21]. Much recent attention has focused on understanding how individuals use combinations of public, private and social information [22,23]. For example, private information is often prioritized over public or social information [24–26], but social information can gain importance when private information becomes unrewarding or outdated in some species and situations [27,28], but not in others [29]. The relative importance of public and social information will depend strongly of the ecology of the organism. For example, ants specializing in retrieving small, non-replenishing food items may require no recruitment or long-term memory. Ants specializing in retrieving large items collectively may rely heavily on social information [30]. Ants from small colonies may be below the threshold for successful use of social information [31], so rely purely on private information. Animals that exploit replenishing semi-permanent food sources, such as honeybees and Lasius niger ants, may use both information sources [25,26]. Public, social and private information may be used in a hierarchical order [32] or they may be used additively or synergistically [33,34]. Social information may also be used to trigger private information use [35,36]. Progress is being made in understanding how individuals use public, private and social information, and the need for such a unification of individual- and group-level decision-making has been recently discussed [37].
Recent models have attempted to understand how characteristics of individual social insects affect group-level decision-making [38,39]. However, the interaction between individual cognitive abilities, such as memory use, and collective decision-making systems, such as recruitment using pheromones, is only beginning to be tackled. Informed individuals may be able to use their private information to drive or speed up group decisions [40,41]. Similarly, informed individuals can communicate their information to the group, improving collective decision-making. For example, Schürch & Grüter [42] recently simulated honeybee colonies in which social information sharing was disrupted, and found that individuals who received social information went on to exploit it and rapidly collect large amounts of food. Here, we first collect empirical data on memory formation and persistence of returning to unrewarding food sources in the ant L. niger. We then use these data to model memory and pheromone use, either alone or in combination, at a colony level, in a changing environment with limited resources. We find that private and social information use complement each other: when individuals could use both information sources, colonies achieved higher food intake due to a more efficient exploitation of their environment.
2. Material and methods
(a). Defining the foraging persistence of ants
The aim of this experiment was to explore how rapidly individual L. niger foragers can switch their attention from a newly unproductive to a newly productive feeder, and how the presence of trail pheromones affects such switching, so as to understand how individual foragers respond to a changing environment. A detailed description of the empirical data collection and statistical analysis is provided in electronic supplementary material S1. In brief, individual ants were trained to a feeder at one end of a T maze and allowed to make repeated visits to this feeder. After 1, 3, 5, 10 or 15 visits the feeder was moved to the other arm of the T maze. The ants would begin searching in the old feeder location, but eventually find the new feeder. We tracked the continued repeat visits of the ants until they had each performed three consecutive correct decisions (i.e. heading directly for the new feeder location). Trail pheromone was either removed continuously from the apparatus or allowed to accumulate.
(b). Examining the role of memory and trail pheromones on colony-level performance using an agent-based model
As reliably preventing ants from depositing pheromone or forming memories is not possible, we examined the roles of route memory and trail pheromones, alone and in combination, in silico, using an agent-based model implemented in netlogo v. 5.0.3 [43]. Agent-based models are well suited to investigating emergent spatial systems such as ant trail networks [44] and have been used successfully to model many aspects of collective organization [45–48].
In our model, an ant colony was presented with an environment containing a variable number of food sources. Each food source can be exploited by one ant before it becomes exhausted, but refills after a certain time delay. These food sources represent replenishing food patches such as aphid colonies or nectar sources, which are the main food source of many ant species, including L. niger. Ants leave the nest and begin scouting. An ant that finds a full food source returns to the nest and is then ready to exploit another food source. If memories are enabled, the ant immediately returns to the feeder it has memorized (for details see electronic supplementary material S1), ignoring trail pheromones. The foraging persistence of the ants was based directly on the empirical data we gathered, with a look-up table populated with the empirical data we gathered on foraging persistence available to the ants. The ants compared the number of rewarding visits to their memorized feeder with the number of unrewarding visits and used the look-up table to draw a probability of changing their memorized feeder (see electronic supplementary material S1 for details). In half of the trials, foraging persistence was based directly on the empirical data, with the memory of the number of repeated visits to a food source being tallied up to a maximum of 15. In other trials, foraging persistence was reduced by only allowing the memories of repeated visits to a feeder to tally up to one. When social information (e.g. pheromone trails) and private information (e.g. memories) conflict, most (but not all) social insects—including L. niger—follow the private information [24–26]. Thus, when the two information sources conflict in our model, private information (memory) is followed. Ants who reach an empty feeder wait a variable length of time and then begin scouting. Scouting ants who encounter a pheromone trail follow it [30]. The number of ants is linked to the number of food sources and their refill rates, so that in principle each food source could be almost fully exploited—the moment the food source refills an ant should be available to exploit it. The location of the food sources is either random or fixed in a ring a set distance from the nest entrance (figure 1). Environmental change is implemented by having the rate at which food sources refill, and in some cases their location, change a varying number of times throughout each simulation. Models were run with both memory and pheromone trails enabled, only memory or pheromone trails enabled, or neither information source enabled. A complete list of the variables and the levels modelled is provided in table 1. Each possible combination of these variables was tested 500 times. A detailed description of the model is provided in electronic supplementary material S1, following the ‘overview, design concepts, details’ (ODD) protocol [49,50]. Figure 1 shows an annotated screenshot of the model. The fully annotated model code is provided in electronic supplementary material S2, via Dryad (doi:10.5061/dryad.9k219). A sensitivity analysis, in which fixed variables of the main model are systematically varied, is presented in electronic supplementary material S1.
