Abstract
The semicircular canals (SCs) of the inner ear detect angular acceleration and are located in the bony labyrinth of the petrosal bone. Based on high-resolution computed tomography, we created a size-independent database of the bony labyrinth of 50 mammalian species especially rodents of the squirrel-related clade comprising taxa with fossorial, arboreal and gliding adaptations. Our sampling also includes gliding marsupials, actively flying bats, the arboreal tree shrew and subterranean species. The morphometric anatomy of the SCs was correlated to the locomotion mode. Even if the phylogenetic signal cannot entirely be excluded, the main significance for functional morphological studies has been found in the diameter of the SCs, whereas the radius of curvature is of minor interest. Additionally, we found clear differences in the bias angle of the canals between subterranean and gliding taxa, but also between sciurids and glirids. The sensitivity of the inner ear correlates with the locomotion mode, with a higher sensitivity of the SCs in fossorial species than in flying taxa. We conclude that the inner ear of flying and gliding mammals is less sensitive due to the large information flow into this sense organ during locomotion.
Keywords: inner ear, semicircular canals, vestibular system, locomotion, morphometry, Rodentia
1. Introduction
In 1873, Hyrtl [1, p. 228] denied that any locomotory evidence could be deducted from the anatomy of the inner ear inside the bony labyrinth of the petrosal bone, which has the double function of spatial orientation in three-dimensional space and detecting sound transmissions. However, with the benefits of modern non-invasive imaging techniques, such as high-resolution computed tomography (µCT), the first aspect has become a favourite topic for functional morphological analyses (e.g. [2–13]), but also for phylogenetic approaches [14–20].
The membranous organs of the vestibular system are composed of the semicircular ducts, the utricle and saccule, and are enclosed by bony structures of the petrosal bone. Utricule and saccule detect linear velocity, whereas the three semicircular canals (SCs) (anterior semicircular canal, ASC; posterior semicircular canal, PSC; and lateral semicircular canal, LSC) detect angular acceleration of the head and correlate in their anatomy with the mode of locomotion and agility. Previous studies on birds and mammals revealed that small curvatures of SCs are correlated with sluggish head movements, whereas canals with large curvatures are found in highly maneuverable species [3,4,8,21–24]. Physical requirements, like a reasonable response speed, a high sensitivity and a low Reynolds number as a dimensionless proxy for laminar flow situations [25] in canal systems must guarantee an undisturbed laminar flow in the SCs. This is also influenced by the highly constrained shape of the inner ear with no significant intraspecific variation in the shape of the SCs [26,27], which stays in contrast to the vestibular system of extant sloths, where the highly variable bony labyrinth is correlated to their sluggish behaviour [8,28,29].
For investigating the correlation of the inner ear with mode of locomotion and agility, previous studies have mainly focused on small taxonomic groups like extant and extinct primates and cetaceans as well as single extant species (e.g. [4,5,9,30]). Here, for the first time to our knowledge, a rodent clade is investigated in a broadly conceived study in order to detect the anatomical adaptations for the respective type of locomotion.
The squirrel-related clade is characterized by different types of locomotion [31,32] and comprises the families Sciuridae, Aplodontidae and Gliridae [33–36]. The relationship between these three families is contradictory and subject of many morphological (e.g. [37,38]) and molecular studies (e.g. [32,34,36]).
Sciurids are morphologically diverse, which is caused by a radiation in response to habitat and geological changes, along with an increase in the number of species [39]. Besides arboreal living tree squirrels (Sciurinae) and terrestrial living groundsquirrels (Sciurinae, Xerinae), sciurids are also capable of gliding (Pteromyinae) [31,40], caused by a high adaption of their postcranium, e.g. the forearm musculature [41]. Extant taxa of aplodontids are represented by a single North American taxon, Aplodontia rufa, the mountain beaver; this species shows postcranial adaptations to a fossorial lifestyle [31,42] and is in sister-group relationship to sciurids, forming the Sciuroidea [33,43]. Extant glirids are exclusively arboreal and are distributed only in the Palaearctic and the African faunal region [31,40]. Traditionally, glirids had been classified with the Myomorpha based on similarities in their masseter musculature [44,45]. However, morphological [37,38,46,47] and molecular studies [34,36] indicate a sister-group relationship of sciurids and aplodontids (=sciuroids) with glirids.
