Abstract
Insects have developed different structures to adhere to surfaces. Most common are smooth and hairy attachment pads, while nubby pads have also been described for representatives of Mantophasmatodea, Phasmida and Plecoptera. Here we report on the unusual combination of nubby and smooth tarsal attachment structures in the !nara cricket Acanthoproctus diadematus. Their three proximal tarsal pads (euplantulae) have a nubby surface, whereas the most distal euplantula is rather smooth with a hexagonal ground pattern resembling that described for the great green bush-cricket Tettigonia viridissima. This is, to our knowledge, the first report on nubby euplantulae in Orthoptera and the co-occurrence of nubby and smooth euplantulae on a single tarsus in a polyneopteran species. When adhering upside down to a horizontal glass plate, A. diadematus attaches its nubby euplantulae less often, compared to situations in which the animal is hanging upright or head down on a vertical plate. We discuss possible reasons for this kind of clinging behaviour, such as morphological constrains, the different role of normal and shear forces in attachment enhancement of the nubby and smooth pads, ease of the detachment process, and adaptations to walking on cylindrical substrates.
Keywords: adhesion, friction, locomotion, insect, morphology, Hetrodinae
1. Introduction
Insects have developed specialized structures on their feet for adhering to surfaces. These attachment structures can be smooth, as those reported in Blattodea [1] and Orthoptera [2], or hairy as found in Coleoptera [3,4] and Diptera [5]. A third type of surface, the nubby-shaped one, was found in representatives of Phasmida [6–8], Blattodea [9] and Plecoptera [10]. These nubs can be distinguished from hairs by having a lower aspect ratio (relation of length to width). According to their development, they may be called acanthae [8,11], although in many cases, it has not been clearly determined whether the nubs are unicellular cuticular projections (acanthae) or subcellular protuberances (microtrichia; for definitions, see [12]). Such specialized attachment structures may occur directly on the ventral surface of the foot (tarsus) or be situated on specialized attachment organs, such as arolia (a median lobe of the pretarsus) and euplantulae (pad-like structures on the ventral side of tarsomeres [13]). Arolia and euplantulae are usually smooth. However, nubby surfaces were reported from the euplantulae of phasmid species [7,11] and from the arolia of blattodean species. Hair-like structures occur, among other taxa, on the euplantulae of mantophasmatodean [11,14] and plecopteran [10] species.
Smooth and nubby attachment structures seem to have different functions. Smooth arolia of the phasmid species Carausius morosus generated high adhesive forces when shear movements were applied, whereas high loads increased the friction forces of its nubby euplantulae [8]. Smooth euplantulae of Cuniculina impigra (Phasmida) generated high adhesive and friction forces on smooth surfaces, while the nubby euplantulae of C. morosus seem to be adapted to different surface roughness [6]. Such differences in adhesive and friction forces of smooth and nubby attachment structures should correlate with their different use in different behavioural situations, when insects rely on either high adhesive or frictional forces. Indeed, when C. morosus stood upright, the first or the first two nubby euplantulae and part of the smooth arolium were in surface contact. When the insects were hanging upside down, contact was never formed by the nubby euplantulae but always by the arolium [8].
Here, we describe the unusual distribution of surface microstructure on tarsal attachment organs of the !nara cricket Acanthoproctus diadematus (Stål, 1858). We compare our results with the attachment surface microstructures found in other polyneopteran insects. Finally, we test the hypothesis that A. diadematus uses its two types of euplantulae to a different extent in various behavioural situations and discuss the function of differently shaped euplantulae.
2. Material and methods
(a). Animals
The distribution of A. diadematus is restricted to the main southern Namib dune sea [15], where the endemic !nara plant, Acanthosicyos horridus, grows in coastal areas and in the vicinity of ephemeral streams [16]. According to the literature, A. diadematus lives and feeds on the !nara plant (e.g. [17]). However, we also found individuals several hundred metres away from the plants in dune grass Stipagrostis sabulicola.
Male and female A. diadematus were caught in January 2011 close to the Gobabeb Training and Research Centre (23°34′31″ S, 15°2′31″ E) and identified according to a key written by John Irish [15]. They were subsequently maintained and bred in a climate chamber at 24°C and 30% humidity under a 14 L : 10 D cycle including 30 min dawn and dusk periods. All results reported in this paper were obtained from wild-caught adults and their first-generation offspring.
(b). Scanning electron microscopy
Tarsi of two females, one male and two larvae were fixed in 70–80% ethanol and critical-point dried. We cleaned some of the samples in an ultrasonic bath beforehand to reduce contamination by sand particles on their surface. This cleaning procedure was successful and no other differences between bathed and non-bathed samples were observed. The tarsi were then sputter-coated with a layer of approximately 10 nm of gold-palladium. To visualize the inner structures of pads, we fractured a few samples with tweezers and sputtered them again. Images were taken with the scanning electron microscope (SEM) Hitachi S4800 (Hitachi High-Technologies Corp., Tokio, Japan) at 3 kV, Hitachi SU3500 and Hitachi TM3000 at 5 kV of acceleration voltage.
