Abstract
Studies on small and declining populations dominate research in conservation biology. This emphasis reflects two overarching frameworks: the small-population paradigm focuses on correlates of increased extinction probability; the declining-population paradigm directs attention to the causes and consequences of depletion. Neither, however, particularly informs research on the determinants, rate or uncertainty of population increase. By contrast, Allee effects (positive associations between population size and realized per capita population growth rate, rrealized, a metric of average individual fitness) offer a theoretical and empirical basis for identifying numerical and temporal thresholds at which recovery is unlikely or uncertain. Following a critique of studies on Allee effects, I quantify population-size minima and subsequent trajectories of marine fishes that have and have not recovered following threat mitigation. The data suggest that threat amelioration, albeit necessary, can be insufficient to effect recovery for populations depleted to less than 10% of maximum abundance (Nmax), especially when they remain depleted for lengthy periods of time. Comparing terrestrial and aquatic vertebrates, life-history analyses suggest that population-size thresholds for impaired recovery are likely to be comparatively low for marine fishes but high for marine mammals. Articulation of a ‘recovering population paradigm’ would seem warranted. It might stimulate concerted efforts to identify generic impaired recovery thresholds across species. It might also serve to reduce the confusion of terminology, and the conflation of causes and consequences with patterns currently evident in the literature on Allee effects, thus strengthening communication among researchers and enhancing the practical utility of recovery-oriented research to conservation practitioners and resource managers.
Keywords: Allee effects, depensation, rebuilding, population growth rate, marine fishes
1. Introduction
Depleted populations follow one of three trajectories: they decline further, they stabilize or they increase. Two themes central to conservation biology focus in particular on the first of these. The small-population paradigm encompasses the dynamics of populations that are at sufficiently low numbers or densities that their probability of extinction is substantially increased owing to demographic, environmental or genetic stochasticity [1–3]. The declining-population paradigm addresses ways of detecting decline, diagnosing its causes and evaluating its consequences [3].
Despite being the dominant paradigms of conservation biology over the past half-century, neither says a very great deal from a broad theoretical or empirical perspective about a key element of conservation biology: recovery. In large part, this was the result of a proliferation of interest and funding for research on the determinants of increased extinction probability, the opposite of recovery. The paucity of studies on general correlates and targets of recovery can also be attributable to the case-study approach that typifies much of the literature. Two decades ago, the paradigms were criticized for comprising ‘mainly case-by-case ecological investigations and recovery operations, often short on scientific rigour’ for which the ‘taxonomy of the species of concern is tacitly considered more important than the process under investigation’ [3]. Research on recovery remains dominated by species-by-species and population-by-population studies that, albeit valuable, have contributed little to a comprehensive understanding of the determinants, rate and uncertainty of recovery [4,5]. (Hereafter, the words ‘species’ and ‘population’ are used interchangeably.)
Often on the fringes of these paradigms, occasionally taking on a more central role, are Allee effects. These are part of a strongly theoretical, sparingly empirical framework that encompasses the dynamics of small, declining and recovering populations. An Allee effect describes a positive relationship between population abundance (or density) and realized per capita population growth rate, rrealized, the latter also representing the average fitness of individuals in a population [6,7]. Also termed a demographic Allee effect, the concept stems from Warder Allee's classical laboratory work in the 1930s on how ‘certain aspects of survival values’ can increase with density when populations are at low abundance [8].
An Allee effect is manifest by a reduction in rrealized with reductions in population size, N (figure 1). This pattern of ‘positive density dependence’ distinguishes an Allee effect from ‘negative density dependence’; under the latter's compensatory dynamics, as populations decline, the lower the effects of density-dependent intraspecific competition and the higher rrealized. In the presence of compensation, per capita population growth rate attains its maximum (rmax) when abundance is lowest. This pattern of change in rrealized with abundance, representing a fundamental assumption of classic models of population growth [9], underpins most models of the dynamics of harvested species, especially fishes [10–12]. (Allee effects are termed ‘depensation’ in the fisheries literature, presumably to distinguish it from compensation; the word has no other meaning.)
Figure 1.
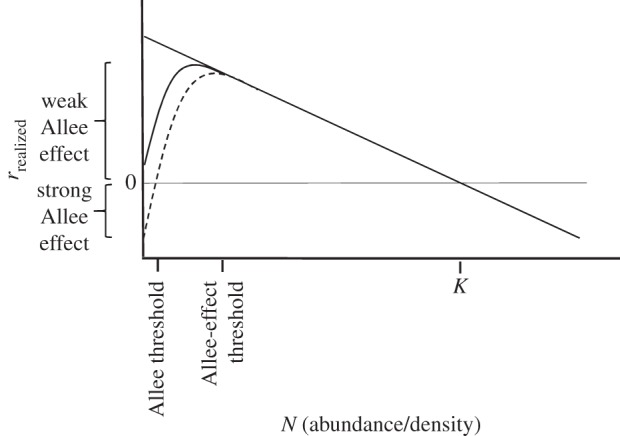
Patterns of association between realized per capita population growth rate (rrealized) and population size (N). The solid straight line represents negative density dependence or compensation. The curvilinear functions reflect the presence of strong (dashed line) and weak (solid line) Allee effects (positive density dependence or depensation). The population size associated with maximum per capita population growth rate (rmax) differs in the presence and absence of Allee effects.
Links between Allee effects and the small- and declining-population paradigms are apparent. A ‘strong’ Allee effect (figure 1), manifested when rrealized falls below zero at low levels of abundance (culminating in extinction), is the focus of a very considerable amount of research in the ecological, conservation-oriented and biomathematical literature [6,13–15]. Allee effects are described as ‘weak’ if rrealized remains positive.
The conceptual and empirical literature on Allee effects provides a potentially instructive point of departure for the articulation of thresholds below which recovery is impaired, absent or impossible. My purpose here is threefold: (i) review the literature on Allee effects as it pertains to population thresholds; (ii) compare population-size minima between species that have and have not recovered following mitigation of the primary threat responsible for their initial decline; and (iii) suggest how generalized thresholds for impaired recovery might be estimated within and among species. For clarification, the descriptor ‘impaired’ is used to mean slower recovery than that generated by stochasticity under the assumption of linear negative density dependence (cf. the solid linear function in figure 1).
The intent is to identify impaired-recovery thresholds of general application, an objective divergent from the species- and population-specificity that permeates much of the current literature on abundance thresholds. A related question is whether there are temporal thresholds that similarly impede recovery. One might reasonably predict, for example, that the longer a depleted population remains small, the longer and more uncertain its recovery, if for no other reason than that the longer the depletion period the more likely it is that the ecosystem or environment will change in ways that are unfavourable to the depleted species.
Another point of clarification pertains to the terms ‘recovery’ and ‘recovering’. Both are often used exceedingly loosely and imprecisely to refer to any depleted population or species exhibiting a positive abundance trajectory, irrespective of its level relative to a target. Notwithstanding the multiple means by which it can be defined [16], the word ‘recovery’ is used here to indicate a reversal of decline and achievement of a predefined target of some metric of abundance.
2. Allee effects: confusion, conflation, complication
The literature on Allee effects, albeit rich and diverse, is not always germane or helpful from a management or conservation perspective. The reasons for this are varied.
Inconsistent and often incorrect definitions create confusion. Some authors unhelpfully conflate cause with pattern by defining Allee effects to be one of its postulated underlying mechanisms, such as ‘the difficulty in finding mates’ [17]. Others conflate consequence with pattern by unduly associating Allee effects with extinction, failing to distinguish strong from weak Allee effects. The literature variously and inappropriately defines Allee effects as ‘extinction thresholds’ [18], ‘a minimum size below which deterministic extinction should follow’ [19], making ‘small populations particularly vulnerable to extinction’ [20], and as being present ‘if there is some population size … below which the population fails to replace itself’ [21].
