Abstract
In animal populations, as in humans, behavioural differences between individuals that are consistent over time and across contexts are considered to reflect personality, and suites of correlated behaviours expressed by individuals are known as behavioural syndromes. Lifelong stability of behavioural syndromes is often assumed, either implicitly or explicitly. Here, we use a quantitative genetic approach to study the developmental stability of a behavioural syndrome in a wild population of blue tits. We find that a behavioural syndrome formed by a strong genetic correlation of two personality traits in nestlings disappears in adults, and we demonstrate that genotype–age interaction is the likely mechanism underlying this change during development. A behavioural syndrome may hence change during organismal development, even when personality traits seem to be strongly physiologically or functionally linked in one age group. We outline how such developmental plasticity has important ramifications for understanding the mechanistic basis as well as the evolutionary consequences of behavioural syndromes.
Keywords: behavioural syndrome, personality, development, genetic correlation, pleiotropy, genotype–age interaction
1. Introduction
Personality refers to a measure of behaviour that shows repeatable differences between individuals [1]. A remarkable number of studies in a wide variety of animal taxa indeed find individual consistency in behaviour, and personality hence is a widespread phenomenon in nature [2]. One further striking feature in personality research is that different behaviours tend to be correlated [3], forming what are termed behavioural syndromes [4]. However, despite personality showing plasticity across ages (e.g. [5,6]), individuals are typically assumed, implicitly or explicitly, to maintain their relative ranking in one or more aspects of personality over age, producing consistent behavioural differences and consistency in the magnitude and sign of the behavioural syndrome correlation across development [1] (figure 1a). Surprisingly, this assumption is not based on a solid empirical ground. On the one hand, psychology studies agree that personality is relatively stable over the ontogeny in humans [7–10]. On the other hand, the few studies conducted in other species typically find contrasting results for single traits [11–17]. In addition, behavioural syndromes may appear or disappear as individuals age [18–25].
Figure 1.
Theoretical plot illustrating the notions of consistency over the ontogeny, GAIs and selection on the genetic correlation between trait 1 and trait 2. In (a,b), each line represents one individual, which is reported as a point in (c,d). In (a), the rank order of the individuals' breeding values for trait 1 remains stable across ontogeny. In (b), the rank order of the breeding values for trait 2 is different in young and adults because of GAI. As a consequence, the positive genetic correlation between trait 1 and trait 2 in young individuals (c) disappears in adults (d). Figures (e) and (f) represent the breeding values of nestlings that recruited (red) or not (grey), assuming a 5% recruitment probability. In (e), the breeding values of the two traits are not negatively correlated in recruits, which is why the genetic correlation is 0 when these individuals are measured as adults. In (f), the individuals are selected randomly and thus the correlation stays negative when they are measured as adults. (Online version in colour.)
A major shortcoming is that most of the human and animal studies have considered personality only on the phenotypic level. As a consequence, observed phenotypic changes in personality and behavioural syndromes may largely reflect age-related changes in non-heritable factors. Therefore, phenotypic patterns do not necessarily inform us of underlying, intrinsic causes of observed age-related changes in personality, which are needed to gain a proper mechanistic or evolutionary understanding of age-related changes in personality. In particular, increased understanding of ontogenetic changes in the additive genetic (co)variances of personality traits is needed for properly understanding the potential of evolutionary forces in shaping these traits [26]. In this paper, we use, for the first time to our knowledge, a quantitative genetic approach to bring new insight into the genetic basis of personality and behavioural syndromes during development.
Variation in behaviour is likely to be caused by the joint effects of many genes (polygenicity), and a behavioural syndrome is likely to arise from the genetic correlation of two behaviours [27]. A genetic correlation between behaviours arises when (part of the) genes underlying both behaviours are the same (pleiotropy) or the genes are (largely) different but are associated through linkage disequilibrium (physical or not). Genetic correlations between behaviours during development reflect the joint effects of many genes expressed by the same individuals (i.e. genomes) at different ages. A behavioural syndrome will show consistency over development when the effects of the genes underlying both behaviours are correlated across development, either because the causal genetic architecture is strictly maintained over ontogeny, or due to a strong functional link between them, referred to as structured pleiotropy [28]. A genetic correlation underlying a behavioural syndrome can change over the ontogeny as a result of two possible mechanisms. First, the expression of genes determining one or both behaviours might change over time as a result of genotype–age interaction (GAI; figure 1b). For instance, some genes underlying the focal behaviours can be turned on or off during development, or the effect sizes of genes differ when expressed at different ages, determining behaviour in ‘young’ versus ‘old’ individuals. Whenever GAI occurs in one or more traits, the genetic correlation between two traits is likely to change over the developmental trajectory (figure 1c,d), unless the two traits are determined through structured pleiotropy, causing the relative ranking of genotypes to be maintained for both traits across age classes [29]. Lastly, selection may lead to not all individuals expressing behaviours during the entire developmental trajectory; many juveniles do not survive to adulthood, especially in wild populations [30]. Selection, by favouring a particular combination of breeding values, therefore has great potential to alter a genetic correlation between behaviours during development, independently of the presence of GAI [31] (figure 1e). For example, linkage disequilibrium between genes underlying two behaviours, and hence a genetic correlation, can arise during development through correlational selection, or linkage disequilibrium between two traits in juveniles may be broken down by selection, and hence disappear in adults (figure 1e).
