Abstract
The evolutionary maintenance of same-sex sexual behaviour (SSB) has received increasing attention because it is perceived to be an evolutionary paradox. The genetic basis of SSB is almost wholly unknown in non-human animals, though this is key to understanding its persistence. Recent theoretical work has yielded broadly applicable predictions centred on two genetic models for SSB: overdominance and sexual antagonism. Using Drosophila melanogaster, we assayed natural genetic variation for male SSB and empirically tested predictions about the mode of inheritance and fitness consequences of alleles influencing its expression. We screened 50 inbred lines derived from a wild population for male–male courtship and copulation behaviour, and examined crosses between the lines for evidence of overdominance and antagonistic fecundity selection. Consistent variation among lines revealed heritable genetic variation for SSB, but the nature of the genetic variation was complex. Phenotypic and fitness variation was consistent with expectations under overdominance, although predictions of the sexual antagonism model were also supported. We found an unexpected and strong paternal effect on the expression of SSB, suggesting possible Y-linkage of the trait. Our results inform evolutionary genetic mechanisms that might maintain low but persistently observed levels of male SSB in D. melanogaster, but highlight a need for broader taxonomic representation in studies of its evolutionary causes.
Keywords: Drosophila melanogaster, evolutionary genetics, overdominance, quantitative genetics, same-sex sexual behaviour, sexual antagonism
1. Introduction
Studies of same-sex sexual behaviour (SSB) have focused on a diverse range of animal taxa, from deep sea squid to insects [1–6]. The core of such research hinges on the assumption that SSB imposes a direct fitness cost on individuals that express it, and therefore represents an ‘evolutionary paradox’ demanding explanation (e.g. [7–10]). However, SSB is no different from any other trait that might appear inexplicably costly when benefits are not immediately obvious. Historically, similar traits have included aggression, altruism and sexual ornamentation [11].
Characterizing the genetic basis of SSB in a broad range of species is critical to better understanding its evolutionary persistence, but biologists studying non-human animals are hampered by a lack of empirical genetic data. We are aware of only a pair of artificial selection experiments using the flour beetle Tribolium castaneum [12,13], a report of intersexual correlation for SSB in the seed beetle Callosobruchus maculatus [14], plus a number of candidate gene studies in Drosophila melanogaster that document male–male courtship as an incidental effect of mutations affecting sex recognition (see [3] or [6] for reviews). The latter have elegantly illuminated proximate neurogenetic mechanisms that influence the expression of SSB in Drosophila, but they have limited power to explain the evolutionary forces that shape this complex, quantitative trait in natural populations [15]. The deficit of genetic data on SSB in non-human animals is compounded by the limited number of theoretical studies that quantitatively model its genetic basis (reviewed in [4]; see also [16–20]).
Theoretical work by Gavrilets & Rice [16] formulated explicit predictions to detect modes of selection maintaining SSB. Their models focus on two genetic hypotheses for SSB—overdominance and sexual antagonism—that have garnered recent attention in the literature, though their conceptual origins date back to the 1950s or earlier [4,16,21]. The Gavrilets & Rice [16] models are formulated in the context of human sexual orientation, but they are applicable to SSB in any diploid dioecious organism. Under overdominance, costly SSB could be maintained in a population if alleles that increase an individual's tendency to exhibit SSB in the homozygous state confer a balancing fitness advantage when expressed in heterozygotes. By contrast, sexual antagonism could maintain costly SSB if alleles increasing its expression in one sex cause a countervailing fitness advantage when expressed in the opposite sex. The hypotheses are not mutually exclusive, and they yield predictions about the inheritance and fitness effects of alleles influencing SSB (table 1).
Table 1.
Predictions for overdominance and sexual antagonism models of SSB (adapted from [16]) evaluated in this study.
| predictionsa |
||
|---|---|---|
| traits | overdominance | sexual antagonism |
| chromosomes | autosomal inheritance | strong X-linkage |
| dominance | dominance effects | no dominance effects |
| fecundity | heterozygote fitness advantage | male SSB correlated with female fitness |
| sex ratio | no sex ratio bias | male SSB correlated with female-biased sex ratio |
aThese and other genetic models for the maintenance of SSB are not necessarily mutually exclusive.
Here, we empirically test predictions outlined by Gavrilets & Rice [16]. We used the Drosophila Genetic Reference Panel (DGRP) [22], which consists of inbred D. melanogaster lines originally derived from the wild. Male–male courtship in D. melanogaster is well documented, it occurs in wild-type flies at low but persistent levels, and SSB phenotyping protocols have been developed and validated [23,24]. SSB in insects is often thought to be caused by poor sex recognition [6,25,26]. In D. melanogaster, flies express sex-specific cuticular hydrocarbons (CHCs), and wild-type flies can detect and differentiate these cues [27]. We designed our study to minimize misidentification that can occur when young adult flies have not yet developed sex-specific CHC profiles, because we were interested in SSB that occurs despite the presence of cues for sexual identity [28].
First, we screened inbred lines to establish the existence of genetic variation for SSB. Second, we identified and validated lines showing consistently high levels of SSB (‘high-SSB’) and lines showing consistently low levels of SSB (‘low-SSB’) for use in crosses. Third, we performed experimental crosses using these high and low lines to test predictions about parental contributions to offspring SSB and levels of dominance. Finally, we estimated female fecundity, an important fitness component, from the crosses to test predictions about the fitness of different genotypic combinations under each model. Our results reveal inheritance patterns and fitness effects that provide mixed support for both models, but in aggregate are most consistent with overdominance. We also uncovered an unexpected paternal effect on the expression of SSB.
2. Material and methods
(a). Origin and maintenance of fly lines
We used 50 inbred lines from the DGRP as focal test flies in SSB assays. The DGRP was derived from a wild population in Raleigh, NC, USA. Lines were subjected to a minimum of 20 generations of full-sib mating and have an estimated inbreeding coefficient of F = 0.986 [22], although this is now likely to be an underestimate owing to their maintenance in laboratory culture for additional generations after 2012. It is likely that rare allelic variants were lost during the production of the inbred lines, limiting the power to detect small-effect loci in association studies [29]. However, this means that any phenotypic differences we found in our screen represent a conservative assessment of genetic variation for male SSB. Establishing which lines show consistent variation in male SSB enabled us to then perform crosses and evaluate modes of inheritance and fitness effects.