A second issue concerns the quality of evidence for and against Allee effects. Some claims for the existence of Allee effects are over-stated or simply incorrect. Take, for example, one assertion [22] that ‘there is widespread evidence for the Allee effect in mammals (e.g. [23]), birds (e.g. [24]) and fish (e.g. [25])’. Based on the citations provided, the ‘widespread evidence’ for mammals is based only on reintroduction data for nine closely related species (representing a single order) of large terrestrial mammals [23]. The cited evidence for birds is an analysis of the establishment probability of non-native species [24], a study with questionable applicability to small or declining natural populations. Regarding the purported evidence for fishes, rather than reporting that Allee effects are evident, the researchers concluded ‘that there is a significant amount of uncertainty about whether depensation exists and to what degree’ [25].
Focusing additional scrutiny on the literature for marine fishes, general reviews have documented little empirical evidence of the underlying causes of Allee effects [12,26,27]. Although the impression left by one review [28] is that Allee effects are widespread, the supportive studies are not cited, rendering the basis for the assertion unclear at best. A recent book [6] identifies difficulty in mate finding as a cause for an Allee effect on reproduction in Atlantic cod (Gadus morhua); having co-authored the study in question [29], I can attest to the fact that the work provided highly circumstantial evidence only.
I suspect that if one delved deeper, the evidence for Allee effects might not be as well-substantiated as the literature would otherwise lead one to believe. There is certainly compelling evidence in some species [6,30], but the evidence for others is much less so. This does not mean that Allee effects do not exist or are unimportant, but it might well speak to the difficulties that exist in detecting them in native wild populations [6].
It is also legitimate to question whether the complexity of the literature on Allee effects has unhelpfully superseded its practical utility. At least 14 types of Allee effect have been identified [30]. From a theoretical perspective, such complexity can be highly advantageous, as the rich biomathematical literature on Allee effects will attest. But, from a practical perspective, it is not clear that such complexity is warranted given the arguably limited empirical evidence for the existence of Allee effects. As noted by the most recent authoritative review [6], ‘It is hard to confirm or refute definitively the hypothesis that a species has Allee effects; in many cases it is probably not worth trying.’
Among these definitions is one affiliated to several studies of putative Allee effects that might or might not be indicative of a positive association between population size and rrealized or fitness. This is the component Allee effect (as opposed to the demographic Allee effect), defined as ‘a positive relation between any component of individual fitness and either numbers or density of conspecifics [italics added]’ [7]. The term ‘component Allee effect’ has practical merit for researchers studying the dynamics of small populations; there is the logical attraction of presuming that a fitness component reflects fitness itself, and metrics of fitness are considerably easier to quantify.
But the degree to which a component Allee effect is likely to be indicative of a positive association between fitness and population size is problematic. Firstly, it requires acceptance of the assumption that an increase in one component of fitness automatically leads to an increase in overall fitness. This assumption is unlikely to be generally true because of the trade-offs ubiquitous among fitness-related traits [9]. Offspring production, for example, is not related to rmax in most vertebrates [31]. A recent review on insects concluded that ‘no study has yet clearly demonstrated a causal relationship between mating failure [at low population sizes] and lower rates of [per capita] population growth’ [32]. Secondly, by focusing attention on only one aspect of fitness (such as offspring production) and finding no association with population size, one can fail to detect Allee effects generated by other components of fitness (such as adult survival). Allee effects can be present even if offspring per individual increases as population size declines [33].
The confusion and conflation of Allee effects in the literature complicates efforts to communicate the science underlying Allee effects among scientists, managers and decision-makers. This has almost certainly contributed to the near-absence of their explicit practical application. The International Union for Conservation of Nature (IUCN) mentions Allee effects sparsely in its guidelines for using the Red List Categories and Criteria to assess species conservation status [34]. Unfortunately, it does so in a way that sows some of the confusion described above. When discussing the reversibility of population declines for Criterion A (the declining-population criterion), the guidelines note that ‘the population size must not be so low that factors such as Allee effects make it impossible or unlikely to recover’. The emphasis here is on strong rather than weak Allee effects; the same is true of the reference to Allee effects in relation to setting extinction thresholds for quantitative analyses encompassed by the IUCN's Criterion E [34]. Guidelines for assessing species status in Canada by COSEWIC (Committee on the Status of Endangered Wildlife in Canada) are more circumspect [35], stipulating that status assessments will incorporate ‘the likelihood that the wildlife species is vulnerable to the Allee effects of density dependence’. It is unclear, however, how often Allee effects are explicitly accounted for in COSEWIC's listing advice to government.
3. The nature of Allee-related thresholds
The concept of ‘threshold’ is central to Allee effects. If the pattern between rrealized and N changes from a negative to a positive relationship as populations decline, an obvious and fundamental question emerges: at what level of abundance are Allee effects likely to be manifest in natural populations? Despite the logical necessity in posing this question, surprisingly little work has been devoted to answering it. This might reflect an influence of the prevailing conservation-biology paradigms and their emphasis on extinction rather than recovery, resulting in greater study being directed to strong rather than weak Allee effects ([13,30,36]; ISI Web of Science searches).
The concentration on strong Allee effects in the literature has carried over into the realm of specifying population thresholds. An ‘Allee threshold’ is defined as the population size or density below which rrealized becomes negative [6]. This conflation of terminology sows additional confusion. The Allee threshold is describing a population-extinction threshold that is caused by, rather than being characteristic of, an Allee effect. Logically, given that an Allee effect simply reflects a decline in rrealized with declining N, one might reasonably expect an Allee threshold to identify the population size at which this takes place. But it does not.
Although highly relevant to species reintroduction studies [23] and assessments of species-invasion probabilities [37], Allee thresholds might not be similarly advantageous to studies of recovery. Knowing the Allee threshold is akin to having identified a point-of-no-return. Managing species to maintain their abundance above that level might prevent extinction, but it might have far less likelihood of leading to the ‘successful conservation’ of a species (sensu [16]).
There would seem to be a need then for an ‘Allee-effect threshold’, defined as the population size or density below which rrealized begins to decline relative to the negatively density-dependent pattern exhibited at larger population sizes (cf. figure 1). Allee-effect thresholds are likely to be of greater practical utility than Allee thresholds. For those wishing to take meaningful action to slow or halt population decline, identification of the threshold at which recovery probability is likely to be negatively affected or impaired (rather than a threshold at which recovery probability is negligible) would be useful.
It would also be useful if such thresholds were defined in such a way as to be broadly applicable across species. In general, they are not. Most studies focus on absolute numbers of individuals (e.g. moths per trap, dogs per pack, mice per hectare) rather than population size relative to some standard, such as carrying capacity (K) or maximum observed abundance (Nmax) (table 1). This has contributed to the specification of absolute Allee-related thresholds relevant only to the population or species under study, a practice consistent with the small-population paradigm's focus on absolute numbers of individuals, as opposed to the declining-population paradigm for which population size is of no great relevance [3]. The case-study approach can be helpful from a narrow taxonomic or spatial perspective, but such thresholds have limited utility in a broad general context. And, as argued elsewhere for the establishment of recovery targets [4,5], species-independent thresholds (i.e. those that can be applied across different species) are perhaps better specified in relative rather than absolute terms.
Table 1.