(c). Behavioural experiments
In order to investigate how A. diadematus uses its different euplantulae during locomotion, we let larvae adhere to a glass plate in different orientations: (i) horizontal with the animal standing upright, (ii) horizontal with the animal hanging upside down, (iii) vertical with the animal facing up, and (iv) vertical with the animal facing down. It was then determined which euplantulae were in surface contact (see the electronic supplementary material, text S1 and figure S2).
Within each type of attachment structure, we statistically compared, between the four situations, the proportion of euplantulae of the corresponding type being in contact with the plate. Therefore, data from the fore-, middle and hind legs were pooled together and, for the nubby euplantulae, we additionally pooled the data for all three euplantulae. We used a two-sample test for equality of proportions with continuity correction (function prop.test in the statistical software R) and adjusted for multiple comparisons according to Holm [18]. Within each position (i)–(iv), we compared the per cent observations in that at least one of the nubby euplantulae was in contact with the glass plate with the per cent observations in that the smooth euplantula was in contact. We again used a two-sample test for equality of proportions with continuity correction (function prop.test in the statistical software R). We did not use the data that were pooled for the three nubby euplantulae for this test to account for the fact that there were three nubby but only one smooth euplantula.
3. Results and discussion
(a). Morphology
(i). Adults
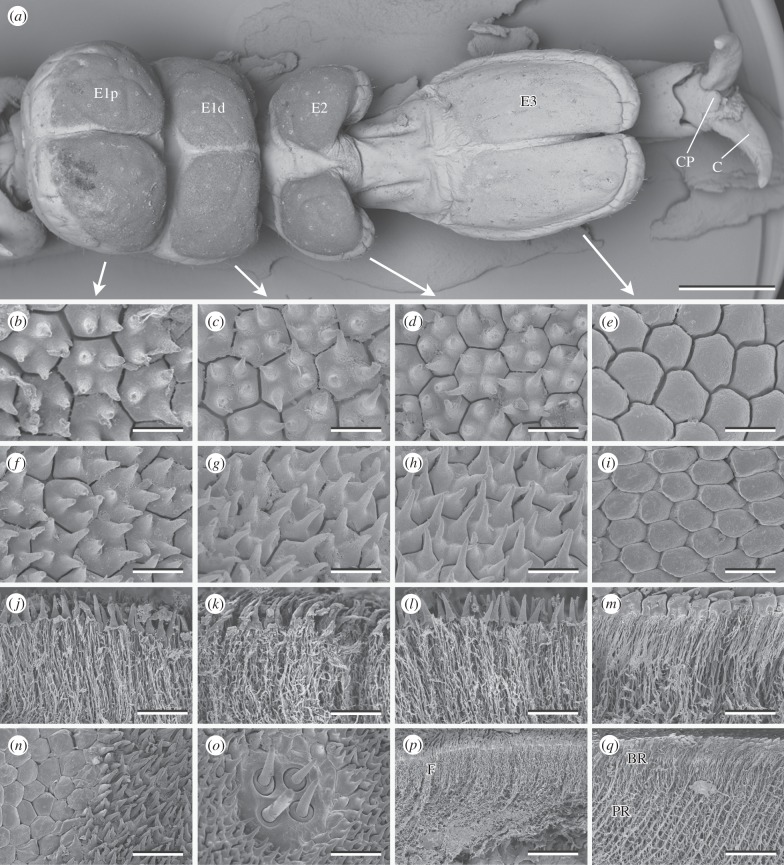
All tarsi of adult A. diadematus consist of four tarsomeres, the last (most distal) one of which ends in a paired claw (figure 1 and electronic supplementary material, figure S1). Between the claws, there is a little pad-like structure that seems to be homologous to arolia and resembles a similar structure in the great green bush-cricket Tettigonia viridissma [19]. The dorsal surface of the first (proximal) tarsomere is convex, while its ventral side is split, by a deep notch, into a proximal and a distal half, each of which bears a paired euplantula (E1p and E1d, respectively). The two lateral halves of E1p and E1d are separated by a shallow, median notch. On the second tarsomere, we find a paired euplantula (E2) with two pads that are farther apart than those of E1p and E1d. The paired euplantula on the following tarsal segment (E3) is the most distal one. It is divided by a shallow notch and considerably longer than all the other euplantulae. Generally, the tarsi of A. diadematus resemble those of the fore- and middle leg of T. viridissma as described by Henning [19].
Figure 1.
Tarsal structures of A. diadematus. (a) Ventral view of the tarsus. Surface structures (b–i,n,o) and fractures (j–m,p,q) of the proximal portion of the first euplantula (b,f,j,n,o), distal portion of the first euplantula (c,g,k), second (d,h,l,p), and third (e,i,m,q) euplantula. (n) Transition from the outer zone to the nubby structures. (o) Aggregate of sensilla. E1p, E1d, proximal and distal euplantula of tarsomere 1; E2 and E3, euplantulae of tarsomeres 2 and 3; C, claw; CP, claw pad; BR, layer of branching rods; F, layer of fine filaments; PR, layer of principal rods. Scale bars: (a) 1 mm, (b–i) 5 µm, (j–m) 10 µm, (n,o) 15 µm and (p,q) 30 µm.