We used an additional D. melanogaster strain carrying a yellow-body mutation on a wild-type background, Hmr2, as a consistent genotype against which to test DGRP individuals in paired trials. The yellow-body strain was used so that each fly within a vial could be distinguished and assigned specific behaviours. Hmr2 flies originated from the Bloomington Stock Center (FlyBase ID: FBal0144848) [30]. The yellow-body mutation could conceivably exert pleiotropic effects on behavioural traits [31], although prior work suggests this is not likely to have a strong effect in our trials [24]. Furthermore, we avoided confounding our experimental design by always pairing focal DGRP flies with the Hmr2 strain.
Stock flies were kept in large vials (25 × 95 mm) on cornmeal agar medium seeded with yeast. They were maintained at 18°C on a 12 L : 12 D photoperiod. During experiments, virgin males were collected under light CO2 anaesthesia from stock vials, whereupon they were transferred individually to small vials (16 × 95 mm) and allowed to recover. Experimental flies were kept at 23°C until they were used in assays. We were specifically interested in situations where the sex of interacting partners was unambiguous and readily detectable, so we only used virgin yellow-body males 3–5 days old and virgin DGRP males 6–8 days old in SSB trials.
(b). Initial same-sex sexual behaviour screen and validation
Some of the data below have been reported in a previous study focusing on indirect genetic effects on male tapping behaviour in yellow-body flies [32]. These data are the tapping behaviour of yellow males, and orienting, following, tapping, licking, singing, abdomen curling and general activity of DGRP males, for both the initial screen and validation (Dryad doi:10.5061/dryad.d4s1k). Here, we focus on variation in same-sex courtship elements exhibited by DGRP males while interacting with yellow-body partners in paired trials. We focused on male SSB only. Female sexual behaviour in D. melanogaster is generally assessed in the context of mate rejection, and while females will partly determine the outcome of any male mating attempt, quantifying female courtship is problematic owing to the lack of observable active courtship elements such as can be readily scored in males [33]. We used the behavioural assay described by Bailey et al. [24] to quantify male SSB. All DGRP lines were screened against the common strain to enable comparison among the inbred lines. We quantified three male courtship behaviours that characterize same-sex sexual interactions: licking, singing and abdomen curling (i.e. attempted mounting). We also scored orienting, following and tapping behaviours, but restrict our focus here to licking, singing and abdomen curling. These behaviours are unambiguously expressed during the context of opposite-sex courtship and copulation interactions, whereas orienting, following and tapping are known to function in non-sexual contexts such as aggression [34,35]. Detailed descriptions and links to videos of exemplar behaviours can be found in Bailey et al. [24].
We recorded behaviours exhibited by both the focal DGRP male and his interacting yellow partner using an interval sampling technique [24,32]. One DGRP male and one Hmr2 male were introduced into a small (16 × 95 mm) vial oriented horizontally, and behaviour was observed for 3 min spread over three evenly spaced 1 min observation periods. Five trials were run simultaneously under fluorescent interior lighting and indirect sunlight between 19.4°C and 24.9°C during morning hours. We performed 39 or 40 trials for each DGRP line. Five trials were excluded from analysis after it was discovered they were performed at too low a temperature (17.1–17.2°C); their exclusion did not qualitatively affect the results.
To validate our behavioural assay, we repeated the above procedure on a subset of eight DGRP lines, with the observer blind to line identity. We selected three validation lines that exhibited high levels of SSB in the initial assay, and four that exhibited low levels of SSB. One intermediate line (RAL_897) was selected as it showed an unusual pattern of reaction norm variation in a different experiment (N.W.B. 2015 unpublished data). The selection was made only with respect to the behaviours involved in SSB: licking, singing and abdomen curling. Maintenance, rearing and behavioural observations (n = 40 per line) were performed as before.
We quantified SSB in each trial using a binary assessment of whether licking, singing or abdomen curling occurred. If any of those behaviours were exhibited by a male during the 3 min trial period, then he received an SSB score of 1. We calculated line mean trait values as the proportion of trials in which the DGRP male exhibited SSB. There are advantages and disadvantages to using this system of quantifying behaviour [24]. Estimating the intensity of SSB within different lines (i.e. the number of bouts of SSB during trials) had the potential to create a bias owing to the proportionally heavier weighting of data from lines in which very few males exhibited the behaviour. Following analysis of the validation data, overall SSB line means for the eight retested lines were calculated by combining the original and validation data.
(c). Behaviour diallel
Our validation study indicated that SSB among lines could be consistently classified as ‘high-SSB’ or ‘low-SSB’. We selected two lines that exhibited high levels of SSB (RAL_149 and RAL_75) and two lines that exhibited low levels of SSB (RAL_223 and RAL_38) to perform a complete diallel cross. The identities of these lines remained blind to the observer throughout the diallel experiments. Maintenance and rearing procedures were as described above. The complete diallel included diagonal (intra-line) and off-diagonal (inter-line) crosses, including reciprocals. Each of the 16 crosses was established by housing 10 virgin males and 10 virgin females from the designated parental lines. Virgin male F1 offspring from these crosses were collected and maintained individually in small (16 × 95 mm) vials as before. For each of the 16 crosses, we performed behavioural observations on F1 males (n = 39–50 F1 males per cross), using the same protocol with yellow-body males as a standard strain against which to quantify the expression of SSB.
(d). Fecundity diallel
We estimated a component of female fitness by measuring early fecundity of the F1 diallel offspring. We set up F2 crosses using the diallel F1 offpsring as parents. Virgin male and female full sibs were mated, with 10 replicate full-sib matings set up for each of the 16 cross types. One-day-old virgin parents were kept in small vials (16 × 95 mm) for 2 days to enable mating and oviposition, whereupon they were transferred to a fresh vial for an additional 2 days and then removed. Once eclosion commenced, adults were counted and sexed daily until no new adults were observed to eclose. Total offspring numbers were calculated by pooling the counts across all collections for each cross replicate. Two blocks were run approximately two weeks apart to allow for uncontrolled environmental effects. While our estimate of fitness only captured early-life fecundity, early-life fitness appears to be genetically correlated with later-life fitness in D. melanogaster [36].
(e). Analysis
Statistical analyses focused on (i) the correspondence between original and validation SSB screens, (ii) inter-line variation in SSB, (iii) comparison of SSB levels in F1 offspring from diallel crosses, and (iv) fecundity differences among the diallel crosses. Analyses were performed in Minitab v. 12.21 and SAS v. 9.3.