Empirical studies of population thresholds related to Allee effects. Thresholds are expressed as absolute numbers (N) or relative to maximum observed abundance (Nmax).
| species | threshold | units | reference |
|---|---|---|---|
| gypsy moth (Lymantria dispar) | Allee | absolute (N trap−1) | [13] |
| African wild dogs (Lycaon pictus) | Allee | absolute (N pack−1) | [38] |
| greenlip abalone (Haliotis laevigata) | Allee | absolute (N m−2) | [39] |
| polar bear (Ursus maritimus) | Allee | absolute (N 1000 km−2) | [14] |
| flour beetle (Tribolium confusum) | Allee-effect | absolute (N mg flour−1) | [8] |
| deer mouse (Peromyscus maniculatus) | Allee-effect | absolute (N ha−1) | [40] |
| red-backed vole (Clethrionomys gapperi) | Allee-effect | absolute (N ha−1) | [40] |
| mammals (9 Artiodactylans) | Allee-effect | absolute (N = 20) | [23] |
| Atlantic cod (Gadus morhua) (multiple populations) | Allee-effect | relative (10%Nmax) | [10] |
| pink salmon (Oncorhynchus gorbuscha) (Sashin Creek, Alaska) | Allee-effect | relative (12%Nmax) | [17] |
| Atlantic herring (Clupea harengus) (Icelandic spring-spawning population) | Allee-effect | relative (10%Nmax) | [17] |
4. Population thresholds for impaired recovery
There has been little empirical work on Allee-related thresholds, a conclusion drawn from a collation of studies (table 1) that encompasses the examples proffered by recent reviews [6,27,30] and related searches on the ISI's Web of Science (J. A. Hutchings 2015, personal observation). The paucity of work might well reflect inherent difficulties in detecting Allee effects in wild populations [6,12,25,41]. These include insufficient (and often highly uncertain) data at the low population sizes at which Allee effects are likely to be manifest, the complication that population decline is often associated with habitat alteration and destruction (thus making it difficult to disentangle the effects of declining abundance on rrealized from habitat change and other factors), and low statistical power.
To overcome some of these limitations, a recent meta-analysis was undertaken on 207 marine fish populations (median number of years per time series was 32) that encompassed periods during which many had remained at low abundance (less than 10%Nmax), increasing the data resolution at which Allee effects might be evident [10]. Based on the results of a Bayesian hierarchical model (an approach that provides modelling flexibility with no strong assumptions about the relationship between metrics of abundance and rrealized), it was reported that: (i) some species (25 of 104; 24%) exhibit strong compensatory population dynamics (rrealized increasing at low N, i.e. less than 10%Nmax); (ii) many exhibit weak (38%) or no compensation (34%) at low population size (rrealized changing little or not at all at low N); and (iii) some (4%) exhibit patterns consistent with the presence of an Allee effect (rrealized decreasing at low N). Tellingly perhaps, the species for which evidence of Allee effects was strongest (Atlantic cod) was also the species for which there were the greatest amount of data at low N.
This meta-analysis is one of four or five meta-analyses undertaken on marine fishes for the purpose of detecting Allee effects [10,11,17,25]. Despite an impressive temporal breadth of abundance data and taxonomic breadth of species affinity, the work has yielded little substantive evidence of Allee effects. However, for several of the reasons identified above, it remains difficult to conclude that Allee effects truly do not exist in this group of vertebrates. At least one analysis was beset by issues related to low statistical power and few data at low N [17]. A recent analysis [11] applied an arguably unduly high threshold for the inclusion of data; Allee effects were analysed for all populations that had declined to less than 20% of Nmax. A lower threshold would have increased the probability of detecting an Allee effect—should one exist—but would presumably have also limited the number of populations available for analysis.
There is perhaps another approach to the study of Allee effects, one that focuses on empirical evidence of their predicted consequences, rather than on the statistical model-fitting of data on metrics of rrealized and N characteristic of the literature on marine fishes.
There are overfished populations that have exhibited little or no recovery despite massive reductions in fishing-related mortality. These might provide sufficient information to generate estimates of where Allee-effect thresholds might be manifest relative to maximum observed population size (table 2). The compilation provided here includes 14 populations for which little or no increase has been documented since fishing on the depleted population ceased or had been greatly reduced (in some cases, the populations have continued to decline). Although these ‘thresholds’ of impaired recovery need not have been caused by an Allee effect, the slow or absent population recovery trajectories, following mitigation of the threat responsible for the declines, is certainly consistent with the expected consequences of an Allee effect [6,55].
Table 2.
Marine fish populations that have exhibited negligible recovery following the year in which the primary threat (targeted or bycatch-related fishing mortality) ceased or was greatly reduced. Abundance (N) is expressed as a percentage of maximum observed population size (Nmax).
| species | population | N when threat was mitigated | year of threat mitigation | consecutive years below 10%Nmax | current N | reference |
|---|---|---|---|---|---|---|
| Atlantic cod (Gadus morhua) | Northern cod | 2%Nmax | 1992 | 25 | 5%Nmax | [42,43] |
| Southern Gulf of St Lawrence | 27%Nmax | 1994 | 2 | 5%Nmax | [42,44] (DP Swain, personal communication, 17 March 2015) | |
| Eastern Scotian Shelf | 12%Nmax | 1994 | 9 | 9%Nmax | [42] (DP Swain, personal communication, 11 March 2015) | |
| Kattegat | 13%Nmax | 2002 | 3 | 18%Nmax | http://ices.dk/community/advisory-process/Pages/Latest-Advice.aspx | |
| eastern gemfish (Rexea solandri) | Western Australia | 5%Nmax | 1992 | 9 | 15%Nmax | [45,46] |
| cusk (Brosme brosme) | Eastern Canada | 9%Nmax | 1996 | 8 | 12%Nmax | [47] |
| winter skate (Leucoraja ocellata) | Southern Gulf of St Lawrence | 13%Nmax | 1991 | 16 | 2%Nmax | [44,48] |
| American plaice (Hippoglossoides platessoides) | Southern Gulf of St Lawrence | 24%Nmax | 1999 | 0 | 13%Nmax | [44,49] |
| South Newfoundland | 6%Nmax | 1994 | 15 | 12%Nmax | [47,50] | |
| white hake (Urophycis tenuis) | Southern Gulf of St Lawrence | 6%Nmax | 1994 | 1 | 6%Nmax | [44,51] |
| Northern Gulf of St Lawrence | 8%Nmax | 1994 | 1 | 15%Nmax | [52] | |
| roundnose grenadier (Coryphaenoides rupestris) | Labrador Shelf/Northeast Newfoundland | 8%Nmax | 1992 | 10 | 8%Nmax | [52] |
| bocaccio (Sebastes paucispinis) | British Columbia | 6%Nmax | 2003 | 17 | 6%Nmax | [53] |
| porbeagle (Lamna nasus) | Eastern Canada | 15%Nmax | 2003 | 0 | 15%Nmax | [54] |
The impaired-recovery thresholds implied in table 2 average 11.0%Nmax for the 14 populations (nine species), a relative-abundance metric very similar to the 10–12%Nmax Allee-effect thresholds that have been estimated for a limited number of marine fishes (table 1). An impaired-recovery threshold near 10%Nmax is further supported by a recent meta-analysis on 153 depleted marine fish populations [56] that predicted recovery to be prolonged and highly uncertain when population size fell below 20% of the population biomass at which maximum sustainable yield (Bmsy) would be obtained, a level of reduction that would correspond to approximately 6–8%Nmax (assuming a range in Bmsy of between 30 and 40% of unfished biomass) [57].