In this study, we quantify the genetic correlations underlying a behavioural syndrome across the ontogeny from juvenile to adult. We hence test the stability of a behavioural syndrome during development. We study a wild pedigreed population of blue tits (Cyanistes caeruleus) and use a sophisticated quantitative genetic approach to estimate all relevant additive genetic (co)variances in addition to considering the putative role of selection in shaping the genetic correlations across ontogeny. The behavioural syndrome we study is composed of two personality traits measured during handling in nestlings and adults, which are heritable and correlated on the phenotypic and genetic level in nestlings [32]. We find that this genetic correlation disappears in adults and demonstrate that GAIs are underlying this developmental change.
2. Material and methods
(a). Study species and measures of behaviour
The study was conducted on a population of blue tits (C. caeruleus) breeding in nest-boxes in southwestern Finland near the city of Tammisaari (60°01′ N, 23°31′ E). The nest-boxes were made available for breeding starting from 2003 in an area of approximately 10 km2 of mixed boreal forest. Each year, the birds were monitored during the breeding season (April–July). All individuals were identified by a metal ring placed on them when they were 9 days old, or during first capture as an unringed adult. During handling, the behaviour of adults and 16-day-old nestlings was scored. Handling aggression (HA) is a score (ranging from 1 to 5) reflecting whether the individual is passive and docile when held by the observer (low HA score) or whether it fights back (high HA score) [32]. Breath rate (BR) is quantified by timing with a stopwatch how long it takes an individual to breathe 30 times, carried out two consecutive times. BR (calculated as the average breaths per second over the two measures) is considered an indication of stress in birds, where a higher BR is associated with a higher stress response [33]. The details of the method of handling nestlings prior to assaying their behaviours changed in 2011 (all placed in one large paper bag) relative to before 2011 (all placed in individual paper bags), but the genetic correlation in nestling behaviour before and after 2011 did not differ from unity (electronic supplementary material S1) and we hence consider the two approaches equivalent. In our population, HA and BR scores are in adults associated with fecundity (in males) and survival (in females), and are hence ecologically important behaviours [34]. In addition, work on the stress response to handling in the closely related great tits Parus major selected for extreme exploration scores suggests that BR is genetically correlated to exploration score [35]. The latter is an important aspect of personality in wild birds [36–38].
(b). Quantitative genetics
The focus of our analysis was to contrast the genetic correlation underlying the HA–BR behavioural syndrome in 16-day-old nestlings with the genetic correlation in adults (more than or equal to 1-year-old birds). We estimated the additive genetic variance–covariance G matrix of the four traits (HA and BR in adults and in nestlings) using the following linear mixed model (animal model [36,39]):
| 2.1 |
where y is a vector of all the information on all the individuals, β is a vector of one or more fixed effects, X is a design matrix (of zeros and ones) relating the appropriate fixed effects to each individual, uA the vector of additive genetic (random) effects and ZA the design matrix relating the appropriate additive genetic effect to each individual. We included the sex of the individual, year and identity of the observer as fixed effects. The summation ∑Zkuk allows for more random effects such as permanent environment and common environment effects. Permanent environment effects were included for adult behaviours in order to capture the (co)variances between individuals that are not due to additive genetic effects, but are caused by other environmental or non-additive genetic (e.g. dominance) effects that are conserved across repeated records [26]. Common environmental effects on nestlings' behaviours due to the environment (biotic and abiotic conditions) shared by nestlings were modelled by including the ID of their nest. A reciprocal cross-fostering procedure was carried out where part of the nestlings were swapped between two nests at 2 days post-hatching between 2006 and 2010 (see [40] for a detailed description), and we used the ID for the nest of rearing as the common environment for cross-fostered nestlings. Finally, e is a vector for residual errors, which represents the difference between the trait values observed and the values expected on the basis of the fixed and random effects. This mixed model was implemented in ASReml (VSN International, Hemel Hempstead, UK) and solved using restricted maximum likelihood.