(i) We tested whether the subset of validated DGRP lines showed the same relative levels of male SSB in a blind validation block as they did in our original screen using a binary logistic regression with a logit link function. There were only two factor levels in ‘block’, preventing accurate covariance estimates if modelled as a random effect, so we modelled it as a fixed effect. The subsequent experiments required us to cross lines that displayed high levels of SSB with lines that displayed low SSB. Because we selected high- and low-SSB lines to validate, we tested whether the three high-SSB and four low-SSB lines yielded consistently high and low estimates of SSB across experimental blocks by including ‘SSB level’ and the ‘block × SSB level’ interaction as fixed effects. To account for variation arising from lines within ‘high-SSB’ and ‘low-SSB’, we nested ‘line’ within ‘SSB level’. Temperature was included as a covariate. As a secondary verification that our measurement of line means for SSB was consistent across blocks, we regressed line mean SSB from the validation block on line mean SSB from the original block for all eight retested lines.
(ii) Finding no evidence for experimental block effects, we assessed variation in SSB across all 50 lines, combining the original and validation data for those eight lines that had been retested. We used a mixed-model binary logistic regression in which ‘line’ was modelled as a random effect and temperature was included as a covariate. A logit link was used and degrees of freedom were estimated using the Satterthwaite method. We also estimated broad-sense heritability by calculating H2 = Vg/Vp. We obtained Vg using variance components from a standard analysis of variance (ANOVA), where Vg = (MSa − MSw)/[(1/a − 1)(ΣNi − Σ(Ni2)/ΣNi)], MSa is mean squares among groups, MSw is means squares within groups, a is the number of groups and Ni is each group size. Vp is the overall phenotypic variance.
(iii) SSB expression was compared among male F1 offspring of our diallel crosses using a binary logistic regression and a logit link. The aim was to estimate the relative contributions of maternal versus paternal genotypes to the expression of SSB in offspring, so the model included ‘maternal line’, ‘paternal line’ and the ‘maternal × paternal’ interaction as fixed factors. Temperature was modelled as a covariate. Parental lines were not modelled as random effects for the same reasons given previously, and also because they had been selected for use in planned contrasts between ‘low-SSB’ and ‘high-SSB’ lines [37].
(iv) Fecundity and offspring sex ratio (daughters/total offspring) of the diallel families was assessed using general linear models (GLMs). Offspring sex ratio data were natural-log-transformed prior to analysis. The key comparison for testing our predictions was among offspring from the four types of inter-line crosses, but we first evaluated the difference between inbred crosses (diagonal of the diallel) and all outbred crosses (off-diagonals). We therefore modelled ‘inbreeding’ as a fixed effect with two factor levels. Experimental block and its interaction with ‘inbreeding’ were modelled as fixed effects. Because the same lines were used in multiple crosses, we included maternal and paternal line identity as fixed effects to assess the impact of ‘inbreeding’ above and beyond any line-specific effects. Including the interaction between maternal and paternal line identity was hindered by the fact that we lacked data from one cross in one of the experimental blocks due to failed matings, so it was not included.
Inbreeding was a major source of variation in fecundity, so we proceeded to examine fecundity of the inter-line crosses only. A post hoc GLM was performed on the same dataset excluding information from the inbred crosses. We tested for variation in fecundity among high–high, high–low, low–high and low–low inter-line crosses, modelled as ‘cross type’, and included the interaction between ‘cross type’ and ‘block’. The same model structures were applied to the natural log-transformed offspring sex ratio data. In both analyses, we excluded data from replicates for which three or fewer offspring collections could be made (n = 13).
3. Results
(a). Behavioural screen and validation
We performed 2320 behavioural trials. The interaction between ‘SSB level’ and ‘block’ in our validation analysis was a key indicator of how consistently we were able to quantify variation in SSB across blocks (figure 1). We found neither a significant ‘block’ effect (binary logistic regression: Wald 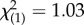 , p = 0.310) nor a significant ‘block × SSB level’ interaction (binary logistic regression: Wald
, p = 0.310) nor a significant ‘block × SSB level’ interaction (binary logistic regression: Wald 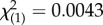 , p = 0.948), which provided confidence that our scoring technique reliably distinguished high-SSB and low-SSB lines across independent experiments. Line effects nested within each SSB level were similarly non-significant (binary logistic regression: Wald
, p = 0.948), which provided confidence that our scoring technique reliably distinguished high-SSB and low-SSB lines across independent experiments. Line effects nested within each SSB level were similarly non-significant (binary logistic regression: Wald 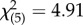 , p = 0.427). As expected, the ‘SSB level’ term in our model indicated that high-SSB and low-SSB lines differed significantly (binary logistic regression: Wald
, p = 0.427). As expected, the ‘SSB level’ term in our model indicated that high-SSB and low-SSB lines differed significantly (binary logistic regression: Wald 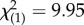 , p = 0.0016). Temperature did not affect the expression of SSB (binary logistic regression: Wald
, p = 0.0016). Temperature did not affect the expression of SSB (binary logistic regression: Wald 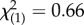 , p = 0.415). We confirmed the overall consistency of SSB measurements in a follow-up regression comparing all eight line means in the original versus validation blocks, which showed variation in SSB among lines to be positively correlated across experiments (linear regression: adjusted r2 = 0.461, F1,6 = 6.98, p = 0.038).
, p = 0.415). We confirmed the overall consistency of SSB measurements in a follow-up regression comparing all eight line means in the original versus validation blocks, which showed variation in SSB among lines to be positively correlated across experiments (linear regression: adjusted r2 = 0.461, F1,6 = 6.98, p = 0.038).
Figure 1.