Trajectories of populations exhibiting impaired recovery can be compared with those that have recovered (table 3 and figure 2). Among 21 marine fish populations that had attained their recovery targets as of the end of 2014 (including all 18 ‘rebuilt’ marine fish populations in the US (www.nmfs.noaa.gov/sfa/fisheries_eco/status_of_fisheries/)), one-third recovered after having declined below 10%Nmax although the targets for some of these are relatively modest when compared with maximum observed abundance, three of seven being less than 25%Nmax (table 3). The relative abundance of recovered populations at the time of threat mitigation (21%Nmax) was more than double that of non-recovered populations (10%Nmax) (figure 2). The most recent data indicate that recovered populations have more than doubled as threats were mitigated (49%Nmax), whereas the average current abundance of non-recovered populations has remained essentially unchanged (10%Nmax) over time spans in excess of 20 years for most species (table 2).
Table 3.
Formerly overfished marine fish populations that have attained their recovery targets. Nmax is the maximum observed population size prior to the population minimum (Nmin). Those associated with the websites specified in the following parentheses (http://www.nefsc.noaa.gov/saw, http://sedarweb.org, http://www.pcouncil.org, https://www.iccat.int/en/assess.htm) are US populations considered to have been ‘rebuilt’ as of the end of 2014. (Note that one species, butterfish (Peprilus triacanthus), is deemed ‘rebuilt’ despite an estimated decline. This reflects uncertainty as to whether the population had previously been in an overfished condition. The stock assessment available at (http://www.nefsc.noaa.gov/saw) provides more details. It is included here because it is considered ‘rebuilt’.)
| species | population | Nmin | consecutive years below 10% Nmax | target and year achieved | reference |
|---|---|---|---|---|---|
| Atlantic cod (Gadus morhua) | Northeast Arctic | 9%Nmax | 1 | 41%Nmax (2002) | http://ices.dk/community/advisory-process/Pages/Latest-Advice.aspx |
| Iceland | 13%Nmax | 0 | 24%Nmax (2005) | http://ices.dk/community/advisory-process/Pages/Latest-Advice.aspx | |
| black sea bass (Centropristis striata) | Mid-Atlantic coast | 26%Nmax | 0 | 90%Nmax (2009) | http://www.nefsc.noaa.gov/saw/ |
| Southwest Atlantic | 28%Nmax | 0 | 71%Nmax (2013) | http://sedarweb.org | |
| Acadian redfish (Sebastes fasciatus) | Gulf of Maine/Georges Bank | 2%Nmax | 22 | 47%Nmax (2012) | http://www.nefsc.noaa.gov/saw/ |
| windowpane flounder (Scophthalmus aquosus) | Southern New England/Mid-Atlantic | 2%Nmax | 2 | 20%Nmax (2012) | http://www.nefsc.noaa.gov/saw/ |
| yellowtail flounder (Pleuronectes ferruginea) | Southern New England/Mid-Atlantic | 3%Nmax | 16 | 14%Nmax (2012) | http://www.nefsc.noaa.gov/saw/ |
| haddock (Melanogrammus aeglefinus) | Gulf of Maine | 4%Nmax | 7 | 27%Nmax (2011) | http://www.nefsc.noaa.gov/saw/ |
| summer flounder (Paralichthys dentatus) | Mid-Atlantic Coast | 26%Nmax | 0 | >100%Nmax (2011) | http://www.nefsc.noaa.gov/saw/ |
| widow rockfish (Sebastes entomelas) | Pacific coast | 24%Nmax | 0 | 32%Nmax (2011) | http://www.pcouncil.org |
| pollock (Pollachias virens) | Gulf of Maine/Georges Bank | 21%Nmax | 0 | 29%Nmax (2010) | http://www.nefsc.noaa.gov/saw/ |
| spiny dogfish (Squalus acanthias) | Atlantic coast | 17%Nmax | 0 | 58%Nmax (2010) | http://www.nefsc.noaa.gov/saw/ |
| scup (Stenotomus chrysops) | Atlantic coast | 21%Nmax | 0 | >100%Nmax (2009) | http://www.nefsc.noaa.gov/saw/ |
| swordfish (Xiphias gladius) | North Atlantic | 37%Nmax | 0 | Not established (2009) | https://www.iccat.int/en/assess.htm |
| Atlantic herring (Clupea harengus) | Norwegian spring-spawning | 2%Nmax | 19 | 43%Nmax (1997) | [58] |
| monkfish (Lophius americanus) | Gulf of Maine/Northern Georges Bank | 41%Nmax | 0 | 62%Nmax (2008) | http://www.nefsc.noaa.gov/saw/ |
| Southern Georges Bank/Mid-Atlantic | 68%Nmax | 0 | 68%Nmax (2008) | http://www.nefsc.noaa.gov/saw/ | |
| bluefish (Pomatomus saltatrix) | Atlantic coast | 29%Nmax | 0 | 49%Nmax (2008) | http://www.nefsc.noaa.gov/saw/ |
| king mackerel (Scomberomorus cavalla) | Gulf of Mexico | 32%Nmax | 0 | 35%Nmax (2008) | http://sedarweb.org |
| golden tilefish (Lopholatilus chamaeleonticeps) | Mid-Atlantic Coast | 5%Nmax | 16 | 19%Nmax (2014) | http://www.nefsc.noaa.gov/saw/ |
| butterfish (Peprilus triacanthus) | Gulf of Maine/Cape Hatteras | 53%Nmax | 0 | 42%Nmax (2014) | http://www.nefsc.noaa.gov/saw/ |
Figure 2.
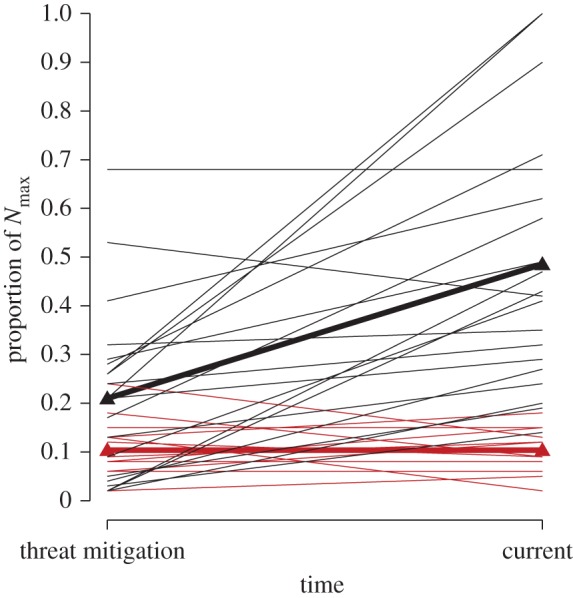
Trajectories of marine fish populations that did (black lines) and did not (red lines) recover following threat mitigation. Triangles represent average population sizes as proportions of observed maxima (Nmax).
5. Generalizing impaired-recovery thresholds across species
It is difficult to judge the extent to which these impaired-recovery metrics are applicable to other species. Meta-analyses of large mammals [59] and birds [60] have been unable to detect Allee-effect thresholds for populations that had declined to less than 10 and 15%, respectively, of Nmax. However, as these studies were undertaken two decades ago, they bear re-examination, given that additional data have been forthcoming in the interim [61].