Here, we investigate the correlation between the shape of the SCs and the locomotion mode of squirrels. In order to detect a trend for the functional morphological adaptation of the inner ear of mammals and their respective locomotion modes, we also included actively flying bats and subterranean taxa into this study. Our data matrices are size independent, so that large and small animals can be functionally compared in the same analysis. The effect of the phylogeny in the squirrel-related clade is investigated with phylogenetically controlled comparative methods.
2. Material and methods
For a better comparability with previous studies and as data matrices for future studies focusing on extant but also extinct mammals, we were focusing on the bony labyrinth instead of the membranous system even if there are functional morphological limitations. The bony labyrinths of 41 sciurid species representing 29 genera, five genera of glirids, and A. rufa, and for comparative investigations, additional selected taxa of gliding marsupials, actively flying bats, subterranean taxa and the arboreal Tupaia sp., were investigated by µCT with the v|tome|x s scanner (GE phoenix|x-ray) at the Steinmann-Institut für Geologie, Mineralogie und Paläontologie, Universität Bonn, Bonn, Germany. The subterranean taxa were scanned with a SkyScan 1173 (Bruker) at the Department of Palaeontology, University of Vienna, Vienna, Austria (for detailed list of investigated taxa, see the electronic supplementary material). The identification of species is based on collection data. The allocation of the locomotion mode of the investigated species follows previous studies [31,48].
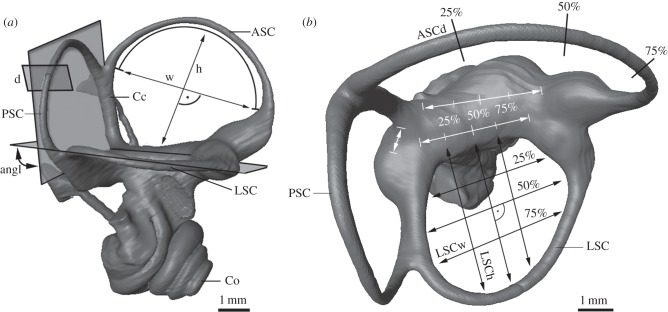
The bony labyrinths were visualized by using stacks of digital μCT tiff images. Owing to different states of preservation, different threshold values were reconstructed virtually by manual segmentation and unconstrained smoothing with Avizo v. 6.1.1 software (Visualization Sciences Group). Measurements were taken directly from the virtual reconstruction with Avizo v. 6.1.1., Amira v. 5.4.1. (Visualization Sciences Group) and Polyworks v. 10.0.5 (Innovmetric Inc.) (figure 1).
Figure 1.
Measurements on the bony labyrinth represented by P. petaurista. (a) Lateral view of the right bony labyrinth. (b) Dorsal view. angl, angle between the SCs; ASC, anterior semicircular canal; ASCd, diameter of anterior semicircular canal; Co, cochlea; d, diameter of SC; h, height of SC; l, length of SC; LSC, lateral semicircular canal; LSCh, height of lateral semicircular canal; LSCw, width of lateral semicircular canal; PSC, posterior semicircular canal; w, width of SC.
The measurements were taken as follows (for detailed list of measurements, see the electronic supplementary material).
— ASCh, PSCh, LSCh—the maximal height of each SC as the distance of the surface of the vestibulum to the inner wall of the bony canal; taken at 25, 50 and 75% of the distance between the ampulla of the canal and the crus commune (figure 1b); the height corresponds to the space between the vestibulum and the bony canal arcs.
— ASCw, PSCw, LSCw—the maximal width between the inner walls of the bony tubes of the SC was measured at 25, 50 and 75% of the height of the corresponding canal in a right angle to the canals’ height (figure 1b); the width corresponds to the space between the bony canal arcs.
— ASCd, PSCd, LSCd—diameter of SC was taken at 25, 50 and 75% of the length of the corresponding canal (figure 1b).
— ASCl, PSCl, LSCl—lengths of the SC were measured following previous studies [49].
— ASCr, PSCr, LSCr—radii of curvature of SC were calculated following previous studies [3].
— Ccl—length of the crus commune (Cc).
— anglASC/PSC, anglASC/LSC anglPSC/LSC—bias angle of best-fitting planes were computed with a set of points characterizing the deflection of the SCs (figure 1a).