Figure 1.
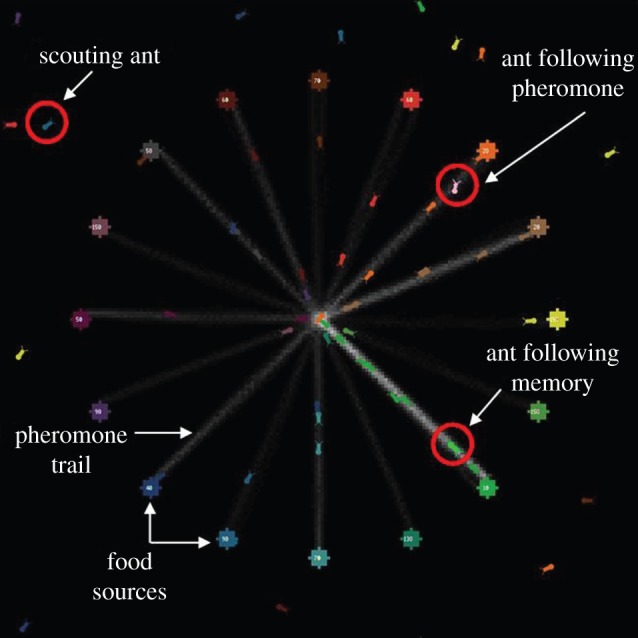
Annotated screenshot of the model. The nest entrance is located in the centre of the world and is surrounded by 16 food sources, which are non-randomly distributed. This screenshot was taken at time-step 2000. (Online version in colour.)
Table 1.
Variable levels tested in the agent-based model.
| variable | levels modelled |
|---|---|
| memory enabled? | ‘true’, ‘false’ |
| pheromone enabled? | ‘true’, ‘false’ |
| random feeder locations? | ‘true’, ‘false’ |
| maximum memory tally (=foraging persistence) | 1 (low foraging persistence), 15 (realistic foraging persistence) |
| number of food sources | 4, 8, 16, 32 |
| number of environmental changes | 1, 3, 5, 7, 9 |
| time-steps agents wait at a depleted food source before searching for other food sources | 1, 30 |
The efficiency of a colony at exploiting its environment was recorded in two ways: by measuring the average time a feeder remained full (the lower, the better) and by measuring the total food intake of the colony (the higher, the better).
3. Results
(a). Empirical experiments: foraging persistence
Ants who had made more training visits to the initial feeder location tended to return to the unrewarding location for longer than those with fewer training visits (figure 2). This increase in persistence plateaued at around 10 visits. The presence or absence of trail pheromone had little effect on the overall pattern. For a detailed presentation of the empirical results, see electronic supplementary material S1.
Figure 2.

The mean proportion of correct decisions made by ants depends on the number of visits they made to the new food location (visit number—x-axis) and the number of previous training visits to the old food location (different colours and symbols). Visit 2 to the new food location is the first visit in which the ants can potentially know that the food location has changed. Whiskers represent 95% CIs for the means. Data from trials in which pheromone was removed or allowed are merged in this figure. Only the first 12 visits post-switch, and only training visit numbers 1, 3, 5, 10 and 15, are shown for clarity. (Online version in colour.)