However, varying from that of T. viridissima, the ventral surface of the euplantulae E1p, E1d and E2 in A. diadematus is densely covered with nubs (figure 1b–d,f–h). These nubs are about 5 µm in height with an aspect ratio of 2–3. There are no great differences in size or shape of the nubs on different euplantulae. The nubs disappear at the outer zone of the pads, where the hexagonal surface pattern becomes smooth (figure 1n). In all cases, on an average, four (minimum 1, maximum 6) nubs are located on individual hexagonal plates, each plate being roughly 4 µm in diameter. We assume that a single hexagonal plate corresponds to a single epidermal cell and that hence, the nubs of A. diadematus are microtrichia.
In contrast to the three euplantulae on the first two tarsomeres, the euplantula on the third tarsomere is solely composed of smooth hexagons (figure 1e,i). They are larger than the hexagons of the other euplantulae, around 6 µm in diameter (area 21.4 µm2; s.d. = 2.2 µm2; n = 10). Similar hexagonal structures have been described for the attachment pads of T. viridissima [19,20]. With an area of 14.7 µm2 (s.d. = 1.96 µm2; n = 22), the hexagons of the most distal euplantula of T. viridissima are smaller than those of A. diadematus [21]. This might correlate with the different body size of both species.
The euplantulae of A. diadematus bear scattered aggregates of 2–4 sensilla (figure 1o) that closely resemble those described for T. viridissima [19]. We found neither obvious differences between the tarsal structures of females and males, nor differences within a single euplantula or between euplantulae, except for E3 (see above). There was also no general gradient in the length of the nubs, as described for Mantophasma zephyrum [11], nor a gradient in the size of the hexagons within an euplantula.
(ii). Larvae
Larval and adult tarsi are similar in their general appearance (electronic supplementary material, figure S1). The ventral side of the three proximal euplantulae is nubby, while the most distal one has a smooth appearance with a clearly visible hexagonal pattern. In relation to the total size of the tarsus, the euplantulae of larvae seem to be less bulky compared with those of adults. This is most obvious for the nubby euplantulae because their magnitude can easily be detected in the SEM images: their surface appears slightly darker than the dorsal surface of the tarsi.
(iii). Inner structure
Below their nubby surface, the proximal three euplantulae are composed of long, fine filaments that are interconnected by cross-links (figure 1j–l,p). The filaments are packed more densely in the upper layer, down to a depth of about 15 µm, than further below. A slightly similar architecture has previously been found in Locusta migratoria [20].
Below the smooth surface of the distal euplantula of A. diadematus, two strikingly different layers can be distinguished. Thick principal rods occur in a zone from about 15 to 30 µm below the surface. In the layer above, these rods branch into filaments (figure 1m,q). All branches of a single principle rod end in one hexagon. As for the filaments of the nubby euplantulae, principal and branched rods are interconnected by cross-links. The principal rods of A. diadematus are 1.13 µm (s.d. = 0.15 µm; n = 20) in diameter, which is about the same thickness as in T. viridissima (1.12 µm [21]), another tettigoniid species with a similar cuticular architecture of euplantulae [2,19–21]. Thick principal and thin branching rods have also been described for the arolia of the caeliferan species Schistocerca gregaria [22], the phasmid C. morosus [23] and different cockroach species [24,25]. This pattern of the pad's internal architecture has been shown to strongly influence the attachment properties of smooth pads by increasing their adhesion, especially on rough substrates [2,20,23].
In A. diadematus, the rods are oriented distally at an inclination of roughly 50°–80° to the surface, which varies to a great extent in different areas of the euplantulae (mean = 63.7°; s.d. = 6.9°; n = 20). Such a slope was likewise found in the euplantulae of T. viridissima (45°–70° [21]) and in the arolia of C. morosus ([23]; 71° [26]). By contrast, the rods in the arolia of S. gregaria are arranged almost perpendicular to the surface [22]. The angle of the rods decreases under load, resulting in an increased contact area [26] and a higher frictional force, when the pad is moved proximadly [21].
(b). Attachment structures in Polyneoptera
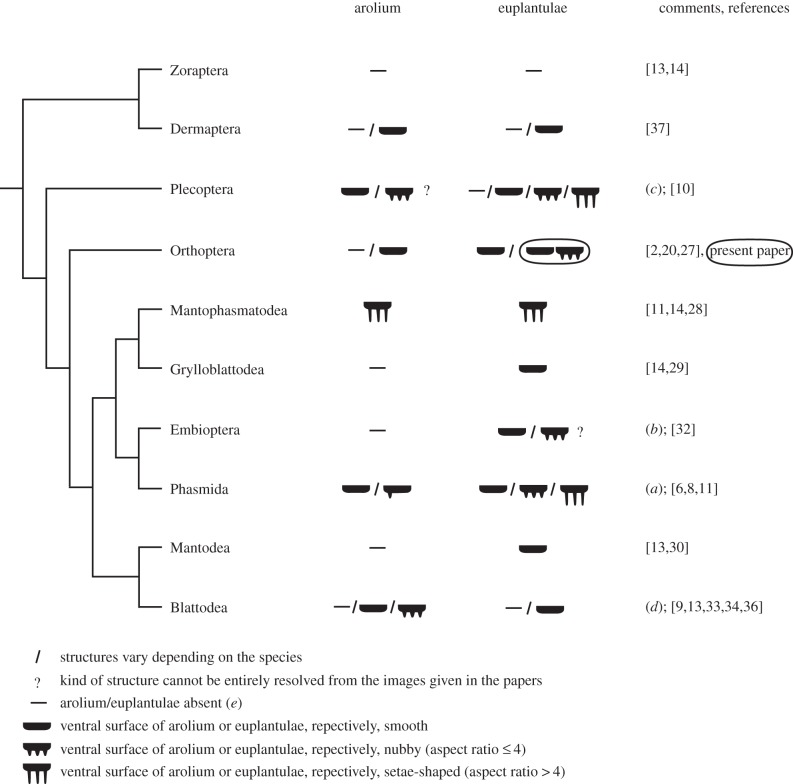
In polyneopteran species, arolia and euplantulae have been described to be smooth, covered with protuberances or completely absent (figure 2) [2,6–11,13,14,20–30,32–34,36,37]. Different outgrowths of tarsal adhesive pads have been previously described: nubs, acanthae, microtrichia and setae. If euplantulae are absent, the tarsomeres can be ventrally covered with densely standing setae as, for example, in Dermaptera [37]. In some taxa, such as Grylloblattodea, Mantophasmatodea, Zoraptera and Mantodea, there is no pronounced variation in the attachment structures between different species. By contrast, Nelson [10] described a high morphological variety for the surface structures of arolia and euplantulae in Plecoptera, which might be partly due to the large amount of different taxa he studied. In general, within and across insect orders, the aspect ratio (relation of length to width) of the protuberances can vary greatly.