Two studies suggest how a generic impaired-recovery threshold might be estimated for different species. One draws on basic density-dependent population dynamics, incorporates first principles related to population growth and implicitly accounts for changes in habitat quality and quantity [5]. The protocol for specifying a generic recovery target is based on the premise that, prior to being threatened, a population growing at its maximum rate, (∂N/∂t)max, will have low extinction probability and good survival prospects at an abundance (relative to K, considered here to be related to Nmax) likely to be associated with meaningful ecological and evolutionary functionality. Based on the commonly assumed linear decline between rrealized and N (figure 1; assuming that population growth is logistic), the default recovery target would be 0.5 K, i.e. the population size relative to K corresponding to (∂N/∂t)max, termed here δmax. But for many populations (∂N/∂t)max is estimated to occur at population levels other than 0.5 K. Among marine teleost fishes, δmax is thought to better correspond to a value of approximately 0.3 rather than 0.5 [57].
Turning to the second study, δmax is predicted to vary with per capita population growth. Based on data collated for 17 species of disparate phylogenetic affinity (e.g. Paramecium sp.; fin whale, Balaenoptera physalus), δmax has been shown to decline with rmax per generation, according to δmax = 0.633–0.187(ln(rmaxT)), where T is generation time [62]. Here, I apply Fowler's equation to estimates of rmax and generation time (calculated as 1/M + age at maturity, where M is the instantaneous rate of natural mortality [63]) for 188 populations (151 species) of vertebrates [31] (electronic supplementary material, table S1). The results suggest that δmax for chondrichthyan fishes (e.g., sharks, skates, rays), terrestrial mammals and marine mammals is higher than that estimated for marine teleost fishes (table 4).
Table 4.
Estimated proportions of carrying capacity (K) at which population growth rate is maximized (δmax) and Allee-effect thresholds are manifest (see also electronic supplementary material, table S1).
Available data, albeit limited, provide a means by which impaired-recovery thresholds might be estimated for these different taxonomic groups, based on empirical data collated for marine fishes. As noted previously, there is evidence that thresholds for impaired recovery for marine fishes can be evident at about 10%Nmax. This is one-third of the estimate of δmax = 0.3Nmax for marine teleosts [57]. Applying this conversion to the δmax estimates for other vertebrates yields impaired-recovery thresholds of 0.13Nmax, 0.15Nmax and 0.19Nmax for chondrichthyans, terrestrial mammals and marine mammals, respectively (table 4).
6. Temporal thresholds for impaired recovery
It seems important to distinguish the passing of an abundance threshold for impaired recovery from the period of time that a population spends below the threshold. The latter may be considerably more influential than the former on rate and probability of recovery, although the topic does not seem to have been formally addressed in the literature. The conceptual basis for this suggestion is that the longer a population remains depleted, the more likely it is that the environment around it will change in ways that are unfavourable to its persistence [12].
Such temporal thresholds are likely to involve alterations to inter-specific interactions, such as predation and competition. Changes in the abundance of interacting species to which an increasingly depleted population becomes increasingly vulnerable can lead to emergent Allee effects [6,33,44,64]. Examples of probable emergent Allee effects have been reported in eastern Canadian waters. The natural (non-fishing) mortality of fishes in the Southern Gulf of St Lawrence has increased to levels that threaten the persistence of species depleted by overfishing [44,65,66]. This has been attributed to predation by grey seals (Halichoerus grypus) which have increased more than tenfold [64]. Mounting evidence suggests that the natural mortality of marine fishes can increase as abundance declines in overfished populations [44,65–67]. And, all else being equal, increased mortality can readily generate an Allee effect in depleted populations [33].
The idea of a temporal threshold for impaired recovery garners additional support when comparing marine fish populations that have recovered (table 3) from those that have not (table 2). Using 10%Nmax as a numerical threshold benchmark, the median period of time spent below 10%Nmax is 0 years among 21 rebuilt populations and 8.5 years for 14 populations that have exhibited little or no recovery.
Although Allee effects were not examined per se, a recent study [56] of recovery in marine fishes also supports the hypothesis that temporal thresholds for impaired recovery exist. Using a Bayesian regression model based on stochastic population dynamics (and accounting for multiple potential correlates of recovery), the meta-analysis found that recovery by depleted populations was forecast to occur within 10 years but only if fishing mortality was reduced immediately after the depletion had been detected. Uncertainty about recovery time increased exponentially with applied fishing pressure. In other words, the longer it takes for fishing mortality to be meaningfully reduced once depletion has been detected, the longer the recovery period and the greater the uncertainty of recovery.
Prolonged recovery times and increased uncertainty in recovery is what one would expect if the depleted populations had been subjected to Allee effects. This was recently demonstrated by simulations of a density-dependent, stochastic, individual-based model that tracked changes in life history, survival and rrealized in Atlantic cod [55]. Following fishing-induced depletions to 0.05Nmax, the study documented time and probability of recovery to carrying capacity under scenarios of low to negligible fishing mortality in the presence and absence of an empirically documented Allee effect [10]. Both time to recovery and uncertainty of recovery were predicted to increase dramatically in the presence of an Allee effect [55].
7. Discussion
(a). Thresholds for impaired recovery
A paucity of studies on Allee-related thresholds for declining populations generated the primary impetus for the exploratory work presented here. Its empirical legitimacy hinges on a limited number of examples of Allee effects and several instances of negligible recovery in marine fishes. These data provided a basis for identifying relative levels of abundance below which mitigation of threats responsible for population decline might not be sufficient to effect recovery. Among marine fishes, the analysis suggested that recovery might be negligible, delayed and increasingly uncertain when populations fall below 10% of maximum observed abundance (Nmax). There is also reason to believe that relative-abundance thresholds for impaired recovery are likely to differ among species, increasing as per capita population growth rate decreases.
The suggested impaired-recovery threshold of 10%Nmax for marine fishes is broadly consistent with previous work. For example, the most recent meta-analysis on this taxonomic group could not discount the possibility that Allee effects are manifest when abundance falls below 5% of historical maximum biomass [11]. Then again, it might not be productive to focus solely on the question of whether Allee effects exist or not. It is clear that some populations do not exhibit meaningful recovery despite threat mitigation. In marine fishes, the lowest average abundance experienced by such populations is approximately 10%Nmax. Overfished populations classified as having recovered (independently of where the rebuilding targets are in relation to Nmax) have, with some exceptions, either not declined below 10%Nmax or spent much time at such low levels. The same might be true for other organisms, although this empirical work has yet to be undertaken.
(b). Allee effects
An Allee effect is a pattern in data. It does not distinguish populations that are absolutely small (tens to hundreds of individuals) from those that are absolutely large (hundreds of thousands to millions of individuals) but comparatively small (relative to a metric such as historical maximum or carrying capacity). Despite this, there is far greater interest in studying strong Allee effects under the small-population paradigm rather than weak Allee effects under the declining-population paradigm. One probable reason for the attention on absolutely small populations is that they are more likely to be subjected to the predominant postulated causes of an Allee effect [6,27,68] such as reduced probability of fertilization, lower chance of finding a mate, impaired group dynamics (e.g. reduced anti-predator vigilance), increased incidence of inbreeding or genetic drift and group conditioning of the environment.
Unlike most depleted vertebrates, marine fishes often remain numerically large despite massive reductions (more than 90%) in abundance. Estimates of the number of mature individuals at their respective population minima (Nmin) include: Southern Gulf white hake (Nmin = 977 000); northern Atlantic cod (Nmin = 22 million); Southern Gulf cod (Nmin = 60 million); winter skate (Nmin = 0.5 million); Eastern Scotian Shelf cod (Nmin = 1 million); Southern Gulf American plaice (Nmin = 100 million) (references in table 2).