(b). Agent-based model
As the model has multiple variables, each with multiple levels, which are all systematically varied, presenting an exhaustive comparison of all combinations of variables would be excessive. Thus, we present the comparisons that we believe have the greatest significance. However, we also provide the entire dataset online via Dryad (doi:10.5061/dryad.9k219), so as to allow readers to examine any combination of factors they wish. Readers may also explore changes in variable levels we did not vary by running the model themselves, using the original model file also provided.
Overall, when neither memory nor pheromone trails are enabled, ant colonies retrieved relatively little food. When either memory or trail pheromones are enabled, food retrieval increases dramatically (figure 3). As predicted, memory and trail pheromones can act in concert, increasing food intake beyond what either effect can achieve alone. These results are mirrored in the average proportion of feeders unexploited at any given time (electronic supplementary material, figure S2).
Figure 3.

Average total food returned after 4000 time-steps is affected by whether memory and/or trail pheromones are enabled. In this and all following figures, symbols are means and whiskers are 95% CIs. All variables not explicitly separated in the figures are pooled.
Two factors that interacted strongly were the location of the food patches (random or fixed) and the rate of environmental change. These two factors strongly influenced the value of route memory and trail pheromones. When food patch locations were fixed, the rate of environmental change only slightly affected the utility of memory or pheromone, with pheromone trails becoming more effective in more static environments (figure 4a). However, if feeder locations were randomly distributed, pheromone trails became much less effective as environmental changes became more frequent (figure 4b). Indeed, pheromone hindered food exploitation in rapidly changing environments in which food locations, as well as quality, changed. Likewise, but to a lesser extent, individual memory was also less effective in such changing environments.
Figure 4.
The effect of pheromone and memory on food retrieval in a changing environment in which either (a) food patch quality (but not location) changes or (b) both food patch quality and location change.
Food patch location also interacted with the number of food patches and affected the utility of trail pheromone. When food patch locations were non-random, trail pheromone was always effective at reducing the average time food patches remained unexploited, consequently increasing food retrieval (figure 5a). Indeed, when the number of food patches was very low and their location fixed, pheromone trails were more effective than memory. However, when food patches were randomly placed, trail pheromone was beneficial at low food patch numbers, but disruptive at high food patch numbers (figure 5b).
Figure 5.
The effect of memory and pheromone on the average proportion of unexploited feeders, depending on the number of food patches in an environment where food patch location (a) does not or (b) does change. In a changing environment with a large number of low-quality food patches, trail pheromones disrupt colony performance.
In the main model, varying the level of foraging persistence has relatively little impact on food intake. Decreasing the foraging persistence caused a decline in colony-level performance, especially in slowly changing environments (figure 6). With a more realistic level of foraging persistence, the ants are more capable of efficiently distributing their workforce, resulting in higher food intake. However, during the sensitivity analysis of experiment duration, we discovered that the effect of realistic foraging persistence is enhanced if the experiment is allowed to run longer than the standard run (16 000 time steps, ≈4 h). In such longer model runs, colonies in which ants had a realistic level of foraging persistence retrieved 9.7% more food than those with a low foraging persistence (see electronic supplementary material S1).
Figure 6.

How foraging persistence (maximum memory) affects colony-level performance. In more static environments, a realistic level of foraging persistence (the tally of repeated visits to a feeder can reach 15) results in better colony-level performance than a lower level of persistence (memory tally limited to one). This effect becomes more pronounced if the model is allowed to run longer (see electronic supplementary material S1). Only data in which memories are enabled are presented in this figure.
4. Discussion
Social animals, especially eusocial insects such as ants and bees, have both private and social information available to them [25,26,51]. The manner in which individuals and groups use these information sources individually has been the focus of intense study [5,10,23,26,52]. However, the information available to individuals is often ignored when studying group decision-making, even if the decisions of individuals are studied [5,8]. While this is often done intentionally in the name of parsimony [5], our results provide an example where over-simplification of the individuals (in this case by assuming no memory) causes important effects on collective decision-making to be missed. The way in which the dual use of private and social information by individuals affects group-level decision-making is still poorly understood, and the fields of group- and individual-level decision-making would benefit from being linked [37].