Figure 2.
Structures on the attachment organs (arolia and euplantulae) in Polyneopteran orders. The cladogram follows Misof et al. [31]. Findings of the present paper are circled. (a) In Timema nevadense (Timematidae, the most basal family within the Phasmida), the central area of the arolium is smooth and euplantulae have setae-shaped structures [11]; representatives of the Euphasmatodea (all other phasmid families) have an entirely smooth arolium and smooth or nubby euplantulae [6,8,11]. (b) Small semicircular protuberances (papillae) are present on meso- and metathoracic tarsomeres in Embioptera [32]; they cover only a small proportion of the tarsomeres. These papillae look similar to euplantulae, however, their origin is not yet solved. (c) In the exhaustive study of Nelson [10], 39 plecopteran species were analysed and a large variety of structures and their combinations were described for the ventral tarsomeres. The nubs on the arolia seem to vary in length, so that some of them might have an aspect ratio > 4. (d) Cockroaches: arolia and euplantulae absent, smooth or nubby [9,33,34], reduction of the arolium often occurs in cavernicolous species [33]; termites (Epifamily Termitoidae within Blattodea [35]): arolia are present in alate adults of some species and absent in workers [36], euplantulae are absent [13]. (e) If specialized attachment organs are lacking, the ventral side of the tarsomeres is usually covered with long setae-like structures.
In most taxa, the aspect ratio of protuberances is below five. However, the structures on the euplantulae of Timema nevadensis (a basal group of Phasmida) have an aspect ratio of 5–10 (estimated from the figure given in [6]) and the euplantulae of M. zephyrum (Mantophasmatodea) even have 10 µm long setae-shaped structures with a tongue-shaped apical part and an aspect ratio of 10–20. In comparison, the nubs on the euplantulae of A. diadematus are 5 µm in length with an aspect ratio of 2–3. They are strikingly similar in shape and density to those found on the euplantulae of the euphasmatodean species Aretaon asperrimus [11], C. morosus [6,8] and Neohirasea maerens [11]. We will subsequently call the surface structures of the arolia and euplantulae ‘nubs’, if their aspect ratio is 4 or less, and ‘setae-shaped structures’ if their aspect ratio is greater than 4. However, it is difficult to estimate aspect ratios from images that were not taken exactly at an angle of 90° to the structure. Additionally, the aspect ratio of the structure might vary within a single euplantula. For example, surface structures on the arolia of Plecoptera could be properly named as soon as suitable images are taken.
Nubby euplantulae have not been found in any other orthopteran species than A. diadematus so far (figure 2). The !nara cricket is also the first species for which an hexagonal pattern with imposing nubs is described. Up to now, smooth and nubby attachment structures have been found together on a single tarsus in representatives of Phasmida, which have smooth arolia and nubby euplantulae (figure 2). It has not been previously described that structures vary between different euplantulae of a single tarsus. The co-occurrence of nubby and smooth euplantulae in A. diadematus is hence a further novelty.
(c). Behavioural experiments
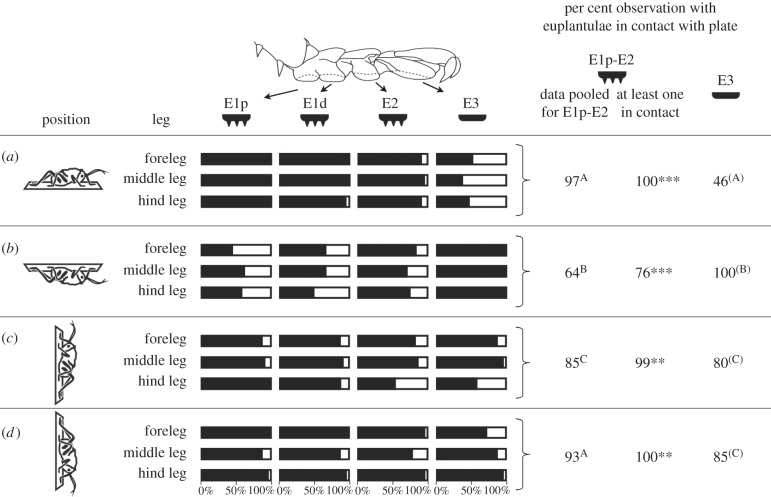
We tested the hypothesis that A. diadematus preferably uses different structures for attachment in different behavioural situations. While standing on a horizontal glass plate in an upright position, the nubby euplantulae were almost always in surface contact (97% of all observations, data pooled from all three nubby euplantulae and from the fore-, middle and hind legs; figure 3). If the animals were hanging upside down on the same plate, their nubby euplantulae were in contact in only 64% of the cases, which is significantly less than in the upright position (p < 0.001) and compared with hanging head up (85%, p < 0.001) or head down (93%, p < 0.001) on a vertical surface.