The mean value calculated at the position 25, 50 and 75% of the parameter height, width and diameter of each canal, was calculated and used for analysis (figure 1b). Size independence of the investigated specimens was carried out with standardizations of the measurements with least squares linear regressions of the residuals of condylobasal length (CBL) to the respective measurements of the SCs before the calculation of the principal component analysis (PCA) [50,51]. In order to explore the functional morphological link between the shape of the SCs and the locomotion mode, a multivariate morphospace of the bony labyrinth was created with the dataset of measurements per species with IBM SPSS Statistics v. 20.0. For clarifying the functional relevance of the SCs to different modes of locomotion, the sensitivity of the SCs was calculated [27]: xmax = (ω/8 × v) × R × r2, where xmax = sensitivity [mm3 s−2]; ω = average value of angular velocity of the head = 1 rad s−1; v = kinematic viscosity of endolymph = 10−6 m s−1; R = radii of curvature of SC; r = diameter of SC.
The signal and effect of phylogenetic relationships is investigated with the Brownian motion model of evolution. To exclude a phylogenetical signal from the diameters of the SC, which have the highest loadings of the principal components, the phylogenetical independent contrasts, Bloomberg's K-value and Pagel's λ were analysed [52–55] with Mesquite v. 3.02 [56], Rstudio v. 0.98.1091 [57], and the R-packages ape 3.2 [55,58], phylobase 0.6.8 [59], grid [60] and phytools 0.4–45 [61] (for detailed list of settings and results, see the electronic supplementary material). Testing the morphological variability by investigating one specimen per species was already calculated in previous studies [8]. Testing for repeatability, all measurements (n = 38) of the bony labyrinth were captured twice at one skull of Funisciurus anerythrus and were investigated with a one-way analysis of variance with measuring repetitions (one-way within-subjects ANOVA: p < 0.05).
3. Results
Detailed measurements of each specimen and results of the effect of phylogeny are listed in the electronic supplementary material, table S1.
(a). Shape of the semicircular canals
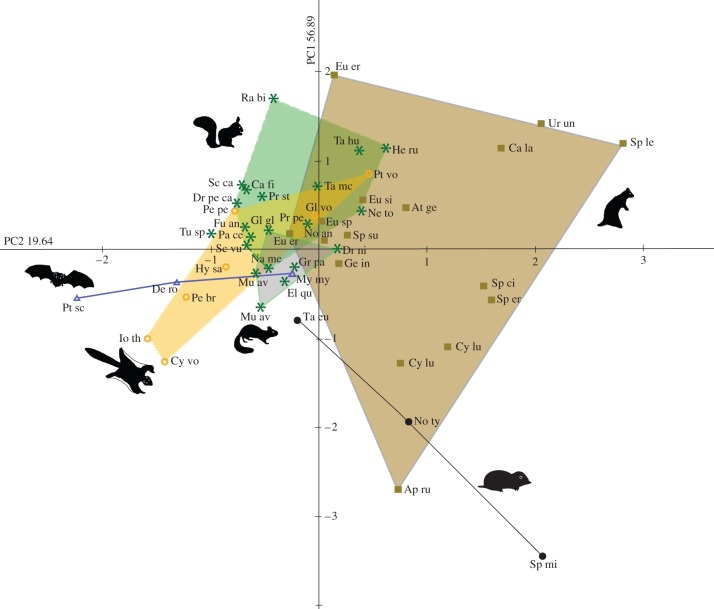
The shape of the bony labyrinth was investigated with PCA. The bony labyrinths in the squirrel-related are distinguishable by their shape and occupy distinct morphospaces (figure 2). The first principal component (PC1) is positively correlated with height, width and length of all three SCs, as well as the length of Cc and negatively correlated with the diameter of ASC, PSC and LSC. The second principal component (PC2) is positively correlated with the diameters and height of all three SCs, the width of PSC and LSC, the length of ASC, PSC and Cc, and negatively correlated with the width of ASC and the length of LSC. A significant morphological differentiation is indicated by MANOVA between the different locomotion types (Wilk's λ-test: value = 0.30, F = 3.301, p > 0.001).
Figure 2.