Our agent-based models demonstrate that individual decision-making based on both private information and social information can lead to improved group performance, allowing a more efficient exploitation of temporally limited resources. Both private and social information come with benefits and costs during group decision-making. In our system, colonies benefit from the use of social information by recruitment to food sites worth exploiting, but suffer from the inability to reallocate workers from over-exploited food sites or to new food locations after an environmental change. The use of private information allows a more efficient distribution of workers among food sites of variable productivity, but suffers from an inability to quickly exploit very productive food sites via recruitment, especially when food sites are rare. The benefits of both recruitment systems are additive when combined, allowing both targeted recruitment and efficient worker distribution. This, in turn, results in higher rates of food return and more efficient exploitation of the environment. Much like a composite material, which gains the benefits of the materials from which it is made, the ant colonies in our model demonstrate composite decision-making, gaining the benefits of both social and private information-based decision-making. Encouragingly, many of the patterns we report are also reported by Schürch & Grüter [42] in a recent model of honeybee foraging. While their model was specifically designed to test the efficacy of experimental manipulation of honeybee waggle dance use, they also found combined private and social information use to be more effective than either in isolation. Moreover, as in this study, they report that social information is of greater benefit with rarer food sources and that social information may be detrimental in rapidly changing environments. Such parallel findings in two different studies designed for different reasons strongly suggests that these findings represent general principles regarding information use in group decision-making.
The value of informed individuals or individuals with differing personalities in leading group decision-making has been well demonstrated [2,19–21,40,53,54]. However, a general assumption usually made is that the cohesion of the group must be maintained. This is not always the case. When the benefits of split decision-making outweigh the benefits of cohesion, group fission can occur [55]. Moreover, in socially foraging animals that share information, exploiting multiple food sources can keep the group informed of other options in the environment [56,57]. The tendency towards assuming a single-state solution may have hampered our understanding of group decision-making. This is the difference between consensus and combined decision-making. In the study of social insects, for example, colonies are often interrogated as to which of multiple options of various qualities they collectively choose [1,10,17,54,58–60]. However, field observations of social insects note the simultaneous exploitation of multiple resources [61]. In effect, the colonies simultaneously choose multiple ‘answers’ to the question of where to allocate foragers. This is reasonable, as by foraging on one food source a forager will make the same food source less valuable, and so less worthy of exploitation by a second forager. In our model, the ants were able to use memory-based foraging persistence to spread themselves out efficiently among the available food sources, thus exploiting the environment more effectively. The ability to use resources more optimally by taking advantage of individual flexibility may be valuable not only for social insects. Group-living animals may respond to a dispersed distribution of food sources by group fission, and groups may fuse back together when large, clumped resources are available for exploitation [55]. Further afield, networking algorithms based on ant colony foraging (e.g. the ant colony optimization metaheuristics, or ACO [62]) may benefit from allowing some degree of combined decision-making as well as consensus decision-making, by allowing individual agents to have private as well as social information. The inclusion of a ‘pseudo-memory’ alongside more conventional social information use in ACO algorithms may, for example, allow data-routing algorithms to make more efficient use of existing telecommunication networks, by routing some traffic via less direct, but underused, portions of the network.
Realistic foraging persistence allowed the colonies to exploit the environment more efficiently, as long as the rate of environmental change was low. This effect is cumulative, and stronger when the model is allowed to run longer (electronic supplementary material S1). The real ants persisted in visiting the unrewarding feeder for longer with increasing training visits to the original food location. This effect plateaus at around 10 visits to a food source. Similarly, honeybees persist in visiting unrewarding food sources, and their persistence increases for higher-quality, or closer, food sources [56]. It seems a certain degree of foraging persistence allows the colonies to achieve a more efficient distribution of workers, and thus increased long-term food retrieval. When persistence is too low, workers ‘give up’ on a patch too quickly, and the system fails to achieve a high-efficiency state. Thus, responding too rapidly to current conditions may be maladaptive at a collective level. This may help explain why foraging ants make use of information on multiple time frames, from real-time trail use information via encounter rates, through use over the last hours via trail pheromones, to use over the previous days via home-range markings [34,63–65].