For these numerically large populations, negligible recovery following threat mitigation (table 2 and figure 2) is suggestive of the existence of an emergent Allee effect made possible by the lengthy periods of time that depleted populations have remained depleted. One example was identified earlier, increased mortality of depleted prey by a numerically increasing predator [33,44]. Another cause of emergent Allee effects has been termed the ‘cultivation/depensation effect’ [69]. Reductions in a predator release prey from a significant source of mortality, allowing the prey to increase in abundance and to prey upon and compete with the young of the formerly abundant predator [70]. Thus, when numerically abundant but relatively depleted populations fail to recover, this might be indicative of an altered ecosystem or regime shift generated by the decline in one species and altered interactions with others, ultimately manifesting itself as an emergent Allee effect [33,44,69,71,72].
(c). Limitations
Criticism can be levelled at the approach adopted here. One might be that it is unduly focused on marine fishes. But there are practical and logical reasons for doing so. There is a wealth of information on trends in abundance in exploited fishes which surpasses that for most organisms. Another advantage is that, as far as can be ascertained, habitat destruction or alteration is not a significant contributing factor to the declines, compared with the influence of excessive fishing mortality (coral reef fishes, which are not included here because of lack of data, might constitute an exception). Thirdly, many marine fish populations have been severely depleted, meaning that there is a comparative abundance of data at small-population sizes, thus increasing one's ability to detect thresholds for impaired recovery, such as an Allee-effect threshold, should they exist. Another concern is that data from marine fishes should not be used to estimate thresholds for impaired recovery in other organisms. But the relative absence of data similar to those presented in table 2 for other organisms not threatened by habitat loss makes it difficult to do otherwise.
The charge could also be levelled that the work presented here downplays the observation that Allee effects have generally not been detected in meta-analyses of marine fishes [10,11,17,25]. However, analyses undertaken prior to 2012 were beset by data-limitation issues, low statistical power and biologically uninterpretable parameters [11,12,25,41]. One meta-analysis of abundance data for insects, birds, mammals and fishes reported that support for models of both negative and positive (Allee effect) density feedback increased with data availability at low population sizes [61]. It might not be coincidental that evidence of Allee effects appears to be strongest in populations and species that have spent long periods of time in a depleted state (e.g. Atlantic cod; Icelandic spring-spawning herring; tables 1–3). Although regime shifts almost certainly play an important role in negatively influencing recovery [73], it is worth contemplating the extent to which the regime shifts that have been documented to affect population productivity are a cause, or a consequence, of the impaired ecological functionality and productivity of depleted populations.
One can also criticize the dependence herein on maximum abundance. The correspondence between Nmax and, say, carrying capacity will not always be clear. Those working on exploited populations might prefer a metric related to a standard such as Bmsy, although the estimation of such a reference point can also be fraught by noisy, uncertain and (or) highly variable data. Two advantages to articulating thresholds in relation to Nmax are that an estimate of maximum abundance can almost always be made for any depleted population and such a metric allows for a comparison of impaired-recovery thresholds across species. For many populations of conservation concern, such a metric might be preferable to others that demand empirically and temporally rich abundance data.
(d). Recovering population paradigm
‘[The small-population paradigm] would contribute immeasurably more if some of the theoretical momentum so generated were channelled into providing a theory of driven population declines, thereby liberating the declining-population paradigm from the inefficiency of case-by-case ecological investigations and recovery operations' [3]. The extinction-related focus of most studies associated with the small- and declining-population paradigms is understandable. Current rates of species loss and endangerment provide well-reasoned justification for such research. That said, research conducted under the auspices of these paradigms has not informed our understanding of species and population recovery nearly to the extent that it could. The vast majority of studies on Allee effects, for example, are either case studies with minimal broader relevance or theoretical studies having unknown significance to the dynamics of depleted natural populations. Remarkably for a concept developed more than 80 years ago, it is a field still in empirical infancy from the perspective of identifying thresholds for impaired recovery.
There may be utility in articulating a recovering population paradigm that provides a theoretical and empirical template for research on the determinants, rate and probability of recovery. The current biodiversity crisis [74] underscores an acute and growing need for a recovery-correlate template applicable across species and different spatial scales [4]. One justification for such a template lies in the observation that threat amelioration, albeit necessary, is often insufficient to effect recovery (table 2 and figure 2) [4,75]. In addition to research on the determinants of recovery, associated studies could include empirical and theoretical work on rates of recovery. There are clearly multiple case studies that could be used as a basis for such a review, some of which are noted here (tables 2 and 3). A recent meta-analytic approach to forecasting rates of recovery for marine fishes [56] could readily be applied to other taxonomic groups. Concomitant with studies on rates of recovery should be work on factors affecting the uncertainty of recovery [55,56].
A recovering population paradigm would benefit immensely by concerted efforts to identify thresholds of abundance below which impaired recovery might be manifest. Such thresholds could readily serve as ‘red flags’ to conservation practitioners and resource managers [4]. The work presented here suggests that such red flags for impaired recovery can be estimated and might be expected to differ among broad taxonomic groups, being comparatively low for marine fishes and higher for marine mammals. The articulation of a recovering population paradigm might also serve to reduce the confusion of terminology and conflation of patterns with mechanisms and consequences that negatively affect both communication among scientists and the practical utility of recovery-oriented research to conservation practitioners and resource managers.
Supplementary Material
Acknowledgements
Part of this article was the Presidential Address to the annual meeting of the Canadian Society for Ecology and Evolution in Montreal, 2014. I am very grateful to the many colleagues with whom I have worked on the research considered here, noting in particular the collaborations or discussions I have had with Julia Baum, Verónica García, Olaf Jensen, David Keith, Anna Kuparinen, Luis Lucifera, Ransom Myers, Philipp Neubauer and Doug Swain. The manuscript was strengthened by the very helpful reviews received from Veijo Kaitala, Anna Kuparinen, Per Lundberg, Doug Swain and an anonymous referee. Ilmoilan Vajatoimisto provided logistical support.
Competing Interests
I declare that I have no competing interests.