Figure 3.
Percentage of observations in which the different euplantulae of A. diadematus larvae were in contact with a glass plate. (a,b) Plate orientated horizontally and animal standing above (a) or hanging below (b) the plate. (c,d) Plate orientated vertically and animal facing upward (c) or downward (d). For further analyses, data from the fore-, middle and hind legs were pooled. Within each column, different letters indicate significant pairwise differences between groups. Within each position (row), asterisks indicate significant differences between the per cent observations in that at least one of the euplantulae E1p-E2 was in contact with the plate and the per cent observations in that E3 was in contact. E1p-E3: Euplantulae numbered according to figure 1 with E1p being the most proximal and E3 the most distal one. ***: significant differences at p < 0.001; **: significant differences at p < 0.01. Four individuals were recorded and each euplantula was observed five to seven times per individual, leg and situation.
The smooth euplantulae had surface contact in just 46% of the observations for the horizontal, upright position. By contrast, if the animals were hanging upside down under the horizontal glass plate, the smooth euplantulae were in contact in all observations (100%). This is also significantly more than on the vertical surface (facing upward: 80%, p < 0.001; facing downward: 85%, p = 0.004).
When attaching below a horizontal surface, it was significantly less often the case that at least a single nubby euplantula was in contact with the plate, if compared to the smooth euplantula (p < 0.001). The opposite was true for all other positions.
These results clearly show that the euplantulae of the two types are differently used in different behavioural situations. The smooth distal euplantulae were used in situations where high adhesive forces are required, i.e. upside down on a vertical surface and above the centre of body mass on a vertical surface. The nubby proximal euplantulae were used when high friction forces were required, i.e. to a higher extend, when walking on vertical surfaces, if compared to the situation of hanging below a horizontal surface. They were also preferably used under compression, when the cricket was walking upright on a horizontal surface.
Several factors might be responsible for the observed contact patterns. The use of the most distal euplantulae, when attaching upside down on the horizontal plate, and the high proportion of contact with the most distal pad of the leg facing upward, when attaching on a vertical plate, might not be a result of ultra-structural pattern but simply a consequence of both morphological and mechanical constraints of the tarsal chain. Two further species attach to vertical surfaces similar to A. diadematus. The cockroach Nauphoeta cinerea has both a smooth arolium and smooth euplantulae and also contacts a vertical plate with the most distal pad of the leg facing upward [24]. The leaf beetle Gastrophysa viridula has no specialized attachment organ like arolium or euplantulae, but mainly spatula-shaped attachment setae on the most distal tarsal segment and pointed as well as discoidal (males only) setae on the two more proximal segments. This species as well contacts a vertical plate with the most distal pad of the leg facing upward [4].
However, the question remains why does the tarsus require both types of structures (smooth and nubby) instead of just one?
The different functional properties of smooth and nubby attachment structures have recently been investigated in the phasmid species C. morosus [8]. In these experiments, attachment forces of nubby euplantulae were compared with those of the smooth arolium. The latter one generated relatively high adhesive forces that increased when shear movements were applied. Friction forces of nubby euplantulae increased when large normal forces were applied. Hence, the high proportion of smooth euplantula in contact with the horizontal plate in our experiments is perhaps being observed because shear forces can be relatively easily generated by pulling the legs to the centre of the body. While hanging upward or downward in a vertical position, the strength of friction forces might be more easily adapted by modifying the pressure on the downward tarsi. To this end, and as adhesion is only required for legs above the centre of body mass, to prevent dropping off due to torque, the upward directed legs need to attach firmly to the surface. Indeed, the smooth distal euplantulae (E3) of the feet facing upward were in contact with the glass plate more often than the smooth euplantulae of the feet facing downward (figure 3c,d).
Interestingly, in both studies comparing smooth and nubby attachment structures [6,8], smooth attachment structures reached at least similar but often even better adhesive and friction forces compared to nubby structures. From these results, it might be surprising that nubby euplantulae occur at all. The maximal force that can be reached might not play a dominant role. It was shown, for example, that the whole surface of a smooth euplantula was only seldom attached to the surface [24]. The poor performance of nubby structures in adhesive and friction tests might thus be compensated by their higher numbers. In general, nubby attachment structures might have the following advantages.
(i). Adjustment of friction forces under different loads
Nubby euplantulae respond in their friction forces to changing load to a higher degree compared with smooth euplantulae [8]. This self-adjustment might be advantageous especially in situations when high friction forces are needed, as for example on vertical surfaces.