One might expect a priori to find a speed–accuracy trade-off when varying foraging persistence, with lower persistence being advantageous in rapidly changing environments, and higher persistence allowing a more accurate, but slower, distribution of foragers in a slowly changing environment. However, we saw no hint of changeable foraging being more effective than a more resilient memory, even in the most rapidly changing environment. It must be noted that in the empirical experiment, there were only two food locations present. It is conceivable, and even likely, that the foraging persistence of the ants would differ if, after their training to one food source, they were allowed to discover several different food sources. Lasius niger foragers can memorize and recall at least two different feeder locations simultaneously [36], and so the data from our empirical experiment represent foraging persistence, not memory-overwriting. In honeybees, inspector bees periodically return to unrewarding food sources they know about and can reactivate exploitation of that food source via recruitment [66]. In the model, ants could only memorize one location. This choice was deliberate, as there are no data available on foraging persistence to multiple food sources. However, we speculate that in reality disappointed foragers may inspect the other food locations they know directly, instead of beginning a random search. This may speed up the process of evenly distributing foragers in line with patch productivity.
A second assumption made in the model, which holds for many but not all cases, is that private information (memories) over-ride social information (pheromones). If the opposite were true, we would expect trail pheromones to interfere more strongly with foraging in more variable environments, as many more ants would be channelled to over-exploited food patches. The memory-based process of forager distribution relative to patch qualities would also be disrupted. Responsiveness to over-exploitation may be slowed, as persistence would be governed by pheromone evaporation and deposition, rather than memory. Predicting the effect of an intermediate choice rule between the two information sources is more difficult. Lastly, pheromones interact with memories and other information sources in a wide variety of ways [18]. For example, pheromones may support learning, or increase walking speed [33,34]. How such interactions affect group-level behaviour is an open question.
Our results demonstrate that utilization of social and private information by individuals can result in increased group efficiency. Such composite collective decision-making systems are likely to be widespread in nature, but have been underappreciated since the link between individual- and group-level decision-making is still poorly understood. By taking this interaction into account, ecologists may better understand collective decisions observed in nature. The realization that private and public decision-making systems are not mutually exclusive may also be usefully exploited in the development of accurate and flexible decision-making algorithms. While collective decision-making algorithms tend to use very simple, homogeneous agents [62], our results imply that a small amount of individual complexity can greatly improve collective decision-making.
Supplementary Material
Acknowledgements
We thank Christoph Grüter, Ofer Feinerman and two anonymous reviewers for constructive comments on previous versions of this manuscript.
Data accessibility
The fully functioning model, as well as the datasets from the model runs and empirical data, are available as electronic supplementary material from Dryad (doi:10.5061/dryad.9k219).
Authors' contributions
T.J.C. conceived and designed the study, collected and analysed the empirical data, co-wrote the model and wrote the manuscript. B.C. co-wrote the model and ran the simulations, and revised the manuscript. C.I. assisted in data collection and revised the manuscript. J.H. coordinated the project and critically revised the manuscript. All authors gave final approval for publication.
Funding
T.J.C. was funded by an Alexander von Humboldt postdoctoral fellowship. B.C., C.I. and J.H. received no funding for work on this project.