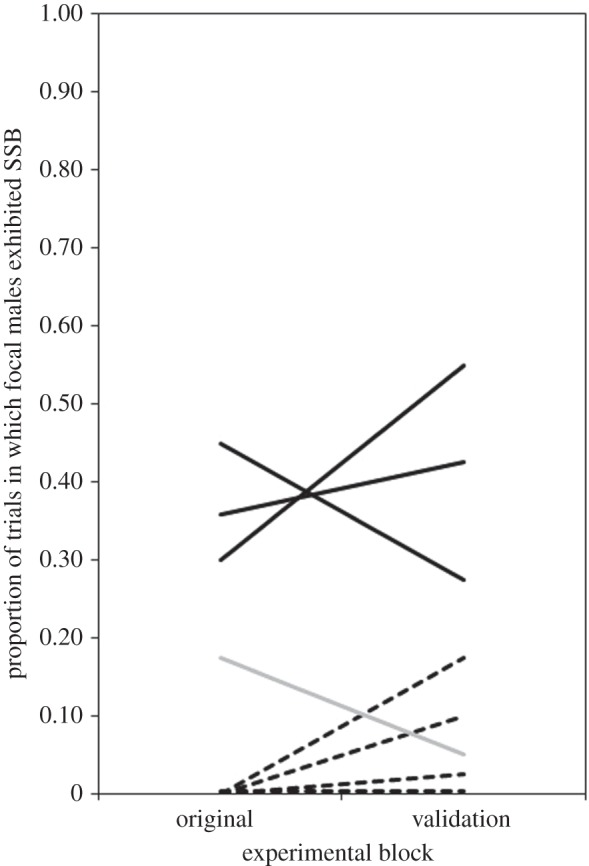
Original SSB screen compared with blind validation screen in eight DGRP lines. Solid black lines indicate DGRP lines that expressed high-SSB in the original screen, whereas dashed black lines indicate lines that expressed low-SSB in the original screen. The intermediate-SSB line is shown in grey. The lowest line has been jittered to aid visualization.
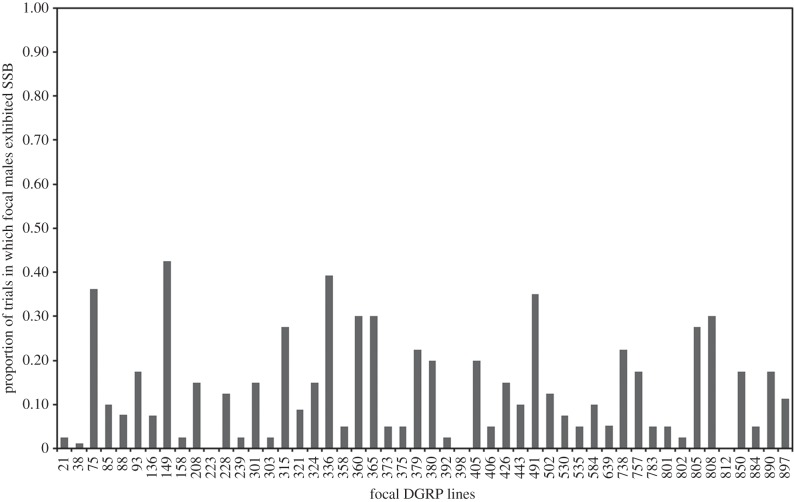
We detected considerable variation in the expression of male SSB across the 50 tested DGRP lines. The proportion of trials in which males displayed SSB ranged from 0.0 to 42.5% (figure 2; mixed-model binary logistic regression: n = 2315, Z = 3.81, p < 0.001). As before, temperature did not affect SSB expression (mixed-model binary logistic regression: F1,2268 = 1.36, p = 0.244). Broad-sense heritability calculated across the lines using a standard ANOVA was H2 = 0.11, but this is probably an underestimate owing to inflated within-group variance relative to among-group variance, caused by the binomial scoring of SSB.
Figure 2.
Variation in the expression of male SSB among focal lines. For lines that were retested in the blind validation procedure, the values indicate the combined incidence of SSB across both blocks. Lines are ordered on the x-axis according to their original numerical identifier.
(b). Behaviour diallel
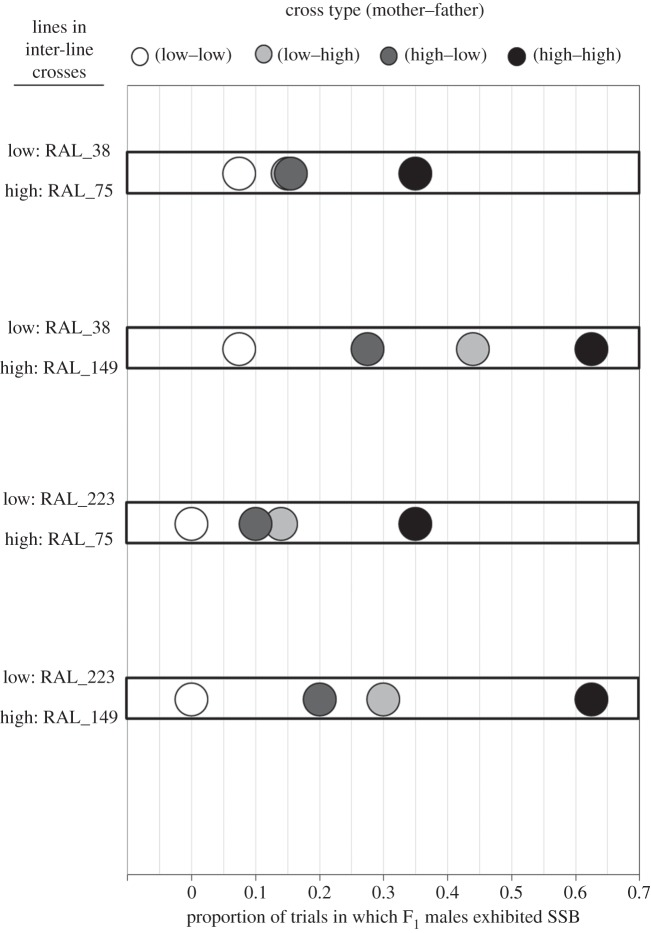
The genotype of fathers, but not mothers, exerted a considerable influence on offspring SSB in diallel crosses: F1 males expressed SSB patterns more similar to their father's line than their mother's line (figure 3). The paternal contribution to offspring SSB expression is evident from a significant ‘paternal line’ effect (binary logistic regression: Wald 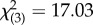 , p = 0.001), while in contrast, ‘maternal line’ did not influence offspring SSB expression (binary logistic regression: Wald
, p = 0.001), while in contrast, ‘maternal line’ did not influence offspring SSB expression (binary logistic regression: Wald 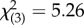 , p = 0.154). Any interaction between maternal and paternal genotypes did not appear to be strong (binary logistic regression: Wald
, p = 0.154). Any interaction between maternal and paternal genotypes did not appear to be strong (binary logistic regression: Wald 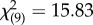 , p = 0.070), and temperature had no effect (binary logistic regression: Wald
, p = 0.070), and temperature had no effect (binary logistic regression: Wald 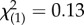 , p = 0.720).
, p = 0.720).
Figure 3.