The (co)variances for nestling and adult traits were estimated on the additive genetic and residual levels. In addition, (co)variances for nest of rearing and permanent environment were estimated for nestling and adult behaviours, respectively. The additive genetic and other random effects for the four traits were assumed to be normally distributed with a mean of zero (i.e. defined relative to the trait-specific fixed-effect mean), and with multivariate normal trait-specific variances and covariances. The G matrix (for vector uA) and its elements (additive genetic variances and covariances) was estimated using the coefficient of co-ancestry θij between individuals i and j, which was derived from the population pedigree. This G matrix contains the trait-specific additive genetic variances and all pairwise genetic covariances. Statistical tests of elements in this G matrix were conducted by comparing the likelihood, using likelihood-ratio tests (LRTs) between a model constraining the elements and the unconstrained model with the degrees of freedom calculated as the difference in variance components between the constrained and unconstrained models. Phenotypic (co)variances were calculated as the sum of all estimated (co)variance components.
The data used in these analyses consisted of 8079 observations made on 7191 individuals between 2006 and 2014, including 744 individuals measured as adults only, 414 recruits (measured as nestling and adult) and 6033 nestlings which have not recruited (detailed in electronic supplementary material, table S1).
The pruned pedigree, which included only individuals for which we have at least one measure for one of the four traits, holds records for 7203 individuals (including 781 founders), 6036 maternities, 6392 paternities, 31 457 pairs of full sibs and 32 422 pairs of half sibs. The mean family size is 12.6 and lineage of multiple generations is recorded with a maximal lineage depth of seven generations. This is a social pedigree, where offspring hatched in one nest were assumed to be full siblings. Half-sibs in a social pedigree arise when a parent produces a recruit with a different partner (e.g. in a different year). Because some social fathers have not sired the offspring for which they provide care, there are likely to be errors in the paternal links in this pedigree. We do not know the proportion of extra-pair paternity in this population. We evaluated the sensitivity of our findings to the inclusion of the uncertainty in paternity using a simulation approach. We assumed the distribution of extra-pair young (EPY) was described by the hierarchical model developed by Brommer et al. [41], parametrized using empirical data on EPY in nine blue tit populations [42] (model values used were m = 0.875, s = 0.156). This parametrized model provided a description of the expected distribution of EPY and was applied to our social pedigree to generate 1000 pedigrees where the paternal links of all randomly drawn ‘EP nestlings’ were assumed to be unknown. These random pedigrees were subsequently used to obtain 1000 estimates of all (co)variances based on the animal model described above, and their mode and 95% credible intervals were calculated using a density kernel (see electronic supplementary material S2 for the R code). In addition, the LRT statistic of the model where the genetic correlation between HA and BR was constrained to be the same versus unconstrained was calculated for each simulated pedigree. We assumed that our findings were robust to the inclusion of uncertainty of unknown extra-pair paternity in case the LRT statistic exceeded the χ2 threshold value for 1 d.f. in more than 95% of model comparisons using the pedigree with simulated extra-pair paternities, and when quantitative genetic estimates based on our social pedigree were within the 95% credible interval of estimates based on the pedigree with simulated extra-pair paternity.
Our analysis contrasts nestlings versus adults. Clearly, an individual's breeding value of HA and BR may undergo changes during adulthood (because of GAI), such that the grouping of differently aged adult individuals in one age class may not fully represent ontogenetic changes in additive genetic (co)variances. There was nevertheless no evidence that the genetic correlations in HA or BR between 1, 2 and 3+ years old was lower than unity (electronic supplementary material, table S2), and the pooling of adults of different ages was hence representative with respect to adults' breeding values for these two behaviours.
(c). Selection
Selection has the potential to make or break a genetic correlation across the developmental trajectory (figure 1e). In our case, we contrast the genetic correlations of 16-day-old nestlings and adults. The putative selection then implies that only nestlings with certain combinations of breeding values for HA and BV would recruit into the breeding population. We tested this hypothesis by extending the above-described multivariate animal model to six character states, estimating the genetic correlations between HA and BR in nestlings that recruited as breeding adults in our population, in nestlings that did not recruit, and in adults. The hypothesis that selection alters the magnitude and/or sign of the genetic correlation in adults compared with its magnitude and/or sign in juveniles predicts that the genetic correlation between HA and BR in recruited nestlings must be similar to the one in adults, but different from the one in non-recruited nestlings. Alternatively, when selection does not ‘pick out’ certain combinations of breeding values, the genetic correlation in recruited offspring is similar to that in non-recruited offspring, but different from the one in adults. Because these hypotheses specify non-nested models, their fit to the data was compared using the Akaike information criterion (AIC) [43]. AIC was calculated as –2 × log(L) + 2 × K, where log(L) is the log-likelihood of the model and K the number of different correlations between HA and BR, which ranged from 1 (all the same) to 3 (all independent). Support for each model was calculated as exp(–½ΔAICi)/Σexp(–½ΔAIC), where a higher value indicates a great support for a particular model relative to the other candidates [44].