(ii). Adaptation to a wider range of surface structures
When the attachment performances of the nubby structures on the euplantulae of the phasmid species C. morosus were compared to the smooth euplantulae of Cuniculina impigra, the nubby structures of C. morosus were less susceptible to different surfaces [6]. In these experiments, the adhesive forces as well as the frictional coefficient (measured in the proximal direction) of the nubby euplantulae did not differ significantly between smooth surfaces, if compared to surfaces with a roughness of Ra = 1.4 µm, while these parameters measured in the smooth euplantulae did differ significantly on different surfaces. In order to generalize these findings, further experiments on surfaces with a broader range of roughness are needed.
(iii). Ease of detachment
Insects might need less force to detach nubby rather than smooth euplantulae. Nubby euplantulae are load sensitive while smooth ones are shear sensitive [8]. To detach nubby structures, it might be sufficient to simply decrease load. If high loads were applied beforehand, the elastic energy that is stored in the deformed nubs may promote the detachment process [38]. The cost of removing smooth euplantulae might be much higher as reverse shear forces need to be applied or the euplantulae need to be peeled off.
(iv). Prevention of stick and slip behaviour
Microstructured surfaces can show strongly reduced stick–slip motions in comparison with smooth surfaces [39]. Although previous experiments were performed with mushroom-shaped structures, it is likely that nubby microstructures possess similar properties. The importance of preventing stick–slip motions in A. diadematus might be indicated by the presence of a hexagonal pattern that was observed on both its smooth and its nubby euplantulae. Such a hexagonal pattern was previously demonstrated to reduce stick–slip behaviour on dry surfaces and to increase friction on wet surfaces [40].
(v). Adaptation to walking on structured surfaces, such as the !nara plant
The twigs of the leafless !nara plant are corrugated. It was previously shown in stick insects that adhesive forces of smooth euplantulae were higher on smooth compared with rough substrates, but there was no significant difference for nubby euplantulae [6]. Hence, nubby euplantulae might be adapted to adhering with similar forces, to a wide variety of surfaces. Up to now, most studies on the attachment properties of euplantulae or arolia were conducted on smooth or rough plate-like surfaces. However, nubby euplantulae might be adapted to attach firmly to surfaces with roughness of far greater magnitudes than those tested so far. For example, in nature, A. diadematus climbs on the twigs of the !nara plant [41], which have a corrugated surface with longitudinal grooves and ridges, and on the dune grass S. sabulicola [42]. The protruding nubs might snuggle more easily against the plant epidermis cells inside the furrows. A detailed microscopic visualization on how nubby and smooth euplantulae form contact with such surfaces and analyses on how this contact affects adhesive and friction forces could be helpful in understanding specific adaptations of the cricket's attachment structures to actual surfaces.
Moreover, for comparison, the structure and performance of euplantulae of further species within the Heterodinae should be analysed. Besides morphological and biomechanical analyses, it would be worth studying general correlations between the structure of attachment devices and the autecology of species in the future. For example, within taxa such as Phasmida and especially Plecoptera, the great diversity in attachment structures could correlate with the diversity of their habitats and the surface structures of substrates found in their habitats.
4. Conclusion
The ventral surface of the three proximal euplantulae of the !nara cricket A. diadematus is nubby, the surface of the most distal euplantula is smooth. Nubby euplantulae in an orthopteran species and the co-occurrence of nubby and smooth euplantulae on one tarsus are described here for the first time, to our knowledge. Our experiments show that the smooth distal euplantula is used in situations in which adhesion is required, whereas the more proximal nubby ones are mostly used when friction is required and under compression. With the morphological description of attachment pads in species of more insect taxa in the future, we expect an even greater variability of patterns to be found than those depicted in figure 2.
Supplementary Material
Acknowledgements
The Namibian Ministry of Environment and Tourism granted the permit to export the crickets (permit no. 1557/2010). We are grateful to O. Gustafsson and E. Appel for technical help, to J. Henschel for advice in the field and to A. Kelber for access to various equipment. Discussions with L. Heepe, A. Kelber and J. Wolff improved this manuscript. Martin Kohler kindly provided the drawings in figure 3. We thank V. Kastner for linguistic corrections of the manuscript and two anonymous reviewers for valuable comments.
Data Accessibility
The dataset associated with the behavioural experiments of this study is provided as electronic supplementary material table S1.
Authors' Contributions
C.G. summarized the attachment structures of polyneopteran orders, carried out the statistical analyses and drafted the manuscript; C.G. and M.H. conducted the behavioural experiments; C.G., M.H. and T.N. provided SEM images; M.H. bred and raised the animals; M.H. and T.N. collected the specimens from the field; M.H., T.N. and S.G. revised the manuscript; T.N. and S.G. conceived the study. All authors gave final approval for publication.
Competing Interests
We have no competing interests.
Funding
This work was supported by the German Research Foundation (grant ‘Function by Switching’, SFB 677-C10) and the Swedish Research Council (grant nos. Vr 621–2009–5683 and Vr 621–2012–2212).