Shape differences of the bony labyrinth calculated with PCA in the squirrel-related clade and selected flying and subterranean taxa. 76.53% of the among group variance. Set of 13 variables. Dashed and solid lines delineate different locomotion types. Allocation of coloured morphospaces are as follows: blue area, flying taxa; brown area, fossorial taxa; green area, arboreal taxa; grey area, glirids; orange area, gliding taxa. Data points are labelled as follows: squares, fossorial; asterisks, arboreal; circles, gliding; triangles, flying. Abbreviations of the taxa are as follows and are sorted according to the respective orders resp. families: squirrel-related clade: Aplodontidae: Ap ru, Aplodontia rufa; Sciuridae: At ge, Atlantoxerus getulus; Ca la, Callospermophilus lateralis; Ca fi, Callosciurus finlaysonii; Cy le, Cynomys leucurus; Cy lu, Cynomys ludovicianus; Dr pe ca, Dremomys pernyi calidior; Eu sp, Eutamias sp.; Eu er, Euxerus erythropus; Eu si, Eutamias sibiricus; Fu an, Funisciurus anerythrus; Ge in, Geosciurus inauris; Gl vo, Glaucomys volans; He ru, Heliosciurus rufobrachium; Hy sa, Hylopetes sagitta; Io th, Iomys thompsonii; Na me, Nannosciurus melanotis; Ne to, Neotamias townsendii; No an, Notocitellus annulatus; Pa ce, Paraxerus cepapi; Pe pe, Petaurista petaurista; Pr pe, Prasadsciurus pennantii; Pr st, Protoxerus stangeri; Pt vo, Pteromys volans; Ra bi, Ratufa bicolor; Sc ca, Sciurus caroliensis; Sc vu, Sciurus vulgaris; Sp ci, Spermophilus citellus; Sp er, Spermophilus erythrogenys; Sp le, Spermophilopsis leptodactylus; Sp su, Spermophilus suslicus; Ta hu, Tamiasciurus hudsonicus; Ta mc, Tamiops mcclellandii; Ur un, Urocitellus undulatus; Xe in, Xerus inauris. Gliridae: Dr ni in, Dryomys nitedula intermedius; El qu, Eliomys quercinus; Gl gl, Glis glis; Gr pa, Graphiurus parvus; Mu av, Muscardinus avellanarius. Spalacidae: Sp mi, Spalax microphthalmus. Talpidae: Ta eu, Talpa europaea. Dermoptera: Cy vo, Cynocephalus volans; Chiroptera: De ro, Desmodus rotundus; My my, Myotis myotis; Pt sc, Pteropus scapulatus; Scandentia: Tu sp, Tupaia sp. Marsupialia: No ty, Notoryctes typhlops; Pe br, Petaurus breviceps. (Online version in colour.)
The range of the morphological space of the investigated fossorial species is larger than in the other taxa. An overlapping region contains taxa of arboreal sciurids (Heliosciurus rufobrachium, Neotamias townsendii, Tamiasciurus hudsonicus, Tamiops mcclellandii, Prasadsciurus pennanti), fossorial species (Eutamias sibiricus, Eutamias sp., Euxerus erythropus) and, additionally, gliding taxa (Glaucomys volans, Pteromy volans). Additionally, an overlapping morphospace with arboreal taxa (F. anerythrus, Paraxerus cepapi, Sciurus vulgaris, Nannosciurus melanotis) and the gliding taxon Petaurista petaurista is present.
The grouping of the investigated arboreal glirids is overlapping with the morphospaces of fossorial, arboreal and gliding sciurids. The distribution of the actively flying mammals and the subterranean taxa are represented in straight lines. The morphospace of the flying taxa is overlapping the gliding and arboreal species, with Myotis myotis found in the cluster of glirids. With the representative of marsupials, Notoryctes typhlops, the investigated subterranean taxa are overlapping with the fossorial species. The investigated specimen of Tupaia sp. is found in the morphospace of the actively flying mammals.