(d). Simulation of expected change in genetic correlation across ontogeny
A second mechanism that can change the magnitude or sign of a genetic correlation between two traits across ontogeny is GAI leading to age-related changes in the relative ranking of the breeding values in one or both traits. As a result, a positive, but not perfect (i.e. rA < 1), cross-ontogeny correlation in each of the two traits forming a behavioural syndrome will cause a decrease in the magnitude of the genetic correlation between these two traits when comparing their correlation in the juvenile stage with older ontogenetic stages. This is an unavoidable consequence, because imperfect genetic correlations across ontogeny of each trait imply that a certain amount of ‘noise’ is added to the covariance between the two traits across ontogeny. The magnitude of the correlation between two traits then decreases across ontogeny. We wanted to investigate the extent to which this phenomenon was responsible for changes in the genetic correlation between our two behaviours expressed at the juvenile versus the adult stages. To this end, we generated an expectation of the genetic correlation between HA and BR in adults, based on the estimated genetic correlations between nestlings and adults for HA and BR alone. We first generated breeding values for 1000 individuals according to the genetic (co)variance matrix for these traits in nestlings, and then applied the estimated genetic correlations for HA and BR across ontogeny to generate expected breeding values in adults (see electronic supplementary material S3 for the R script). Genetic correlations were calculated as the correlations between the simulated breeding values, between traits across ontogeny and within the adult age class. This procedure was repeated 1000 times, and the expected correlation and 95% credible intervals of the genetic correlations were calculated as the mode and the 95% interval using a density kernel (see electronic supplementary material S3).
3. Results and discussion
The G matrix obtained from the animal model showed that all four traits are moderately heritable (table 1; electronic supplementary material, table S3). There was a negative genetic correlation (–0.49 ± 0.09) between HA and BR in nestlings and a low, positive genetic correlation (0.07 ± 0.16) in adults (table 1). On the phenotypic level, these correlations were –0.36 ± 0.03 and 0.07 ± 0.03, respectively (see electronic supplementary material, table S4, for correlations on all levels). The model corrected for the fixed-effect differences between observer, years and sexes (electronic supplementary material, table S5). The genetic correlations underlying the behavioural syndrome between HA and BR differed significantly between nestlings and adults (LRT: 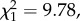 p < 0.01). Thus, we find clear evidence that the genetic underpinning in a behavioural syndrome changes during development. These findings were robust to inclusion of uncertainty in pedigree links due to extra-pair paternity (electronic supplementary material, figures S1 and S2).
p < 0.01). Thus, we find clear evidence that the genetic underpinning in a behavioural syndrome changes during development. These findings were robust to inclusion of uncertainty in pedigree links due to extra-pair paternity (electronic supplementary material, figures S1 and S2).
Table 1.
Genetic correlations and heritabilities of handling aggression and breathing rate in nestlings (HAn, BRn) and in adults (HAa, BRa). Genetic correlations (upper triangle) and heritabilities (diagonal) are represented as estimate ±s.e. and are derived from the matrix of additive genetic effects estimated by a multivariate animal model. Information on variances and correlations for other components than the additive genetic component are provided in the electronic supplementary material, tables S3 and S4. Fixed effects are reported in the electronic supplementary material, table S5. The significance of a particular genetic correlation was tested by comparing the unconstrained model with models where that genetic correlation (rA) was fixed at 0 using an LRT. The genetic correlations describing the HA–BR behavioural syndrome in the different ontogenetic stages are printed in bold.
| HAn | BRn | HAa | BRa | |
|---|---|---|---|---|
| HAn | 0.26 ± 0.04 | –0.49 ± 0.09a | 0.38 ± 0.10b | 0.08 ± 0.11 |
| BRn | 0.28 ± 0.04 | –0.13 ± 0.11 | 0.50 ± 0.11c | |
| HAa | 0.29 ± 0.06 | 0.07 ± 0.16 | ||
| BRa | 0.27 ± 0.06 |
aLRT:  , p < 0.001.
, p < 0.001.
bLRT: 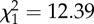 , p < 0.001.
, p < 0.001.
cLRT: 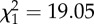 , p < 0.001.
, p < 0.001.