Funding
The bulk of the research undertaken here was funded by the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Gilpin ME, Soulé ME. 1986. Minimum viable populations: processes of species extinction. In Conservation biology: the science of scarcity and diversity (ed. Soulé ME.), pp. 19–34. [Google Scholar]
- 2.Lande R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927. ( 10.1086/285580) [DOI] [PubMed] [Google Scholar]
- 3.Caughley G. 1994. Directions in conservation biology. J. Anim. Ecol. 63, 215–244. ( 10.2307/5542) [DOI] [Google Scholar]
- 4.Hutchings JA, Butchart SH, Collen B, Schwartz MK, Waples RS. 2012. Red flags: correlates of impaired species recovery. Trends Ecol. Evol. 27, 542–546. ( 10.1016/j.tree.2012.06.005) [DOI] [PubMed] [Google Scholar]
- 5.Hutchings JA, Kuparinen A. 2014. A generic target for species recovery. Can. J. Zool. 92, 371–376. ( 10.1139/cjz-2013-0276) [DOI] [Google Scholar]
- 6.Courchamp F, Berec L, Gascoigne J. 2008. Allee effects in ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Stephens PA, Sutherland WJ, Freckleton RP. 1999. What is the Allee effect? Oikos 87, 185–190. ( 10.2307/3547011) [DOI] [Google Scholar]
- 8.Allee WH. 1931. Animal aggregations, a study in general sociology. Chicago, IL: Universtiy Chicago Press. [Google Scholar]
- 9.Roff DA. 2002. The evolution of life histories: theory and analysis. Sunderland, MA: Sinauer. [Google Scholar]
- 10.Keith DM, Hutchings JA. 2012. Population dynamics of marine fishes at low abundance. Can. J. Fish. Aquat. Sci. 69, 1150–1163. ( 10.1139/f2012-055) [DOI] [Google Scholar]
- 11.Hilborn R, Hively DJ, Jensen OP, Branch TA. 2014. The dynamics of fish populations at low abundance and prospects for rebuilding and recovery. ICES J. Mar. Sci. 71, 2141–2151. ( 10.1093/icesjms/fsu035) [DOI] [Google Scholar]
- 12.Hutchings JA. 2014. Renaissance of a caveat: Allee effects in marine fishes. ICES J. Mar. Sci. 71, 2152–2157. ( 10.1093/icesjms/fst179) [DOI] [Google Scholar]
- 13.Johnson DM, Liebhold AM, Tobin PC, Bjørnstad ON. 2006. Allee effects and pulsed invasion by the gypsy moth. Nature 444, 361–363. ( 10.1038/nature05242) [DOI] [PubMed] [Google Scholar]
- 14.Molnár PK, Lewis MA, Derocher AE. 2014. Estimating Allee thresholds before they can be observed: polar bears as a case study. PLoS ONE 9, e85410 ( 10.1371/journal.pone.0085410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Voorn GAK, Hemerik L, Boer MP, Kooi BW. 2007. Heteroclinic orbits indicate overexploitation in predator–prey systems with a strong Allee effect. Math. Biosci. 209, 451–469. ( 10.1016/j.mbs.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 16.Redford KH, et al. 2011. What does it mean to successfully conserve a (vertebrate) species? BioScience 61, 39–48. ( 10.1525/bio.2011.61.1.9) [DOI] [Google Scholar]
- 17.Myers RA, Barrowman NJ, Hutchings JA, Rosenberg AA. 1995. Population dynamics of exploited fish stocks at low population levels. Science 269, 1106–1108. ( 10.1126/science.269.5227.1106) [DOI] [PubMed] [Google Scholar]
- 18.Nariai Y, Hayashi S, Morita S, Umemura Y, Tainaka K-I, Sota T, Cooley JR, Yoshimura J. 2011. Life cycle replacement by gene introduction under an Allee effect in periodical cicadas. PLoS ONE 6, e18347 ( 10.1371/journal.pone.0018347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart EM, Leticia A. 2014. Reconstructing local population dynamics in noisy metapopulations—the role of random catastrophes and Allee effects. PLoS ONE 9, e110049 ( 10.1371/journal.pone.0110049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castel M, Mailleret L, Andrivon D, Ravigné V, Hamelin F. 2014. Allee effects and the evolution of polymorphism in cyclic parthenogens. Am. Nat. 183, E75–E88. ( 10.1086/674828) [DOI] [PubMed] [Google Scholar]
- 21.Sugeno M, Munch SB. 2013. A semiparametric Bayesian method for detecting Allee effects. Ecology 94, 1196–1204. ( 10.1890/12-0454.1) [DOI] [PubMed] [Google Scholar]
- 22.Kokko H, Sutherland WJ. 2001. Ecological traps in changing environments: ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol. Ecol. Res. 3, 537–551. [Google Scholar]
- 23.Komers PE, Curman GP. 2000. The effect of demographic characteristics on the success of ungulate re-introduction. Biol. Conserv. 93, 187–194. ( 10.1016/S0006-3207(99)00141-X) [DOI] [Google Scholar]
- 24.Green RE. 1997. The influence of numbers released on the outcome of attempts to introduce exotic bird species to New Zealand. J. Anim. Ecol. 66, 25–35. ( 10.2307/5961) [DOI] [Google Scholar]
- 25.Liermann M, Hilborn R. 1997. Depensation in fish stocks: a hierarchical Bayesian meta-analysis. Can. J. Fish. Aquat. Sci. 54, 1976–1984. ( 10.1139/f97-105) [DOI] [Google Scholar]
- 26.Gascoigne J, Lipcius RN. 2004. Allee effects in marine systems. Mar. Ecol. Prog. Ser. 269, 49–59. ( 10.3354/meps269049) [DOI] [Google Scholar]
- 27.Lidicker WZ. 2010. The Allee effect: its history and future importance. Open Ecol. J. 3, 71–82. ( 10.2174/1874213001003010071) [DOI] [Google Scholar]
- 28.Kramer AM, Dennis B, Liebhold AM, Drake JM. 2009. The evidence for Allee effects. Pop. Ecol. 51, 341–354. ( 10.1007/s10144-009-0152-6) [DOI] [Google Scholar]
- 29.Rowe S, Hutchings JA, Bekkevold D, Rakitin A. 2004. Depensation, probability of fertilization, and the mating system of Atlantic cod. ICES J. Mar. Sci. 61, 1144–1150. ( 10.1016/j.icesjms.2004.07.007) [DOI] [Google Scholar]
- 30.Berec L, Angula E, Courchamp F. 2007. Multiple Allee effects and population management. Trends Ecol. Evol. 22, 185–191. ( 10.1016/j.tree.2006.12.002) [DOI] [PubMed] [Google Scholar]
- 31.Hutchings JA, Myers RA, García VB, Lucifora LO, Kuparinen A. 2012. Life-history correlates of extinction risk and recovery potential. Ecol. Applic. 22, 1061–1067. ( 10.1890/11-1313.1) [DOI] [PubMed] [Google Scholar]
- 32.Fauvergue X. 2012. A review of mate-finding Allee effects in insects: from individual behavior to population management. Entomol. Exp. Appl. 146, 79–92. ( 10.1111/eea.12021) [DOI] [Google Scholar]
- 33.Kuparinen A, Hutchings JA. 2014. Increased natural mortality at low abundance can generate a demographic Allee effect. R. Soc. Open Sci. 1, 140075 ( 10.1098/rsos.140075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IUCN Standards and Petitions Subcommittee. 2014. Guidelines for using the IUCN Red List categories and criteria, v. 11. See http://www.iucnredlist.org/documents/RedListGuidelines.pdf.
- 35.Committee on the Status of Endangered Wildlife in Canada. 2012. COSEWIC assessment process, categories and guidelines, Ottawa (www.cosewic.gc.ca)
- 36.Schreiber SJ. 2003. Allee effects, extinctions, and chaotic transients in simple population models. Theor. Pop. Biol. 64, 201–209. ( 10.1016/S0040-5809(03)00072-8) [DOI] [PubMed] [Google Scholar]
- 37.Wang M-H, Kot M. 2001. Speeds of invasion in a model with strong or weak Allee effects. Math. Biosci. 171, 83–97. ( 10.1016/S0025-5564(01)00048-7) [DOI] [PubMed] [Google Scholar]
- 38.Courchamp F, Rasmussen G, Macdonald D. 2002. Small pack size imposes a trade-off between hunting and pup-guarding in the painted hunting dog Lycaon pictus. Behav. Ecol. 13, 20–27. ( 10.1093/beheco/13.1.20) [DOI] [Google Scholar]
- 39.Babcock R, Keesing J. 1999. Fertilisation biology of the abalone Haliotis laevigata: laboratory and field studies. Can. J. Fish. Aquat. Sci. 56, 1668–1678. ( 10.1139/f99-106) [DOI] [Google Scholar]
- 40.Morris DW. 2002. Measuring the Allee effect: positive density dependence in small mammals. Ecology 83, 14–20. ( 10.1890/0012-9658(2002)083[0014:MTAEPD]2.0.CO;2) [DOI] [Google Scholar]
- 41.Shelton PA, Healey BP. 1999. Should depensation be dismissed as a possible explanation for the lack of recovery of the northern cod (Gadus morhua) stock? Can. J. Fish. Aquat. Sci. 56, 1521–1524. ( 10.1139/f99-124) [DOI] [Google Scholar]
- 42.Committee on the Status of Endangered Wildlife in Canada. 2010. COSEWIC assessment and status report on the Atlantic cod Gadus morhua in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 43.DFO. 2014. Northern cod (2J3KL) cod stock update. DFO Can. Sci. Advis. Sec. Sci. Resp. 2014/030. Ottawa, Canada: Dept Fisheries and Oceans.