SSB in male offspring from crosses between low- and high-SSB parents. Paternal influences on the expression of SSB were stronger than maternal influences: offspring show SSB levels that resemble the trait value of their father's line more closely than that of their mother's line. Data from the appropriate within-line crosses (low–low or high–high) are included to allow comparison with maternal and paternal trait values. Note that data from each of the two low–low and two high–high crosses appear twice in the graph. We used two low-SSB and two high-SSB lines in crosses, so there were four possible combinations involving a pair of low and high lines. These are grouped along the horizontal rows, with the lines used indicated to the left. Shading in the circles indicates which parents were low-SSB or high-SSB.
(c). Fecundity diallel
Fecundity of F1 females derived from diallel crosses showed a complex pattern of inheritance (figure 4a). As expected, there was a clear difference between inbred (diagonal) and outbred (inter-line) crosses (table 2a). However, fecundity of inter-line crosses was greater for crosses between lines showing high values of SSB, and F1 crosses between high- and low-SSB lines. Females from crosses involving two low-SSB parents produced on average 25 fewer offspring than those derived from crosses involving either one high-SSB and one low-SSB parent or two high-SSB parents. This key fecundity difference was significant in our post hoc comparison examining only inter-line crosses (table 2b and figure 4a). Overall, fecundity differed across the two experimental blocks, but the non-significant ‘cross type × block’ interaction indicated that differences among cross types occurred in a consistent direction (table 2a). Both maternal and paternal line identities also affected F1 female fecundity, and mothers from high-SSB lines produced more offspring than those from low-SSB lines (figure 4a and table 2a). Although the overdominance model classically predicts that crosses should be most extreme, our results, showing that crosses between different high-SSB lines and high- and low-SSB lines have higher early fecundity, are compatible with directional overdominance maintaining SSB in this population.
Figure 4.
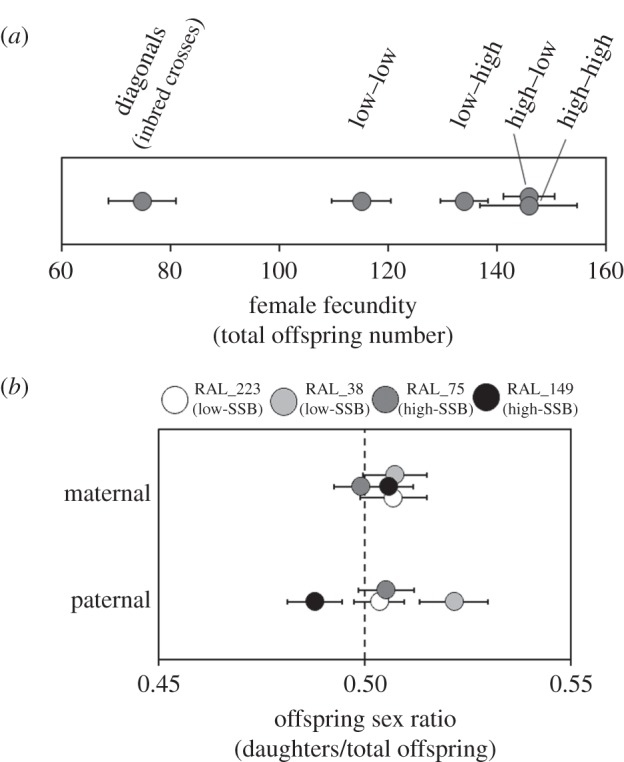
(a) Fecundity of female offspring from diallel crosses. Cross type is indicated above the graph; circles indicate means and error bars indicate 1 s.e. The order of the cross is indicated as mother–father. (b) Maternal and paternal effects on offspring sex ratio. Untransformed sex ratio data are shown, and the dashed line indicates a 1 : 1 offspring sex ratio. Circles indicate means and error bars show 1 s.e. Circle shading corresponds to parental genotypes. In both panels, overlapping data points were jittered to facilitate visualization.
Table 2.
GLM of female fecundity in diallel crosses. (a) Comparison of all crosses, examining differences between diagonal (inbred) crosses and off-diagonal (outbred) crosses. (b) Post hoc analysis examining variation among off-diagonal cross types to assess whether low-SSB × low-SSB crosses show lower fecundity than the rest.
| factor | d.f. | F | p |
|---|---|---|---|
| (a) initial analysis including all crosses | |||
| block | 1 | 23.18 | <0.001 |
| inbreeding | 1 | 96.33 | <0.001 |
| block × inbreeding | 1 | 0.77 | 0.380 |
| maternal genotype | 3 | 11.45 | <0.001 |
| paternal genotype | 3 | 9.52 | <0.001 |
| error | 242 | ||
| (b) post hoc analysis excluding diagonal data | |||
| block | 1 | 75.17 | <0.001 |
| cross type | 3 | 7.36 | <0.001 |
| block × cross type | 3 | 1.86 | 0.138 |
| error | 205 | ||
Offspring sex ratio of mated F1 females was generally unaffected by diallel cross type, although a significant block interaction suggested that patterns of cross-specific variation were inconsistent (figure 4b and table 3a,b). The original maternal lineage did not affect sex ratio, but the original paternal lineage did (figure 4b and table 3a). Despite this paternally induced variation, there was no discernible pattern linking offspring sex ratio to the level of SSB expressed in the paternal line (figure 4b).
Table 3.
GLM of offspring sex ratio in diallel crosses. (a) Comparison of all crosses, examining differences between diagonal (inbred) crosses and off-diagonal (outbred) crosses. (b) Post hoc analysis examining variation among off-diagonal crosses to assess whether offspring sex ratio varied among cross types.
| factor | d.f. | F | p |
|---|---|---|---|
| (a) initial analysis including all crosses | |||
| block | 1 | 2.70 | 0.102 |
| inbreeding | 1 | 0.04 | 0.845 |
| block × inbreeding | 1 | 3.90 | 0.049 |
| maternal genotype | 3 | 0.36 | 0.781 |
| paternal genotype | 3 | 3.61 | 0.014 |
| error | 242 | ||
| (b) post hoc analysis excluding diagonal data | |||
| block | 1 | 0.38 | 0.538 |
| cross type | 3 | 2.27 | 0.081 |
| block × cross type | 3 | 0.56 | 0.644 |
| error | 205 | ||
4. Discussion
We found considerable, and repeatable, variation in male SSB when we screened 50 inbred D. melanogaster lines, which confirms a heritable genetic basis for the trait. Like any other trait that potentially reduces fitness, the evolutionary maintenance of SSB requires a countervailing fitness benefit. Genetic models of SSB [16] have illustrated that such a benefit need not accrue to the individual expressing SSB, but can occur as a result of a fitness advantage specific to the alleles influencing the expression of SSB.