References
- 1.Clemente CJ, Dirks J-H, Barbero DR, Steiner U, Federle W. 2009. Friction ridges in cockroach climbing pads: anisotropy of shear stress measured on transparent, microstructured substrates. J. Comp. Physiol. A 195, 805–814. ( 10.1007/s00359-009-0457-0) [DOI] [PubMed] [Google Scholar]
- 2.Gorb S, Jiao Y, Scherge M. 2000. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J. Exp. Biol. 186, 821–831. ( 10.1007/s003590000135) [DOI] [PubMed] [Google Scholar]
- 3.Gorb EV, Hosoda N, Miksch C, Gorb SN. 2010. Slippery pores: anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 7, 1571–1579. ( 10.1098/rsif.2010.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock JMR, Federle W. 2009. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: effective elastic modulus and attachment performance. J. Exp. Biol. 212, 1876–1888. ( 10.1242/jeb.030551) [DOI] [PubMed] [Google Scholar]
- 5.Niederegger S, Gorb S, Jiao YK. 2002. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J. Comp. Physiol. A 187, 961–970. ( 10.1007/s00359-001-0265-7) [DOI] [PubMed] [Google Scholar]
- 6.Bußhardt P, Wolf H, Gorb SN. 2012. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 115, 135–141. ( 10.1016/j.zool.2011.11.002) [DOI] [PubMed] [Google Scholar]
- 7.Bullock JMR, Drechsler P, Federle W. 2008. Comparison of smooth and hairy attachment pads in insects: friction, adhesion and mechanisms for direction-dependence. J. Exp. Biol. 211, 3333–3343. ( 10.1242/jeb.020941) [DOI] [PubMed] [Google Scholar]
- 8.Labonte D, Federle W, Ivanenko YP. 2013. Functionally different pads on the same foot allow control of attachment: stick insects have load-sensitive ‘heel’ pads for friction and shear-sensitive ‘toe’ pads for adhesion. PLoS ONE 8, e81943 ( 10.1371/journal.pone.0081943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold JW. 1974. Adaptive features on the tarsi of cockroaches (Insecta: Dictyoptera). Int. J. Insect Morphol. Embryol. 3, 317–334. ( 10.1016/0020-7322(74)90026-9) [DOI] [Google Scholar]
- 10.Nelson CH. 2009. Surface ultrastructure and evolution of tarsal attachment structures in Plecoptera (Arthropoda: Hexapoda). Aquat. Insects 31, 523–545. ( 10.1080/01650420802598210) [DOI] [Google Scholar]
- 11.Beutel RG, Gorb SN. 2008. Evolutionary scenarios for unusual attachment devices of Phasmatodea and Mantophasmatodea (Insecta). Syst. Entomol. 33, 501–510. ( 10.1111/j.1365-3113.2008.00428.x) [DOI] [Google Scholar]
- 12.Richards A, Richards PA. 1979. The cuticular protuberances of insects. Int. J. Insect Morphol. Embryol. 8, 143–157. ( 10.1016/0020-7322(79)90013-8) [DOI] [Google Scholar]
- 13.Beutel RG, Gorb SN. 2001. Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J. Zoolog. Syst. Evol. Res. 39, 177–207. ( 10.1046/j.1439-0469.2001.00155.x) [DOI] [Google Scholar]
- 14.Beutel RG, Gorb SN. 2006. A revised interpretation of the evolution of attachment structures in Hexapoda with special emphasis on Mantophasmatodea. Arthropod Syst. Phylogeny 64, 3–25. [Google Scholar]
- 15.Irish J. 1992. The Hetrodinae (Orthoptera: Ensifera: Bradyporidae) of Southern Africa: systematics and phylogeny. Navors. Nas. Mus., Bloemfontein 8, 393–434. [Google Scholar]
- 16.Wilkins-Ellert MH. 2004. Acanthosicyos horridus Welw. ex Hook.f. In Internet record from PROTA4U (eds GJH Grubben, OA Denton). Wageningen, Netherlands: PROTA (Plant Resources of Tropical Africa / Ressources végétales de l'Afrique tropicale) See http://www.prota4u.org/search.asp (accessed 1 April 2014).