References
- 1.Beckers R, Deneubourg JL, Goss S, Pasteels JM. 1990. Collective decision making through food recruitment. Insectes Sociaux 37, 258–267. ( 10.1007/BF02224053) [DOI] [Google Scholar]
- 2.Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403–409. ( 10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 3.Amé J-M, Halloy J, Rivault C, Detrain C, Deneubourg JL. 2006. Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA 103, 5835–5840. ( 10.1073/pnas.0507877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couzin ID. 2009. Collective cognition in animal groups. Trends Cogn. Sci. 13, 36–43. ( 10.1016/j.tics.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 5.Jeanson R, Dussutour A, Fourcassié V. 2012. Key factors for the emergence of collective decision in invertebrates. Front. Neurosci. 6, 121 ( 10.3389/fnins.2012.00121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid CR, Beekman M, Latty T, Dussutour A. 2013. Amoeboid organism uses extracellular secretions to make smart foraging decisions. Behav. Ecol. 24, 812–818. ( 10.1093/beheco/art032) [DOI] [Google Scholar]
- 7.Sumpter DJT, Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280. ( 10.1006/anbe.2003.2224) [DOI] [Google Scholar]
- 8.Lanan MC, Dornhaus A, Jones EI, Waser A, Bronstein JL. 2012. The trail less travelled: individual decision-making and its effect on group behavior. PLoS ONE 7, e47976 ( 10.1371/journal.pone.0047976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald TD, Costa JT. 1986. Trail-based communication and foraging behavior of young colonies of forest tent caterpillars (Lepidoptera: Lasiocampidae). Ann. Entomol. Soc. Am. 79, 999–1007. ( 10.1093/aesa/79.6.999) [DOI] [Google Scholar]
- 10.Goss S, Aron S, Deneubourg JL, Pasteels JM. 1989. Self-organized shortcuts in the Argentine ant. Naturwissenschaften 76, 579–581. ( 10.1007/BF00462870) [DOI] [Google Scholar]
- 11.Krebs JR, Kacelnik A, Taylor P. 1978. Test of optimal sampling by foraging great tits. Nature 275, 27–31. ( 10.1038/275027a0) [DOI] [Google Scholar]
- 12.Marshall JAR, Dornhaus A, Franks NR, Kovacs T. 2006. Noise, cost and speed-accuracy trade-offs: decision-making in a decentralized system. J. R. Soc. Interface 3, 243–254. ( 10.1098/rsif.2005.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosengren R, Fortelius W. 1986. Ortstreue in foraging ants of the Formica rufa group: hierarchy of orienting cues and long-term memory. Insectes Sociaux 33, 306–337. ( 10.1007/BF02224248) [DOI] [Google Scholar]
- 14.Lihoreau M, Chittka L, Raine NE. 2011. Trade-off between travel distance and prioritization of high-reward sites in traplining bumblebees. Funct. Ecol. 25, 1284–1292. ( 10.1111/j.1365-2435.2011.01881.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dussutour A, Nicolis SC. 2013. Flexibility in collective decision-making by ant colonies: tracking food across space and time. Chaos Solitons Fractals 50, 32–38. ( 10.1016/j.chaos.2013.02.004) [DOI] [Google Scholar]
- 16.Latty T, Beekman M. 2013. Keeping track of changes: the performance of ant colonies in dynamic environments. Anim. Behav. 85, 637–643. ( 10.1016/j.anbehav.2012.12.027) [DOI] [Google Scholar]
- 17.De Biseau JC, Deneubourg JL, Pasteels JM. 1991. Collective flexibility during mass recruitment in the ant Myrmica sabuleti (Hymenoptera: Formicidae). Psyche 98, 323–336. ( 10.1155/1991/38402) [DOI] [Google Scholar]
- 18.Czaczkes TJ, Grüter C, Ratnieks FLW. 2015. Trail pheromones: an integrative view of their role in colony organisation. Annu. Rev. Entomol. 60, 581–599. ( 10.1146/annurev-ento-010814-020627) [DOI] [PubMed] [Google Scholar]
- 19.Michelena P, Jeanson R, Deneubourg J-L, Sibbald AM. 2010. Personality and collective decision-making in foraging herbivores. Proc. R. Soc. B 277, 1093–1099. ( 10.1098/rspb.2009.1926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beekman M, Fathke RL, Seeley TD. 2006. How does an informed minority of scouts guide a honeybee swarm as it flies to its new home? Anim. Behav. 71, 161–171. ( 10.1016/j.anbehav.2005.04.009) [DOI] [Google Scholar]