We constructed a multivariate animal model for six traits—HA and BR in nestlings which recruited as breeding adults in our population, in nestlings which did not recruit and in adults—in order to test whether the genetic correlation of recruited offspring differs from that of the non-recruited nestlings (cf. figure 1e). The top-ranked model was one where the genetic correlations in recruited and non-recruited nestlings are the same, but differ from that in adults (model ‘S/S/I’ in table 2). Indeed, the estimated genetic correlations (of the fully unconstrained model ‘I/I/I’ in table 2) underlined that the estimates of the genetic correlation between HA and BR in recruited and non-recruited nestlings were highly similar (–0.59 ± 0.27 and –0.46 ± 0.09, respectively; electronic supplementary material, table S6). Given that selection on the genetic correlation in nestlings was not responsible for the absence of genetic correlation in adults, GAIs are likely to be the main factor behind the change in genetic correlation over development.
Table 2.
Model ranking for hypotheses testing whether selection shapes the genetic correlation between handling aggression and breathing rate across development. Each hypothesis specifies a certain combination of constraints (or not) on the genetic correlation in nestlings which have recruited, in nestlings which have not recruited and in adults. The genetic correlations were constrained to the same value (S) or were independent (I). Models are sorted by ascending order of AIC, and ΔAIC is the difference between the AIC of each model and AIC of the top model.
| hypothesis | recruited/not recruited/adults | log(L) | K | AIC | ΔAIC | support |
|---|---|---|---|---|---|---|
| no selection | S/S/I | −411.850 | 2 | 827.7 | 0 | 0.46 |
| selection | S/I/S | −412.704 | 2 | 829.4 | 1.71 | 0.20 |
| all different | I/I/I | −411.758 | 3 | 829.5 | 1.82 | 0.19 |
| all the same | S/S/S | −413.953 | 1 | 829.9 | 2.21 | 0.15 |
Genetic correlations of BR and HA across ontogeny (nestling to adult) are both positive (0.50 ± 0.11 and 0.38 ± 0.10, respectively; table 1), but these correlations are not perfect as they fall significantly below 1 (LRT: 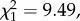 p = 0.002 for BR;
p = 0.002 for BR;  p < 0.001 for HA), indicating some changing in the ranking of an individual's breeding values for these traits expressed across ontogeny. We therefore calculated the genetic correlation between HA and BR we would expect in adults (rA(HAa,BRa)) given these low genetic correlations in HA and BR across ontogeny. Our simulations suggest the observed low genetic correlations in HA and BR across ontogeny act to reduce the genetic correlation for the HA–BR behavioural syndrome in adults we would expect (expected rA(HAa,BRa) is –0.09, 95% credible interval –0.16, –0.04). The 95% CI of the observed genetic correlation between HA and BR in adults indeed encompasses the expected correlation (figure 2). Thus, the change in ranking of the breeding values across ontogeny in both HA and BR (i.e. the GAI) is sufficient to explain the breakdown in the genetic correlation of the syndrome during development. A second striking feature is that the estimated genetic correlation between HA in nestlings and BR in adults rA(HAn,BRa) has a much lower magnitude (i.e. absolute value) than the genetic correlation one would expect (figure 2), which further underlines that GAI uncouples these two traits across ontogeny.
p < 0.001 for HA), indicating some changing in the ranking of an individual's breeding values for these traits expressed across ontogeny. We therefore calculated the genetic correlation between HA and BR we would expect in adults (rA(HAa,BRa)) given these low genetic correlations in HA and BR across ontogeny. Our simulations suggest the observed low genetic correlations in HA and BR across ontogeny act to reduce the genetic correlation for the HA–BR behavioural syndrome in adults we would expect (expected rA(HAa,BRa) is –0.09, 95% credible interval –0.16, –0.04). The 95% CI of the observed genetic correlation between HA and BR in adults indeed encompasses the expected correlation (figure 2). Thus, the change in ranking of the breeding values across ontogeny in both HA and BR (i.e. the GAI) is sufficient to explain the breakdown in the genetic correlation of the syndrome during development. A second striking feature is that the estimated genetic correlation between HA in nestlings and BR in adults rA(HAn,BRa) has a much lower magnitude (i.e. absolute value) than the genetic correlation one would expect (figure 2), which further underlines that GAI uncouples these two traits across ontogeny.
Figure 2.