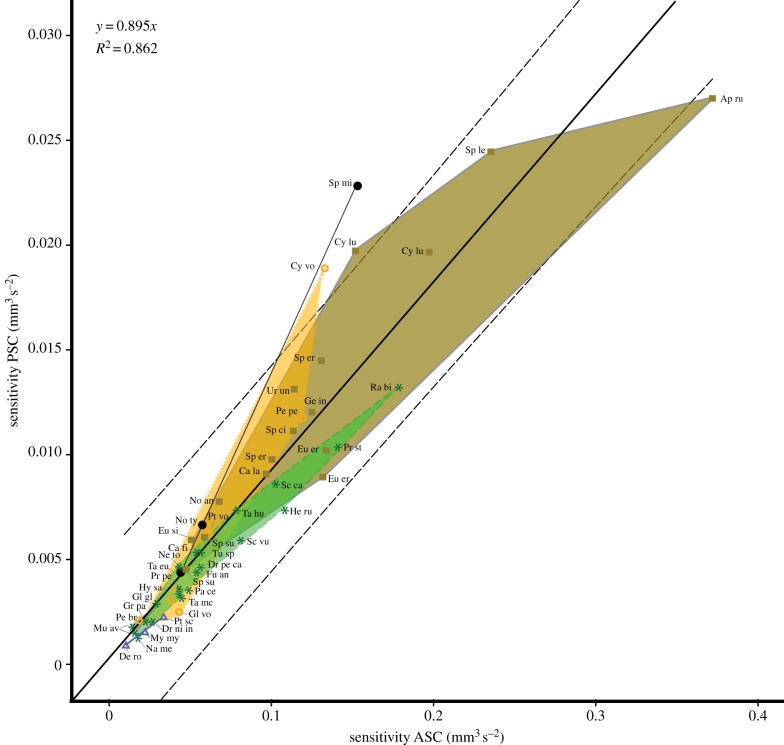
(b). Sensitivity of the semicircular canals depending on the locomotion mode
The sensitivities of the SCs show a correlation of ASC and PSC, with the highest sensibilities among the fossorial species and the lowest in the investigated actively flying mammals, the investigated specimens of the glirids Muscardinus avellanarius and the studied bat Desmodus rotundus (figure 3). Gliding, arboreal and subterranean taxa are found between these two extremes. The investigated arboreal species and the fossorial species Eux. erythropus and A. rufa are found below the regression line, whereas the fossorial and subterranean species are lying above the regression line. A correlation of the sensitivities between ASC/LSC and PSC/LSC is not present.
Figure 3.
Double logarithmic plots of sensitivity between PSC and ASC in the squirrel-related clade, gliding marsupial, and selected subterranean and flying taxa. Dotted lines: 95% CI. For data labels and coloured morphospaces, see figure 2. (Online version in colour.)
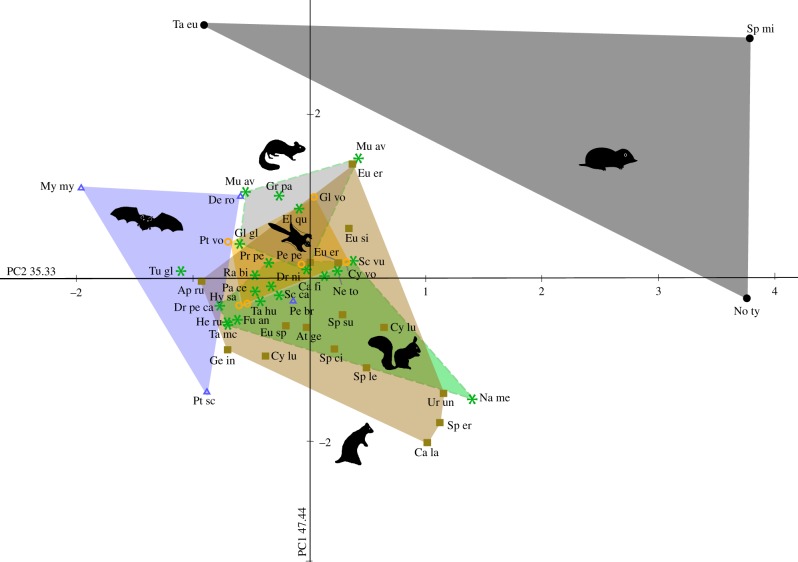
(c). Bias angle between semicircular canals
The intersections of the SCs are characterized with the bias angles of the respective best-fitting planes and were investigated with PCA (figure 4). PC1 is positively correlated with all bias angles between ASC/PSC, PSC/LSC and ASC/LSC. PC2 is positively correlated with the bias angle of ASC/PSC and ASC/LSC, and negatively correlated with PSC/LSC.
Figure 4.
Diagram of bias angles between the SCs. 82.77% of the among group variance. Dashed and solid lines delineate different locomotion types. For data labels and coloured morphospaces see figure 2. (Online version in colour.)
The investigated subterranean taxa represent a separate cluster, whereas the other investigated locomotion modes are overlapping (figure 4). Except for the sciurids Na. melanotis and Pteromys volans, the glirids Mu. avellanarius, Graphiurus parvus and Glis glis, as well as Tupaia sp., all investigated arboreal and gliding taxa are found in the morphospace of the fossorial taxa. The glirid Dryomys nitedula intermedius lies in the morphospace of sciurids. Tupaia sp. is found in the morphospace of the actively flying mammals.
4. Discussion
Our analyses show differences in morphometry and sensitivity of the inner ear in the investigated species of the squirrel-related clade and other mammalian representatives with different modes of locomotion.