- 44.Swain DP, Benoît HP. 2015. Extreme increases in natural mortality prevent recovery of collapsed fish populations in a Northwest Atlantic ecosystem. Mar. Ecol. Prog. Ser. 519, 165–182. ( 10.3354/meps11012) [DOI] [Google Scholar]
- 45.Little R, Rowling K. 2010. 2010 Update of the eastern gemfish (Rexea solandri) stock assessment. Hobart, Australia: CSIRO Mar. Atmos. Res.
- 46.Fisheries Scientific Committee. 2014. Proposed determination. Rexea solandri—gemfish as a vulnerable species. Fisheries Scientific Committee, New South Wales Dept Primary Industries. Ref. No. PD56, File No. FSC 99/23.
- 47.Committee on the Status of Endangered Wildlife in Canada. 2013. COSEWIC assessment and status report on the cusk Brosme brosme in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 48.Committee on the Status of Endangered Wildlife in Canada. 2005. COSEWIC assessment and status report on the winter skate Leucoraja ocellata in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 49.Committee on the Status of Endangered Wildlife in Canada. 2009. COSEWIC assessment and status report on the American plaice (Hippoglossoides platessoides) in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 50.Morgan MJ, Dwyer KS, Healey BP, Rideout RM. 2014. An assessment of the American plaice (Hippoglossoides platessoides) stock in NAFO Subdivision 3Ps. DFO Can. Sci. Advis. Sec. Res. Doc. 2014/098.
- 51.Committee on the Status of Endangered Wildlife in Canada. 2013. COSEWIC assessment and status report on the white hake Urophycis tenuis in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 52.Committee on the Status of Endangered Wildlife in Canada. 2008. COSEWIC assessment and status report on the roundnose grenadier Coryphaenoides ruperstris in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 53.Committee on the Status of Endangered Wildlife in Canada. 2013. COSEWIC assessment and status report on the bocaccio Sebastes paucispinis in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm) [Google Scholar]
- 54.Committee on the Status of Endangered Wildlife in Canada. 2014. COSEWIC assessment and status report on the porbeagle Lamna nasus in Canada, Ottawa (www.registrelep-sararegistry.gc.ca/default_e.cfm). [Google Scholar]
- 55.Kuparinen A, Keith DM, Hutchings JA. 2014. Allee effect and the uncertainty of population recovery. Conserv. Biol. 28, 790–798. ( 10.1111/cobi.12216) [DOI] [PubMed] [Google Scholar]
- 56.Neubauer P, Jensen OP, Hutchings JA, Baum JK. 2013. Resilience and recovery of overexploited marine populations. Science 340, 347–349. ( 10.1126/science.1230441) [DOI] [PubMed] [Google Scholar]
- 57.Hilborn R, Stokes K. 2010. Defining overfished stocks: have we lost the plot? Fisheries 35, 113–120. ( 10.1577/1548-8446-35.3.113) [DOI] [Google Scholar]
- 58.Toresen R, Jakobsson J. 2002. Exploitation and management of Norwegian spring-spawning herring in the 20th century. ICES Mar. Sci. Symp. 215, 558–571. [Google Scholar]
- 59.Fowler CW, Baker JD. 1991. A review of animal population dynamics at extremely reduced population levels. Rep. Int. Whaling Commission 41, 545–554. [Google Scholar]
- 60.Sæther B-E, Ringsby TH, Roskaft E. 1996. Life history variation, population processes and priorities in species conservation: towards a reunion of research paradigms. Oikos 77, 217–226. ( 10.2307/3546060) [DOI] [Google Scholar]
- 61.Gregory SD, Bradshaw CJA, Brook BW, Courchamp F. 2010. Limited evidence for the demographic Allee effect from numerous species across taxa. Ecology 91, 2151–2161. ( 10.1890/09-1128.1) [DOI] [PubMed] [Google Scholar]
- 62.Fowler CW. 1988. Population dynamics as related to rate of increase per generation. Evol. Ecol. 2, 197–204. ( 10.1007/BF02214283) [DOI] [Google Scholar]
- 63.Charnov EL. 1993. Life history invariants. Oxford, UK: Oxford University Press. [Google Scholar]
- 64.Hutchings JA, Rangeley RW. 2011. Correlates of recovery for Canadian Atlantic cod. Can. J. Zool. 89, 386–400. ( 10.1139/z11-022) [DOI] [Google Scholar]
- 65.Benoît HP, Swain DP, Bowen WD, Breed GA, Hammill MO, Harvey V. 2011. Can predation by grey seals explain elevated natural mortality in three fish species in the southern Gulf of St Lawrence? Mar. Ecol. Prog. Ser. 442, 149–167. ( 10.3354/meps09454) [DOI] [Google Scholar]
- 66.Swain DP. 2011. Life-history evolution and elevated natural mortality in a population of Atlantic cod (Gadus morhua). Evol Appl. 4, 18–29. ( 10.1111/j.1752-4571.2010.00128.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Audzijonyte A, Kuparinen A, Gorton G, Fulton EA. 2013. Ecological consequences of body size decline in harvested fish species: positive feedback loops in trophic interactions amplify human impact. Biol. Lett. 9, 20121103 ( 10.1098/rsbl.2012.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stephens PA, Sutherland WJ. 1999. Consequences of the Allee effect for behavior, ecology and conservation. Trends Ecol. Evol. 14, 401–405. ( 10.1016/S0169-5347(99)01684-5) [DOI] [PubMed] [Google Scholar]
- 69.Walters C, Kitchell JF. 2001. Cultivation/depensation effects on juvenile survival and recruitment: implications for the theory of fishing. Can. J. Fish. Aquat. Sci. 58, 39–50. ( 10.1139/f00-160) [DOI] [Google Scholar]
- 70.Swain DP, Sinclair AF. 2000. Pelagic fishes and the cod recruitment dilemma in the Northwest Atlantic. Can. J. Fish. Aquat. Sci. 57, 1321–1325. ( 10.1139/f00-104) [DOI] [Google Scholar]
- 71.De Roos AM, Persson L. 2002. Size-dependent life-history traits promote catastrophic collapses of top predators. Proc. Natl Acad. Sci. USA 99, 12 907–12 912. ( 10.1073/pnas.192174199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Leeuwen A, De Roos AM, Persson L. 2008. How cod shapes its world. J. Sea Res. 60, 89–104. ( 10.1016/j.seares.2008.02.008) [DOI] [Google Scholar]
- 73.Vert-pre KA, Amoroso RO, Jensen OP, Hilborn R. 2013. Frequency and intensity of productivity regime shifts in marine fish stocks. Proc. Natl Acad. Sci. USA 110, 1779–1784. ( 10.1073/pnas.1214879110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butchart SHM, et al. 2010. Global biodiversity: indicators of recent declines. Science 328, 1164–1168. ( 10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 75.Hutchings JA, Reynolds JD. 2004. Marine fish population collapses: consequences for recovery and extinction risk. BioScience 54, 297–309. ( 10.1641/0006-3568(2004)054[0297:MFPCCF]2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