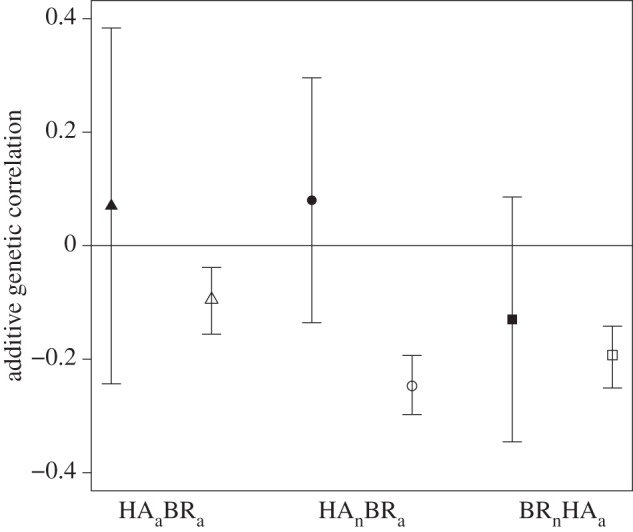
Correlation between HA and BR in adults (rA(HAa,BRa)) and the cross-trait-cross-ontogeny correlations (rA(BRn,HAa) and rA(HAn,BRa)), estimated by the animal model (filled symbols) and derived from the simulation (open symbols). For the simulation, the 95% credible interval were estimated using the highest posterior density distribution of the correlations, and for the estimates derived from the animal model, approximate confidence intervals were obtained by multiplying their s.e. by 1.96.
HA and BR are traits measured in response to the stress of handling, and one would hence intuit these traits to be caused by common mechanisms underlying stress response. In addition, BR is known to be directly linked to the physiological response to stress through the activation of the parasympathetic system and is considered itself as a physiological parameter by some authors [33,45]. Hence, it can be argued that the genetic correlation between HA and BR may reflect a physiological–behavioural correlation. Indeed, major hypotheses explaining syndrome covariance postulate that behaviours, physiological and possibly life-history traits reflect variation along a common axis (e.g. the hypothalamus–pituitary–adrenal axis; coping styles, see [46]) or pace-of-life-syndrome [47].
Here, we document a breakdown of the strong genetic correlation between HA and BR in nestlings as they mature into adults. This finding hence implies that the mechanistic underpinning (in terms of genes and/or physiological mechanisms) of the expression of these behaviours changes across development. Technically, these behaviours are linked through non-structured pleiotropy (cf. [28]). Arguably, research into the mechanistic underpinning of a behavioural syndrome would be particularly interesting for syndromes where the underlying genetic correlation remains consistent in sign and magnitude over development, because such a pattern would signal a mechanism where it is likely that the same genes (expression) or hormonal pathways are underlying the association of personality traits at different ages. Thus, the quantitative genetic approach taken here is a potentially fruitful first step for identifying syndromes where detailed research into the mechanistic underpinning of the behavioural or behavioural–physiological syndrome is attractive.
From an ultimate perspective, we note that genetic correlations act as evolutionary constraints because genetically correlated traits cannot evolve independently in response to selection (e.g. [48]). Meta-analysis underlines that the genetic correlations underlying behavioural syndromes exert strong evolutionary constraints, possible stronger than those acting on life-history traits [49]. Our findings hence suggest that, when the genetic underpinning of behavioural syndromes is explored from a lifetime perspective, behavioural syndromes may be far less evolutionarily constrained than originally perceived on the basis of genetic correlations measured in one particular stage of development. Assuming that genetic correlations are consistent across the ontogeny can hence lead to inaccurate predictions of the evolutionary trajectory of the behaviours or the evolutionary constraints acting upon them.
Our findings underline the importance of studying behaviours during multiple periods in the development of organisms, because a functional, physiological or developmental link between two behaviours expressed at one particular age is not sufficient to demonstrate their validity over the entire development of the organism. This phenomenon, which is likely to occur in other organisms, should be taken into account in future studies of personality. Currently, we know very little about how personality develops and several calls have been made to stimulate research in this direction [4–6,22,50]. After all, studying the development of personality is a necessary step to reach a complete understanding of its evolution and causation.
Supplementary Material
Acknowledgements
We thank the land owners for permission to work on their land. We thank Edward Kluen, Lasse Kurvinen, Jaana Kekkonen, Maaike de Heij and Laura Harjula for their many hours of fieldwork. Two anonymous reviewers are thanked for their constructive comments.
Ethics
All experiments on blue tits described in this paper complied with the Finnish law on animal experiments, and were approved by the relevant authorities: Helsinki University Animal Experiment Committee (2003–2008), Animal Experiment Committee of Southern Finland (2007 onwards).
Data accessibility
Population pedigree and phenotypic data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.443g2.
Authors' contributions
B.C. carried out the statistical analysis, participated in data collection and authored the manuscript. J.E.B. conceived and designed the study, collected data and authored the manuscript.
Competing interests
We declare we have no competing interests.
Funding
B.C. was funded through a CIMO fellowship and Turun Yliopistosäätiö. The Academy of Finland (J.E.B.), Oskar Öflunds Stiftelse and Societas pro Fauna et Flora Fennica funded part of the data collection.