(a). Functional morphological adaptation in the semicircular canals of the bony labyrinth
Fossorial squirrels can be distinguished from arboreal and gliding taxa by the height and diameter of the SCs, the width of the PSC and the LSC, the length of PSC and the length of the Cc. This functional differentiation in the squirrel-related clade is mainly caused by PC2, which has the highest loading on the diameters of the SCs. It can be assumed that the diameter of the SCs is the main factor for adaptive differentiation by investigating the vestibular system. To investigate the phylogenetic signal of this factor, the phylogenetic-independent contrast, Bloombergs’ K-value and Pagel's λ were calculated. The phylogenetic signal in the diameter of the canals cannot be entirely excluded, but between closely related species they resemble each other less than expected under the Brownian motion model. Nevertheless, the adaptive differentiation in the diameter of the SCs is in contrast to previous studies. Either the correlation of radii of curvature to streamline length of each SC [6], or the correlation of radii of curvature to the respective body mass or body size of the investigated mammals were used to detect locomotory adaptations [2–4,62,63]. But rodents are characterized by seasonal changes of weight [64,65], so using the body mass for functional morphological correlations as done in previous studies is not compatible. Therefore, we used the CBL for previous standardization and calculation of the residuals before the statistical analyses.
In the PCAs, we found overlaps of the different morphospaces. The investigated specimens of Eux. erythropus are not found next to each other, as is seen in the investigated specimens of Cynomys ludovicianus. The former is living in open woodland, but it can also occur in a wide range of biotopes [66,67], which may cause a high variability of the vestibular system.
The potential locomotory distinction between arboreal and fossorial sciurids is not just observed in the structure of the SCs. It was also found morphologically in the shape of the tail [68,69], the length of the limbs relative to the vertebral column [70], the social behaviour [71] and the size of the brain [72,73]. The latter suggests a high degree of specialized brain regions, which might correlate with arboreality but also with sociality [74]. Studying the ecological value of the teeth in sciurids is contradictory and does not represent the same ecological indicator as the inner ear. Ecological differences between frugivorous tree squirrels and herbivorous ground squirrels were found by investigating the microwear of teeth [75]. But also a similar generalized dental morphology in sciurids caused by a diet that varies independently from terrestrial, arboreal and gliding habits was postulated [76]. Overall, the anatomy of the inner ear can be used for ecological distinction and respective locomotion modes of sciurids, whereas using the morphology of teeth cannot distinguish between different ecological types of squirrels.
(b). Different sensitivities in the semicircular canals in relation to the locomotion mode
The sensitivities between ASC and PSC are correlated in relation to the locomotion mode in the squirrel-related clade. The highest sensibility is found in the fossorial species and the lowest in the investigated active mammals and glirids, but also in individual sciurids. In the analyses of the inner ear sensitivity, the size of the investigated specimens is not excluded, so the sensitivity of the SCs is not dependent on the size of the specimens [77]. The sensitivity of the inner ear in fossorial sciurids is graduated and higher than in arboreal and gliding squirrels as well as in bats. Previous studies proposed size-dependent differences of the mean radius of the three SCs respective to the body mass as a proxy for the sensitivity of the vestibular system [4,9,21,24,62]. However, these results were contradictory. Larger radius of curvature, higher sensitivity of the SCs and slower movements of the head were found in large animals. But once body size is eliminated, a larger radius of canals is found in faster-moving investigated primates than in slower-moving species [3,5,78,79]. The overall results of these studies indicate differences in the sensitivity of the vestibular system based on the radius of the curvature. However, the results of the distinction of the sensitivity in the squirrel-related clade are caused by the diameter of the SC. Using the equation of sensitivity [27], the diameter of the SC is squared and represents the main component of this formula. However, this has to be validated in further studies and additional analyses [7].
Nevertheless, it can be assumed that preventing an overstimulation of the sense organ in gliding and actively flying mammals is caused by lesser sensitivity of the vestibular system [24,77]. During locomotion, visual signals and information of motion of the three-dimensional space have to be integrated by the brain [80–83]. Therefore, the nervous system of gliding sciurids and actively flying mammals is flooded with information of the inner ear and the changes of the retinal images during locomotion. To prevent an overstimulation during locomotion, the vestibular system of gliding and flying taxa is less sensitive than in fossorial taxa [3]. Furthermore, this result is supported by differences of the sensitivity in the inner ear of P. petaurista and G. volans. Our results suggest that the inner ear of the red giant flying squirrel, P. petaurista, is less sensitive than the inner ear of the southern flying squirrel, G. volans. Caused by the aerodynamic separation, the gliding distance of these two taxa is different. In contrast to smaller flying squirrels, the gliding distance of larger specimens of the flying sciurids is longer and the respective velocity is much faster to maximize their gliding ratio [84]. Additionally, small gliding squirrels require precise manoeuvrability otherwise the gliding phase is affected by air turbulence. Comparing the trend of enlargement of diameter of the SCs of the fossorial to the subterranean taxa and the respective increase of sensitivity, it can be assumed that the vestibular system of subterranean taxa is much more sensitive than in fossorial ones [85].