- 17.Conti E, Viglianisi FM. 2005. Ecology of the calling song of two Namibian armoured ground crickets, Acanthoplus longipes and Acanthoproctus diadematus (Orthoptera Tettigoniidae Hetrodinae). Ethol. Ecol. Evol. 17, 261–269. ( 10.1080/08927014.2005.9522596) [DOI] [Google Scholar]
- 18.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Statistics 6, 65–70. [Google Scholar]
- 19.Henning B. 1974. Morphologie und Histologie der Tarsen von Tettigonia viridissima L. (Orthoptera, Ensifera). Z. Morphol. Tiere 79, 323–342. ( 10.1007/BF00277513) [DOI] [Google Scholar]
- 20.Goodwyn PP, Peressadko A, Schwarz H, Kastner V, Gorb S. 2006. Material structure, stiffness, and adhesion: why attachment pads of the grasshopper (Tettigonia viridissima) adhere more strongly than those of the locust (Locusta migratoria) (Insecta: Orthoptera). J. Comp. Physiol. A 192, 1233–1243. ( 10.1007/s00359-006-0156-z) [DOI] [PubMed] [Google Scholar]
- 21.Gorb S, Scherge M. 2000. Biological microtribology: anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. R. Soc. Lond. B 267, 1239–1244. ( 10.1098/rspb.2000.1133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendall MD. 1970. The anatomy of the tarsi of Schistocerca gregaria Forskål. Z. Zellforsch. Mikrosk. Anat. 109, 112–137. ( 10.1007/BF00364935) [DOI] [PubMed] [Google Scholar]
- 23.Scholz I, Baumgartner W, Federle W. 2008. Micromechanics of smooth adhesive organs in stick insects: pads are mechanically anisotropic and softer towards the adhesive surface. J. Comp. Physiol. A 194, 373–384. ( 10.1007/s00359-008-0314-6) [DOI] [PubMed] [Google Scholar]
- 24.Clemente CJ, Federle W. 2008. Pushing versus pulling: division of labour between tarsal attachment pads in cockroaches. Proc. R. Soc. B 275, 1329–1336. ( 10.1098/rspb.2007.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth LM, Willis ER. 1952. Tarsal structure and climbing ability of cockroaches. J. Exp. Zool. 119, 483–517. ( 10.1002/jez.1401190307) [DOI] [Google Scholar]
- 26.Dirks J-H, Li M, Kabla A, Federle W. 2012. In vivo dynamics of the internal fibrous structure in smooth adhesive pads of insects. Acta Biomater. 8, 2730–2736. ( 10.1016/j.actbio.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 27.Groll EK, Günther KK. 2003. 17. Ordnung Saltatoria (Orthoptera), Heuschrecken, Springschrecken. In Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil: Insecta (ed. Dathe HH.), 2nd edn, pp. 261–290. Berlin, Heidelberg: Spektrum Akademischer Verlag GmbH. [Google Scholar]
- 28.Eberhard MJ, Pass G, Picker MD, Beutel R, Predel R, Gorb SN. 2009. Structure and function of the arolium of Mantophasmatodea (Insecta). J. Morphol. 270, 1247–1261. ( 10.1002/jmor.10754) [DOI] [PubMed] [Google Scholar]
- 29.Rentz D. 1991. Grylloblattodea. In The insects of Australia: a textbook for students and research workers (ed. CSIRO Division of Entomology), pp. 357–359, 2nd edn Carlton, Victoria: Melbourne University Press. [Google Scholar]
- 30.Wieland F. 2013. The phylogenetic system of Mantodea (Insecta: Dictyoptera). Species Phylogeny Evol. 3, 3–222. [Google Scholar]
- 31.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 32.Ross ES. 2000. Embia: contributions to the biosystematics of the insect order Embiidina. Part 1. Origin, relationships and integumental anatomy of the insect order Embiidina. Occas. Pap. Calif. Acad. Sci. 149, 1–53. [Google Scholar]
- 33.Roth LM. 1991. Blattodea. In The insects of Australia: A textbook for students and research workers (ed. CSIRO Division of Entomology), pp. 320–329, 2nd edn Carlton, Victoria: Melbourne University Press. [Google Scholar]
- 34.Bohn H. 2003. 14. Ordnung Blattoptera, Schaben. In Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil: Insecta (ed. Dathe HH.), pp. 197–223, 2nd edn Berlin, Heidelberg: Spektrum Akademischer Verlag GmbH. [Google Scholar]
- 35.Beccaloni G, Eggleton P. 2013. Order Blattodea. In Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness (Addenda 2013), vol. 3703 Zootaxa 3148 (ed. Zhang Z-Q.), pp. 46–48. Auckland, New Zealand: Magnolia Press. [DOI] [PubMed] [Google Scholar]
- 36.Crosland MWJ, Su N-Y, Scheffrahn RH. 2005. Arolia in termites (Isoptera): functional significance and evolutionary loss. Insectes Soc. 52, 63–66. ( 10.1007/s00040-004-0779-4) [DOI] [Google Scholar]
- 37.Haas F, Gorb S. 2004. Evolution of locomotory attachment pads in the Dermaptera (Insecta). Arthropod Struct. Dev. 33, 45–66. ( 10.1016/j.asd.2003.11.003) [DOI] [PubMed] [Google Scholar]
- 38.Labonte D, Williams JA, Federle W. 2014. Surface contact and design of fibrillar 'friction pads’ in stick insects (Carausius morosus): mechanisms for large friction coefficients and negligible adhesion. J. R. Soc. Interface 11, 20140034 ( 10.1098/rsif.2014.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varenberg M, Gorb S. 2007. Shearing of fibrillar adhesive microstructure: friction and shear-related changes in pull-off force. J. R. Soc. Interface 4, 721–725. ( 10.1098/rsif.2007.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varenberg M, Gorb SN. 2009. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 21, 483–486. ( 10.1002/adma.200802734) [DOI] [Google Scholar]
- 41.Kartusch B, Kartusch R. 2008. Stem anatomy of Acanthosicyos horridus (Cucurbitaceae). S. Afr. J. Bot. 74, 647–650. ( 10.1016/j.sajb.2008.04.001) [DOI] [Google Scholar]
- 42.Roth-Nebelsick A, et al. 2012. Leaf surface structures enable the endemic Namib desert grass Stipagrostis sabulicola to irrigate itself with fog water. J. R. Soc. Interface 9, 1965–1974. ( 10.1098/rsif.2011.0847) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset associated with the behavioural experiments of this study is provided as electronic supplementary material table S1.