Phenotypic and fitness patterns from diallel crosses among lines with the highest and lowest SSB trait values supported predictions under both genetic models. The diallel results involved only a sample of extreme lines, and assaying a wider range of female fitness components might yield different results. Taken in aggregate, however, our results lend more support to an overdominant fitness advantage occurring when alleles influencing SSB are present in a heterozygous state in crosses between lines, as opposed to an antagonistic advantage that is only revealed when such alleles are expressed in females. The two models are in fact not mutually exclusive and SSB may be maintained by a combination of mechanisms, a possibility that is highlighted by the fact that we did not find exclusive support for a single model of SSB in D. melanogaster. Instead, we found a complex mix of inheritance patterns and fitness effects, plus an unusual pattern of paternal effects on the expression of male SSB.
A sexually antagonistic mode of selection maintaining male SSB predicts that we are more likely to find X-linkage of loci influencing SSB [16]. We did not find this, as there was no detectable maternal effect on the expression of SSB in sons from diallel crosses. This model also predicts that females from high-SSB lines should experience increased fecundity and contribute to a female-biased sex ratio, the latter owing to greater accumulation of male-deleterious mutations on X chromosomes carrying SSB-increasing alleles. The first of these predictions received support, but the latter did not. Fecundity was influenced by both maternal and paternal genotypes; it was higher in crosses where the mother had a high-SSB genotype (figure 4a), which is what the sexual antagonism model predicts (table 1). Offspring sex ratio was influenced by paternal, but not maternal, genotype, but not in a pattern that related to whether fathers were from high-SSB or low-SSB lines.
We also detected evidence consistent with the heterozygote fitness advantage predicted by the overdominance model. When lines that carried alleles for high levels of SSB were crossed, the resulting offspring had higher fecundity than those from low–low crosses. In offspring from the former crosses, heterozygosity at loci affecting SSB is expected because the parents were derived from different high-SSB lines. Moreover, these effects were not driven purely by heterosis, as we set up high–high and low–low crosses with different high-SSB and low-SSB lines, respectively. Fecundity of offspring from low–low crosses was more than 15% lower than the other crosses. However, sex-specific patterns of dominance in our data might also be consistent with a model in which loci under sexually antagonistic selection are X-linked [38]. Gavrilets & Rice [16] noted that under such a scenario, SSB is expected to be recessive in the sex in which it reduces fitness, and dominant in the sex in which it increases fitness. Consistent with this, male SSB appeared to show recessivity in our crosses (figure 3), whereas loci causing high male SSB had a dominant effect on female fitness in the fecundity assay derived from those crosses (figure 4a).
The paternal effects uncovered in our analyses of male SSB and offspring sex ratio were unexpected, and suggest a promising area for future research. Our screen of all 50 inbred lines revealed modest but significant broad-sense heritability. Crosses between a subset of extreme lines confirmed this genetic variation for SSB by revealing a clear parent-of-origin effect on offspring SSB levels, but surprisingly the paternal genotype exerted a strong influence on the expression of male SSB and on offspring sex ratio, while the maternal genotype did not. Relatively few loci have been identified on the heterochromatic Y chromosome of D. melanogaster, but those that have been studied appear to be strongly implicated in male fitness [39]. In an analysis of polymorphic Y chromosomes crossed into a common wild-type D. melanogaster background, Chippindale & Rice [40] found substantial epistatic fitness effects of variation on the Y. Intriguingly, such male fitness effects may arise from variation in sperm competition and mating behaviour. Several genes with putative spermatogenesis functions have been characterized on the Y [41], and Y-linked effects on male mating behaviours such as courtship song have been documented in Drosophila virilis [42]. Evidence for strong epistatic effects of Y-linked variation on patterns of autosomal gene expression [43] suggests a mechanism whereby Y-linked variation influences SSB: if balancing selection maintains polymorphism on the Y because of fitness benefits in some genetic backgrounds but not others, detrimental epistatic fitness effects mediated by the Y chromosome could manifest as high levels of male SSB. For example, epigenetic modifications disrupting sexually dimorphic gene expression have been suggested as a plausible mechanism underlying the development of SSB [17,44]. If genetic variation on the Y is associated with male SSB, it might be productive to test which autosomal genes interact with such Y-linked variation, and whether they are susceptible to epigenetic modification.
Until further empirical work is performed, the diversity of genetic mechanisms maintaining SSB will remain unknown. Such studies would benefit not only from focusing on different systems, but also from expanding the scope of quantitative genetic experiments to capture a broader range of genetic variation via inbred lines or pedigree-based animal model approaches. Our study focused on male SSB because active courtship behaviour in D. melanogaster is sex-limited, although it would be useful to perform similar genetic analyses in species amenable to studying female SSB. Such work could clarify whether male and female SSB are maintained by similar selective pressures or whether intersexual correlations arise due to incomplete sexual differentiation of sexual behaviours [14,45]. Apart from demonstrating a genetic basis for the trait in Coleopteran beetles [12–14], additional information about the evolutionary genetics of SSB is derived almost exclusively from studies of human homosexuality (in particular, male homosexuality) [46–51]. Despite these comparatively more extensive research efforts, SSB and sexual orientation are obviously not homologous traits [3], and drawing direct parallels between such taxonomically distinct species as human beings and fruitflies is unlikely to be of much value [15]. Nevertheless, with increased research attention in other organisms, it may eventually become feasible to study the genetics of SSB using a comparative approach, which would enable researchers to test the generality of evolutionary hypotheses for its maintenance.
It is debatable whether SSB represents a unified phenomenon across taxa or whether its functions and evolutionary origins are too multifarious to be studied except in the context of a single species or taxonomic group. Some broad themes are beginning to emerge, with reviews of arthropods [16] and work on other invertebrates such as the deep sea squid Octopoteuthis deletron [52] suggesting indiscriminate mate choice may underlie SSB when mating opportunities are limited. In addition, studies in avian taxa have used the comparative method to examine life-history correlates of female–female pair bonding and test phylogenetic signals underlying the expression of SSB [53], and primatologists have studied SSB from a perspective more focused on its role in social transactions in highly social species [2]. These studies suggest different sources of selection maintain this apparently non-adaptive trait with different indirect fitness benefits depending on a variety of ecological and life-history factors. To critically evaluate evolutionary hypotheses about the origins and maintenance of SSB, more genetic research is clearly required across a broader range of organisms.