(c). Different bias angle of semicircular canals in relation to locomotion mode and phylogeny
By investigating the bias angle of the SCs, we detect anatomical differences between taxa with different locomotion modes. Subterranean mammals can be clearly separated from fossorial, arboreal and flying mammals. Arboreal sciurids are completely found in the morphospace of fossorial species but can be clearly separated from arboreal glirids. This distinction between sciurids and glirids and the highly overlapping morphospaces of all investigated sciurids, indicate a phylogenetic signal in the bias angle of the SCs, which is also seen in middle ear characteristics [86,87].
In conclusion, our study demonstrates that inner ear morphology is influenced by the locomotory adaptation and finally refutes Hyrtl's (1873) hypothesis [1, p. 228], who denied this interrelationship. Furthermore, our study provides a base for further investigations on bony labyrinths and locomotory adaptations of extant and extinct mammals.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the following people for providing access to specimens for scanning: C. Beard, A. Henrici and M. Dawson (all Carnegie Museum of Natural History, Pittsburgh), R. Hutterer (Forschungsmuseum Alexander Koenig, Bonn), L. Costeur (Naturhistorisches Museum Basel), W. Maier and E. Weber (Universität Tübingen), G. Rößner (Bayerische Staatssammlung für Paläontologie und historische Geologie, München), F. Zachos and A. Bibl (both Naturhistorisches Museum, Wien). We thank J. Schultz, R. Schellhorn (both Steinmann-Institut, Bonn), G. Billet (Natural History Museum, Paris), U. Anders and J. Kriwet (Institute for Palaeontology, Vienna) for helpful discussions. Many thanks to two anonymous reviewers whose comments helped us to substantially improve the manuscript.
Data accessibility
The dataset supporting this article is available from the Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.dc965.
Authors' contributions
C.P. made the µ-CT scans and the three-dimensional reconstructions, carried out the statistical analyses and drafted the manuscript; T.M. participated in the design of the study, interpretation of the data and helped draft the manuscript; I.R. participated in the data analysis and design of the study, and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This research was supported by FAZIT foundation (Frankfurt am Main, Germany) to C.P.
References
- 1.Hyrtl J. 1873. Die Corrosions-Anatomie und ihre Ergebnisse: mit 18 chromolithographirten Tafeln. Vienna, Austria: Wilhelm Braumüller. [Google Scholar]
- 2.Spoor F, Wood B, Zonneveld F. 1994. Implications of the early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature 369, 645–648. ( 10.1038/369645a0) [DOI] [PubMed] [Google Scholar]
- 3.Spoor F, Zonneveld F. 1998. Comparative review of the human bony labyrinth. Yearb. Phys. Anthropol. 4, 211–251. () [DOI] [PubMed] [Google Scholar]
- 4.Spoor F, Garland T, Krovitz G, Ryan TM, Silcox MT, Walker A. 2007. The primate semicircular canal system and locomotion. Proc. Natl Acad. Sci. USA 104, 10 808–10 812. ( 10.1073/pnas.0704250104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silcox MT, Bloch JI, Boyer DM, Godinot M, Ryan TM, Spoor F, Walker A. 2009. Semicircular canal system in early primates. J. Hum. Evol. 56, 315–327. ( 10.1016/j.jhevol.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 6.Cox PG, Jeffrey N. 2010. Semicircular canals and agility: the influence of size and shape measures. J. Anat. 216, 37–47. ( 10.111/j.1469-7580.2009.01172.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David R, Droulez J, Allain R, Berthoz A, Janvier P, Bennequin D. 2010. Motion from the past. A new method to infer vestibular capacities of extinct species. C. R. Palevol. 9, 397–410. ( 10.1016/j.crpv.2010.07.012) [DOI] [Google Scholar]