References
- 1.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 2.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garamszegi LZ, Markó G, Herczeg G. 2012. A meta-analysis of correlated behaviours with implications for behavioural syndromes: mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evol. Ecol. 26, 1213–1235. ( 10.1007/s10682-012-9589-8) [DOI] [Google Scholar]
- 4.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 5.Stamps JA, Groothuis TGG. 2010. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041. ( 10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamps J, Groothuis TGG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325. ( 10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 7.Roberts BW, DelVecchio WF. 2000. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychol. Bull. 126, 3–25. ( 10.1037/0033-2909.126.1.3) [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, Roberts BW. 2001. Personality development across the life course: the argument for change and continuity. Psychol. Inq. 12, 49–66. ( 10.1207/S15327965PLI1202_01) [DOI] [Google Scholar]
- 9.Donnellan MB, Conger RD, Burzette RG. 2007. Personality development from late adolescence to young adulthood: differential stability, normative maturity, and evidence for the maturity-stability hypothesis. J. Pers. 75, 237–263. ( 10.1111/j.1467-6494.2007.00438.x) [DOI] [PubMed] [Google Scholar]
- 10.McAdams DP, Olson BD. 2010. Personality development: continuity and change over the life course. Annu. Rev. Psychol. 61, 517–542. ( 10.1146/annurev.psych.093008.100507) [DOI] [PubMed] [Google Scholar]
- 11.Sinn DL, Perrin NA, Mather JA, Anderson RC. 2001. Early temperamental traits in an octopus (Octopus bimaculoides). J. Comp. Psychol. 115, 351–364. ( 10.1037/0735-7036.115.4.351) [DOI] [PubMed] [Google Scholar]
- 12.Sinn DL, Gosling SD, Moltschaniwskyj NA. 2008. Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 433–442. ( 10.1016/j.anbehav.2007.05.008) [DOI] [Google Scholar]
- 13.Rensel MA, Schoech SJ. 2011. Repeatability of baseline and stress-induced corticosterone levels across early life stages in the Florida scrub-jay (Aphelocoma coerulescens). Horm. Behav. 59, 497–502. ( 10.1016/j.yhbeh.2011.01.010) [DOI] [PubMed] [Google Scholar]
- 14.Kanda LL, Louon L, Straley K. 2012. Stability in activity and boldness across time and context in captive Siberian dwarf hamsters. Ethology 118, 518–533. ( 10.1111/j.1439-0310.2012.02038.x) [DOI] [Google Scholar]
- 15.Lansade L, Bouissou M-F. 2008. Reactivity to humans: a temperament trait of horses which is stable across time and situations. Appl. Anim. Behav. Sci. 114, 492–508. ( 10.1016/j.applanim.2008.04.012) [DOI] [Google Scholar]
- 16.Lansade L, Bouissou M-F, Erhard HW. 2008. Fearfulness in horses: a temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 115, 182–200. ( 10.1016/j.applanim.2008.06.011) [DOI] [Google Scholar]
- 17.Lansade L, Bouissou M-F, Erhard HW. 2008. Reactivity to isolation and association with conspecifics: a temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 109, 355–373. ( 10.1016/j.applanim.2007.03.003) [DOI] [Google Scholar]
- 18.Kooij Ev.Erp-v.d, Kuijpers AH, Schrama JW. 2002. Can we predict behaviour in pigs? Searching for consistency in behaviour over time and across situations. Appl. Anim. Behav. Sci. 75, 293–305. ( 10.1016/S0168-1591(01)00203-9) [DOI] [Google Scholar]
- 19.Guenther A, Trillmich F. 2012. Photoperiod influences the behavioral and physiological phenotype during ontogeny. Behav. Ecol. 24, 402–411. ( 10.1093/beheco/ars177) [DOI] [Google Scholar]
- 20.Guenther A, Finkemeier MA, Trillmich F. 2014. The ontogeny of personality in the wild guinea pig. Anim. Behav. 90, 131–139. ( 10.1016/j.anbehav.2014.01.032) [DOI] [Google Scholar]
- 21.Janczak AM, Pedersen LJ, Bakken M. 2003. Aggression, fearfulness and coping styles in female pigs. Appl. Anim. Behav. Sci. 81, 13–28. ( 10.1016/S0168-1591(02)00252-6) [DOI] [Google Scholar]
- 22.Bell AM, Stamps JA. 2004. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim. Behav. 68, 1339–1348. ( 10.1016/j.anbehav.2004.05.007) [DOI] [Google Scholar]
- 23.Schürch R, Heg D. 2010. Life history and behavioral type in the highly social cichlid Neolamprologus pulcher. Behav. Ecol. 21, 588–598. ( 10.1093/beheco/arq024) [DOI] [Google Scholar]
- 24.Taylor JH, Mustoe AC, French JA. 2014. Behavioral responses to social separation stressor change across development and are dynamically related to HPA activity in marmosets. Am. J. Primatol. 76, 239–248. ( 10.1002/ajp.22228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson ADM, Krause J. 2012. Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 23, 1316–1323. ( 10.1093/beheco/ars123) [DOI] [Google Scholar]