- 21.Dyer JRG, Johansson A, Helbing D, Couzin ID, Krause J. 2009. Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781–789. ( 10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rendell L, et al. 2010. Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213. ( 10.1126/science.1184719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grüter C, Leadbeater E. 2014. Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. ( 10.1016/j.tree.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 24.Harrison JF, Fewell JH, Stiller TM, Breed MD. 1989. Effects of experience on use of orientation cues in the giant tropical ant. Anim. Behav. 37, 869–871. ( 10.1016/0003-3472(89)90076-6) [DOI] [Google Scholar]
- 25.Grüter C, Balbuena MS, Farina WM. 2008. Informational conflicts created by the waggle dance. Proc. R. Soc. B 275, 1321–1327. ( 10.1098/rspb.2008.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grüter C, Czaczkes TJ, Ratnieks FLW. 2011. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 64, 141–148. ( 10.1007/s00265-010-1020-2) [DOI] [Google Scholar]
- 27.Grüter C, Ratnieks FLW. 2011. Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 81, 949–954. ( 10.1016/j.anbehav.2011.01.014) [DOI] [Google Scholar]
- 28.Wray MK, Klein BA, Seeley TD. 2012. Honey bees use social information in waggle dances more fully when foraging errors are more costly. Behav. Ecol. 23, 125–131. ( 10.1093/beheco/arr165) [DOI] [Google Scholar]
- 29.Leadbeater E, Florent C. 2014. Foraging bumblebees do not rate social information above personal experience. Behav. Ecol. Sociobiol. 68, 1145–1150. ( 10.1007/s00265-014-1725-8) [DOI] [Google Scholar]
- 30.Czaczkes TJ, Ratnieks FLW. 2012. Pheromone trails in the Brazilian ant Pheidole oxyops: extreme properties and dual recruitment action. Behav. Ecol. Sociobiol. 66, 1149–1156. ( 10.1007/s00265-012-1367-7) [DOI] [Google Scholar]
- 31.Beckers R, Goss S, Deneubourg JL, Pasteels JM. 1989. Colony size, communication and ant foraging strategy. Psyche J. Entomol. 96, 239–256. ( 10.1155/1989/94279) [DOI] [Google Scholar]
- 32.Vilela EF, Jaffé K, Howse PE. 1987. Orientation in leaf-cutting ants (Formicidae: Attini). Anim. Behav. 35, 1443–1453. ( 10.1016/S0003-3472(87)80017-9) [DOI] [Google Scholar]
- 33.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. 2011. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 7, 521–524. ( 10.1098/rsbl.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czaczkes TJ, Grüter C, Ratnieks FLW. 2013. Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. J. Exp. Biol. 216, 188–197. ( 10.1242/jeb.076570) [DOI] [PubMed] [Google Scholar]
- 35.Reinhard J, Srinivasan MV, Zhang S. 2004. Olfaction: scent-triggered navigation in honeybees. Nature 427, 411 ( 10.1038/427411a) [DOI] [PubMed] [Google Scholar]
- 36.Czaczkes TJ, Schlosser L, Heinze J, Witte V. 2014. Ants use directionless odour cues to recall odour-associated locations. Behav. Ecol. Sociobiol. 68, 981–988. ( 10.1007/s00265-014-1710-2) [DOI] [Google Scholar]
- 37.Pelé M, Sueur C. 2013. Decision-making theories: linking the disparate research areas of individual and collective cognition. Anim. Cogn. 16, 543–556. ( 10.1007/s10071-013-0631-1) [DOI] [PubMed] [Google Scholar]
- 38.Collignon B, Deneubourg JL, Detrain C. 2012. Leader-based and self-organized communication: modelling group-mass recruitment in ants. J. Theor. Biol. 313, 79–86. ( 10.1016/j.jtbi.2012.07.025) [DOI] [PubMed] [Google Scholar]
- 39.Li L, Peng H, Kurths J, Yang Y, Schellnhuber HJ. 2014. Chaos–order transition in foraging behavior of ants. Proc. Natl Acad. Sci. USA 111, 8392–8397. ( 10.1073/pnas.1407083111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stroeymeyt N, Giurfa M, Franks NR. 2010. Improving decision speed, accuracy and group cohesion through early information gathering in house-hunting ants. PLoS ONE 5, e13059 ( 10.1371/journal.pone.0013059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroeymeyt N, Franks NR, Giurfa M. 2011. Knowledgeable individuals lead collective decisions in ants. J. Exp. Biol. 214, 3046–3054. ( 10.1242/jeb.059188) [DOI] [PubMed] [Google Scholar]
- 42.Schürch R, Grüter C. 2014. Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS ONE 9, e104660 ( 10.1371/journal.pone.0104660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilensky U. 1999. NetLogo See http://ccl.northwestern.edu/netlogo/.