Acknowledgements
We are grateful to Tanya Sneddon, Maureen Cunningham, David Forbes, Harry Hodge and Keith Haynes for technical assistance.
Data accessibility
Behavioural data unique to this study, plus fecundity data, are archived at Dryad (http://dx.doi.org/10.5061/dryad.t0c3s). Additional DGRP phenotype data are archived at http://dgrp.gnets.ncsu.edu/.
Authors' contributions
N.W.B. and M.G.R. designed experiments. J.L.H. executed experiments and collected data. N.W.B. and M.G.R. performed statistical analyses. N.W.B., J.L.H. and M.G.R. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Natural Environment Research Council (NERC) fellowships to N.W.B. (NE/G014906/1 and NE/L011255/1) and a NERC grant to N.W.B. and M.G.R. (NE/I016937/1).
References
- 1.Bagemihl B. 1999. Biological exuberance. New York, NY: St. Martin's Press. [Google Scholar]
- 2.Sommer V, Vasey PL. 2006. Homosexual behaviour in animals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Bailey NW, Zuk M. 2009. Same-sex sexual behaviour and evolution. Trends Ecol. Evol. 24, 439–446. ( 10.1016/j.tree.2009.03.014) [DOI] [PubMed] [Google Scholar]
- 4.Poiani A. 2010. Animal homosexuality. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Caballero-Mendieta N, Cordero C. 2012. Enigmatic liaisons in Lepidoptera: a review of same-sex courtship and copulation in butterflies and moths. J. Insect. Sci. 12, 1–11. ( 10.1673/031.012.13801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharf I, Martin OY. 2013. Same-sex sexual behavior in insects and arachnids: prevalence, causes, and consequences. Behav. Ecol. Sociobiol. 67, 1719–1730. ( 10.1007/s00265-013-1610-x) [DOI] [Google Scholar]
- 7.Santtila P, et al. 2009. Testing Miller's theory of alleles preventing androgenisation as an evolutionary explanation for the genetic predisposition for male homosexuality. Evol. Hum. Behav. 30, 58–65. ( 10.1016/j.evolhumbehav.2008.08.004) [DOI] [Google Scholar]
- 8.Camperio Ciani A, Pellizzari E. 2012. Fecundity of paternal and maternal non-parental female relatives of homosexual and heterosexual men. PLoS ONE 7, e51088 ( 10.1371/journal.pone.0051088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanderLaan DP, Forrester DL, Petterson LJ, Vasey PL. 2012. Offpsring production among the extended relatives of Samoan men and fa'afafine . PLoS ONE 7, e36088 ( 10.1371/journal.pone.0036088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leca J-B, Gunst N, Vasey PL. 2014. Male homosexual behavior in a free-ranging all-male group of Japanese macaques at Minoo, Japan. Arch. Sex. Behav. 43, 853–861. ( 10.1007/s10508-014-0310-6) [DOI] [PubMed] [Google Scholar]
- 11.Bailey NW. 2013. Evolution of apparently non-adaptive behavior. In The Princeton guide to evolution (ed. Losos JB.), pp. 710–717. Princeton, NJ: Princeton University Press. [Google Scholar]
- 12.Serrano JM, Castro L, Toro MA, López-Fanjul C. 1991. The genetic properties of homosexual copulation behavior in Tribolium castaneum: diallel analysis. Behav. Genet. 21, 547–558. ( 10.1007/BF01066681) [DOI] [PubMed] [Google Scholar]
- 13.Castro L, Toro MA, López-Fanjul C. 1994. The genetic properties of homosexual copulation behaviour in Tribolium castaneum: artificial selection. Genet. Sel. Evol. 26, 361–367. ( 10.1186/1297-9686-26-4-361) [DOI] [PubMed] [Google Scholar]
- 14.Burgevin L, Friberg U, Maklakov AA. 2013. Intersexual correlation for same-sex sexual behaviour in an insect. Anim. Behav. 85, 759–762. ( 10.1016/j.anbehav.2013.01.017) [DOI] [Google Scholar]
- 15.Levine JD. 2008. Glia and romance. Nature 11, 8–10. [DOI] [PubMed] [Google Scholar]
- 16.Gavrilets S, Rice WR. 2006. Genetic models of homosexuality: generating testable predictions. Proc. R. Soc. B 273, 3031–3038. ( 10.1098/rspb.2006.3684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice WR, Friberg U, Gavrilets S. 2013. Homosexuality as a consequence of epigenetically canalized sexual development. Q. Rev. Biol. 87, 343–368. ( 10.1086/668167) [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre F, Estep KW. 1993. Sperm competition and the persistence of genes for male homosexuality. BioSystems 31, 223–233. ( 10.1016/0303-2647(93)90051-D) [DOI] [PubMed] [Google Scholar]
- 19.Getz WM. 1993. Invasion and maintenance of alleles that influence mating and parental success. J. Theor. Biol. 162, 515–537. ( 10.1006/jtbi.1993.1102) [DOI] [PubMed] [Google Scholar]
- 20.Camperio Ciani A, Cermelli P, Zanzotto G. 2008. Sexually antagonistic selection in human male homosexuality. PLoS ONE 3, e2282 ( 10.1371/journal.pone.0002282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson GE. 1959. A speculative consideration of certain forms of sexual selection in man. Am. Nat. 93, 81–91. ( 10.1086/282059) [DOI] [Google Scholar]
- 22.Mackay TFC, et al. 2012. The Drosophila melanogaster genetic reference panel. Nature 482, 173–178. ( 10.1038/nature10811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukas R. 2010. Causes and consequences of male–male courtship in fruit flies. Anim. Behav. 80, 913–919. ( 10.1016/j.anbehav.2010.08.017) [DOI] [Google Scholar]
- 24.Bailey NW, Hoskins JL, Green J, Ritchie MG. 2013. Measuring same-sex sexual behaviour: the influence of the male social environment. Anim. Behav. 86, 91–100. ( 10.1016/j.anbehav.2013.04.016) [DOI] [Google Scholar]
- 25.Harari AR, Brockmann HJ, Landolt PJ. 2000. Intrasexual mounting in the beetle Diaprepes abbreviates (L.). Proc. R. Soc. Lond. B 267, 2071–2079. ( 10.1098/rspb.2000.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey NW, French N. 2012. Same-sex sexual behaviour and mistaken identity in male field crickets (Teleogryllus oceanicus). Anim. Behav. 84, 1031–1038. ( 10.1016/j.anbehav.2012.08.001) [DOI] [Google Scholar]