- 26.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 27.Dochtermann NA, Roff DA. 2010. Applying a quantitative genetics framework to behavioural syndrome research. Phil. Trans. R. Soc. B 365, 4013–4020. ( 10.1098/rstb.2010.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong G. 1990. Quantitative genetics of reaction norms. J. Evol. Biol. 3, 447–468. ( 10.1046/j.1420-9101.1990.3050447.x) [DOI] [Google Scholar]
- 29.Stearns S, de Jong G, Newman B. 1991. The effects of phenotypic plasticity on genetic correlations. Trends Ecol. Evol. 6, 122–126. ( 10.1016/0169-5347(91)90090-K) [DOI] [PubMed] [Google Scholar]
- 30.Clutton-Brock TH. 1988. Reproductive success. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), pp. 472–485. Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Sinervo B, Svensson E. 2002. Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338. ( 10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- 32.Brommer JE, Kluen E. 2012. Exploring the genetics of nestling personality traits in a wild passerine bird: testing the phenotypic gambit. Ecol. Evol. 2, 3032–3044. ( 10.1002/ece3.412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carere C, van Oers K. 2004. Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol. Behav. 82, 905–912. ( 10.1016/j.physbeh.2004.07.009) [DOI] [PubMed] [Google Scholar]
- 34.Class B, Kluen E, Brommer JE. 2014. Evolutionary quantitative genetics of behavioral responses to handling in a wild passerine. Ecol. Evol. 4, 427–440. ( 10.1002/ece3.945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fucikova E, Drent PJ, Smits N, van Oers K. 2009. Handling stress as a measurement of personality in great tit nestlings (Parus major). Ethology 115, 366–374. ( 10.1111/j.1439-0310.2009.01618.x) [DOI] [Google Scholar]
- 36.Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ. 2003. Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747. ( 10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korsten P, van Overveld T, Adriaensen F, Matthysen E. 2013. Genetic integration of local dispersal and exploratory behaviour in a wild bird. Nat. Commun. 4, 2362 ( 10.1038/ncomms3362) [DOI] [PubMed] [Google Scholar]
- 38.van Overveld T, Careau V, Adriaensen F, Matthysen E. 2014. Seasonal- and sex-specific correlations between dispersal and exploratory behaviour in the great tit. Oecologia 174, 109–120. ( 10.1007/s00442-013-2762-0) [DOI] [PubMed] [Google Scholar]
- 39.Kruuk LEB. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluen E, de Heij ME, Brommer JE. 2011. Adjusting the timing of hatching to changing environmental conditions has fitness costs in blue tits. Behav. Ecol. Sociobiol. 65, 2091–2103. ( 10.1007/s00265-011-1218-y) [DOI] [Google Scholar]
- 41.Brommer JE, Korsten P, Bouwman KM, Berg ML, Komdeur J. 2007. Is extrapair mating random? On the probability distribution of extrapair young in avian broods. Behav. Ecol. 18, 895–904. ( 10.1093/beheco/arm049) [DOI] [Google Scholar]
- 42.Brommer JE, et al. 2010. Passerine extrapair mating dynamics: a Bayesian modeling approach comparing four species. Am. Nat. 176, 178–187. ( 10.1086/653660) [DOI] [PubMed] [Google Scholar]
- 43.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723. ( 10.1109/TAC.1974.1100705) [DOI] [Google Scholar]
- 44.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 45.David M, Auclair Y, Dechaume-Moncharmont F-X, Cézilly F. 2011. Handling stress does not reflect personality in female zebra finches (Taeniopygia guttata). J. Comp. Psychol. 126, 10–14. ( 10.1037/a0024636) [DOI] [PubMed] [Google Scholar]
- 46.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. ( 10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 47.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh B, Blows MW. 2009. Abundant genetic variation + strong selection = multivariate genetic constraints: a geometric view of adaptation. Annu. Rev. Ecol. Evol. Syst. 40, 41–59. ( 10.1146/annurev.ecolsys.110308.120232) [DOI] [Google Scholar]
- 49.Dochtermann NA, Dingemanse NJ. 2013. Behavioral syndromes as evolutionary constraints. Behav. Ecol. 24, 806–811. ( 10.1093/beheco/art002) [DOI] [Google Scholar]
- 50.Groothuis TGG, Trillmich F. 2011. Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641–655. ( 10.1002/dev.20574) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Population pedigree and phenotypic data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.443g2.