- 44.Fahse L, Wissel C, Grimm V. 1998. Reconciling classical and individual-based approaches in theoretical population ecology: a protocol for extracting population parameters from individual-based models. Am. Nat. 152, 838–852. ( 10.1086/286212) [DOI] [PubMed] [Google Scholar]
- 45.Robinson E, Ratnieks F, Holcombe M. 2008. An agent-based model to investigate the roles of attractive and repellent pheromones in ant decision making during foraging. J. Theor. Biol. 255, 250–258. ( 10.1016/j.jtbi.2008.08.015) [DOI] [PubMed] [Google Scholar]
- 46.Jackson DE, Bicak M, Holcombe M. 2011. Decentralized communication, trail connectivity and emergent benefits of ant pheromone trail networks. Memet. Comput. 3, 25–32. ( 10.1007/s12293-010-0039-2) [DOI] [Google Scholar]
- 47.Czaczkes TJ. 2014. How to not get stuck: negative feedback due to crowding maintains flexibility in ant foraging. J. Theor. Biol. 360, 172–180. ( 10.1016/j.jtbi.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 48.Grüter C, Schürch R, Farina WM. 2013. Task-partitioning in insect societies: non-random direct material transfers affect both colony efficiency and information flow. J. Theor. Biol. 327, 23–33. ( 10.1016/j.jtbi.2013.02.013) [DOI] [PubMed] [Google Scholar]
- 49.Grimm V, et al. 2006. A standard protocol for describing individual-based and agent-based models. Ecol. Model. 198, 115–126. ( 10.1016/j.ecolmodel.2006.04.023) [DOI] [Google Scholar]
- 50.Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J, Railsback SF. 2010. The ODD protocol: a review and first update. Ecol. Model. 221, 2760–2768. ( 10.1016/j.ecolmodel.2010.08.019) [DOI] [Google Scholar]
- 51.Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. ( 10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 52.Couzin ID, Ioannou CC, Demirel G, Gross T, Torney CJ, Hartnett A, Conradt L, Levin SA, Leonard NE. 2011. Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580. ( 10.1126/science.1210280) [DOI] [PubMed] [Google Scholar]
- 53.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 54.Collignon B, Detrain C. 2010. Distributed leadership and adaptive decision-making in the ant Tetramorium caespitum. Proc. R. Soc. B 277, 1267–1273. ( 10.1098/rspb.2009.1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sueur C, et al. 2011. Collective decision-making and fission–fusion dynamics: a conceptual framework. Oikos 120, 1608–1617. ( 10.1111/j.1600-0706.2011.19685.x) [DOI] [Google Scholar]
- 56.Al Toufailia H, Grüter C, Ratnieks FLW. 2013. Persistence to unrewarding feeding locations by honeybee foragers (Apis mellifera): the effects of experience, resource profitability and season. Ethology 119, 1096–1106. ( 10.1111/eth.12170) [DOI] [Google Scholar]
- 57.Moore D, Nest BNV, Seier E. 2011. Diminishing returns: the influence of experience and environment on time-memory extinction in honey bee foragers. J. Comp. Physiol. A 197, 641–651. ( 10.1007/s00359-011-0624-y) [DOI] [PubMed] [Google Scholar]
- 58.Seeley TD, Camazine S, Sneyd J. 1991. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 28, 277–290. ( 10.1007/BF00175101) [DOI] [Google Scholar]
- 59.Grüter C, Schürch R, Czaczkes TJ, Taylor K, Durance T, Jones SM, Ratnieks FLW. 2012. Negative feedback enables fast and flexible collective decision-making in ants. PLoS ONE 7, e44501 ( 10.1371/journal.pone.0044501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reid CR, Latty T, Beekman M. 2012. Making a trail: informed Argentine ants lead colony to the best food by U-turning coupled with enhanced pheromone laying. Anim. Behav. 84, 1579–1587. ( 10.1016/j.anbehav.2012.09.036) [DOI] [Google Scholar]
- 61.Beekman M, Ratnieks FLW. 2000. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 14, 490–496. ( 10.1046/j.1365-2435.2000.00443.x) [DOI] [Google Scholar]
- 62.Dorigo M, Stützle T. 2004. Ant colony optimization. Cambridge, MA: MIT Press. [Google Scholar]
- 63.Czaczkes TJ, Grüter C, Ratnieks FLW. 2013. Negative feedback in ants: crowding results in less trail pheromone deposition. J. R. Soc. Interface 10, 20121009 ( 10.1098/rsif.2012.1009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. 2012. Uncovering the complexity of ant foraging trails. Commun. Integr. Biol. 5, 78–80. ( 10.4161/cib.18209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Detrain C, Deneubourg JL. 2009. Social cues and adaptive foraging strategies in ants. In Food exploitation by social insects (eds S Jarau, M Hrncir), pp. 29–52. Boca Raton, FL: CRC Press. [Google Scholar]
- 66.Granovskiy B, Latty T, Duncan M, Sumpter DJT, Beekman M. 2012. How dancing honey bees keep track of changes: the role of inspector bees. Behav. Ecol. 23, 588–596. ( 10.1093/beheco/ars002) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The fully functioning model, as well as the datasets from the model runs and empirical data, are available as electronic supplementary material from Dryad (doi:10.5061/dryad.9k219).