- 27.Billeter J-C, Atallah J, Krupp JJ, Millar JG, Levine JD. 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–992. ( 10.1038/nature08495) [DOI] [PubMed] [Google Scholar]
- 28.Curcillo PG, Tompkins L. 1987. The ontogeny of sex appeal in Drosophila melanogaster males. Behav. Genet. 17, 81–86. ( 10.1007/BF01066012) [DOI] [PubMed] [Google Scholar]
- 29.Long AD, Macdonald SJ, King EG. 2014. Dissecting complex traits using the Drosophila synthetic population resource. Trends Ecol. Evol. 30, 488–495. ( 10.1016/j.tig.2014.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutter P, Ashburner M. 1987. Genetic rescue of inviable hybrids between Drosophila melanogaster and its sibling species. Nature 327, 331–333. ( 10.1038/327331a0) [DOI] [PubMed] [Google Scholar]
- 31.Bastock M. 1956. A gene mutation which changes a behavior pattern. Evolution 10, 421–439. ( 10.2307/2407002) [DOI] [Google Scholar]
- 32.Bailey NW, Hoskins JL. 2014. Detecting cryptic indirect genetic effects. Evolution 68, 1871–1882. ( 10.1111/evo.12401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferveur J-F. 2010. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr. Opin. Neurobiol. 20, 764–769. ( 10.1016/j.conb.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 34.Spieth HT. 1974. Courtship behavior in Drosophila. Annu. Rev. Entomol. 19, 385–405. ( 10.1146/annurev.en.19.010174.002125) [DOI] [PubMed] [Google Scholar]
- 35.Cobb M, Connolly K, Burnet B. 1985. Courtship behaviour in the melanogaster species sub-group of Drosophila. Behaviour 95, 203–231. ( 10.1163/156853985X00136) [DOI] [Google Scholar]
- 36.Kimber CM, Chippindale AK. 2013. Mutation, condition, and the maintenance of extended lifespan in Drosophila . Curr. Biol. 23, 2283–2287. ( 10.1016/j.cub.2013.09.049) [DOI] [PubMed] [Google Scholar]
- 37.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 38.Fry JD. 2010. The genomic location of sexually antagonistic variation: some cautionary comments. Evolution 65, 1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho AB. 2002. Origin and evolution of the Drosophila Y chromosome. Curr. Biol. 12, 664–668. [DOI] [PubMed] [Google Scholar]
- 40.Chippindale AK, Rice WR. 2001. Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 5677–5682. ( 10.1073/pnas.101456898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charlesworth B. 2001. Genome analysis: more Drosophila Y chromosome genes. Curr. Biol. 11, R182–R184. ( 10.1016/S0960-9822(01)00089-6) [DOI] [PubMed] [Google Scholar]
- 42.Huttunen S, Aspi J. 2003. Complex inheritance of male courtship song characters in Drosophila virilis . Behav. Genet. 33, 17–24. ( 10.1023/A:1021095331850) [DOI] [PubMed] [Google Scholar]
- 43.Jiang P-P, Hartl DL, Lemos B. 2010. Y not a dead end: epistatic interactions between Y-linked regulatory polymorphisms and genetic background affect global gene expression in Drosophila melanogaster . Genetics 186, 109–118. ( 10.1534/genetics.110.118109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice WR, Friberg U, Gavrilets S. 2013. Homosexuality via canalized sexual development: a testing protocol for a new epigenetic model. BioEssays 35, 764–770. ( 10.1002/bies.201300033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gosden TP, Rundle HD, Chenoweth SF. 2014. Testing the correlated response hypothesis for the evolution and maintenance of male mating preferences in Drosophila serrata . J. Evol. Biol. 27, 2106–2112. ( 10.1111/jeb.12461) [DOI] [PubMed] [Google Scholar]
- 46.Camperio Ciani A, Corna F, Capilupi C. 2004. Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proc. R. Soc. Lond. B 271, 2217–2221. ( 10.1098/rspb.2004.2872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camperio Ciani A, Fontanesi L, Iemmola F, Giannella E, Ferron C, Lombardi L. 2012. Factors associated with higher fecundity in female maternal relatives of homosexual men. J. Sex. Med. 9, 2878–2887. ( 10.1111/j.1743-6109.2012.02785.x) [DOI] [PubMed] [Google Scholar]
- 48.DuPree MG, Mustanski BS, Bocklandt S, Nievergelt C, Hamer DH. 2004. A candidate gene study of CYP19 (aromatase) and male sexual orientation. Behav. Genet. 34, 243–250. ( 10.1023/B:BEGE.0000017870.77610.52) [DOI] [PubMed] [Google Scholar]
- 49.Mustanski BS, DuPree MG, Nievergelt CM, Bocklandt S, Schork NJ, Hamer DH. 2005. A genome wide scan of male sexual orientation. Hum. Genet. 116, 272–278. ( 10.1007/s00439-004-1241-4) [DOI] [PubMed] [Google Scholar]
- 50.Långström N, Rahman Q, Carlström E, Lichtenstein P. 2010. Genetic and environmental effects on same-sex sexual behavior: a population study of twins in Sweden. Behav. Genet. 39, 75–80. [DOI] [PubMed] [Google Scholar]
- 51.Bogaert AF. 2006. Biological versus nonbiological older brothers and men's sexual orientation. Proc. Natl Acad. Sci. USA 103, 10 771–10 774. ( 10.1073/pnas.0511152103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoving HJT, Bush SL, Robison BH. 2011. A shot in the dark: same-sex sexual behaviour in a deep-sea squid. Biol. Lett. 8, 287–290. ( 10.1098/rsbl.2011.0680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacFarlane GR, Blomberg SP, Kaplan G, Rogers LJ. 2007. Same-sex sexual behavior in birds: expression is related to social mating system and state of development at hatching. Behav. Ecol. 18, 21–33. ( 10.1093/beheco/arl065) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Behavioural data unique to this study, plus fecundity data, are archived at Dryad (http://dx.doi.org/10.5061/dryad.t0c3s). Additional DGRP phenotype data are archived at http://dgrp.gnets.ncsu.edu/.