- 8.Billet G, Hautier L, Asher RJ, Schwarz C, Crumpton N, Martin T, Ruf I. 2012. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc. R. Soc. B 279, 3932–3939. ( 10.1098/rspb.2012.1212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malinzak MD, Kay RF, Hullar TE. 2012. Locomotor head movements and semicircular canal morphology in primates. Proc. Natl Acad. Sci. USA 109, 17 914–17 919. ( 10.1073/pnas.1206139109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan TM, et al. 2012. Evolution of locomotion in Anthropoidea: the semicircular canal evidence. Proc. R. Soc. B 279, 3467–3475. ( 10.1098/rspb.2012.0939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlin JC, Kirk EC, Rowe TB. 2013. Functional implications of ubiquitous semicircular canal non-orthogonality in mammals. PLoS ONE 8, e79585 ( 10.1371/journal.pone.0079585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgi JA, Sipla JS, Forster CA. 2013. Turning semicircular canal function on its head: dinosaurs and a novel vestibular analysis. PLoS ONE 8, e58517 ( 10.1371/journal.pone.0058517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maddin HC, Sherratt E. 2014. Influence of fossoriality on inner ear morphology: insights from caecilian amphibians. J. Anat. 225, 83–93. ( 10.1111/joa.12190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi M. 1967. Dimensional study of the vestibular apparatus. Laryngoscope 77, 1806–1817. ( 10.1288/00005537-19671000-00003) [DOI] [PubMed] [Google Scholar]
- 15.Wersäll J, Bagger-Sjöbäck D. 1974. Morphology of the vestibular sense organ. In Handbook of sensory physiology: vestibular system (ed. Kornhuber HH.), pp. 123–170. New York, NY: Springer. [Google Scholar]
- 16.Lowenstein O, Saunders RD. 1975. Otolith-controlles response from the first order neurons of the labyrinth of the bullfrog to changes in linear acceleration. Proc. R. Soc. Lond. B 191, 475–505. ( 10.1098/rspb.1975.0140) [DOI] [PubMed] [Google Scholar]
- 17.Igarashi M, O-Uchi T, Alford BR. 1981. Volumetric and dimensional measurements of vestibular structures in the squirrel monkey. Acta Oto-Laryngol. 91, 437–444. ( 10.3109/00016488109138525) [DOI] [PubMed] [Google Scholar]
- 18.Blanks RHI, Curthoys IS, Bennett ML, Markham CH. 1985. Planar relationships of the semicircular canals in rhesus and squirrel monkeys. Brain Res. 340, 315–324. ( 10.1016/0006-8993(85)90928-X) [DOI] [PubMed] [Google Scholar]
- 19.Curthoys IS, Oman CM. 1987. Dimensions of the horizontal semicircular duct, ampulla and utricle in the human. Acta Oto-Laryngol. 103, 254–261. ( 10.3109/00016488709107791) [DOI] [PubMed] [Google Scholar]
- 20.Ghanem TA, Rabbitt RD, Tresco PA. 1998. Three-dimensional reconstruction of the membranous vestibular labyrinth in the toadfish, Opsanus tau. Hearing Res. 124, 27–43. ( 10.1016/s0378-5955(98)00108-7) [DOI] [PubMed] [Google Scholar]
- 21.Gray AA. 1907. The labyrinth of animals: including mammals, birds, reptiles and amphibians, vol. I. London, UK: Churchill. [Google Scholar]
- 22.Turkewitsch BG. 1935. Comparative anatomical investigation of the osseous labyrinth (vestibule) in mammals. Am. J. Anat. 57, 503–543. ( 10.1002/aja.1000570305) [DOI] [Google Scholar]
- 23.Hadziselimovic H, Savkovic LJ. 1964. Appearance of semicircular canals in birds in relation to mode of life. Acta Anat. 57, 306–315. ( 10.1159/000142559) [DOI] [PubMed] [Google Scholar]
- 24.Spoor F. 2003. The semicircular canal system and locomotor behaviour, with special reference to hominid evolution. Cour. Forsch. Senckenberg 243, 93–104. [Google Scholar]
- 25.Antonia RA, Teitel M, Kim J, Browne LWB. 1992. Low-Reynolds-number-effects in a fully developed turbulent channel flow. J. Fluid Mech. 236, 579–605. ( 10.1017/S002211209200154X) [DOI] [Google Scholar]
- 26.Oman CM, Marcus EN, Curthoys IA. 1987. The influence of semicircular canal morphology on endolymph flow dynamics. An anatomically descriptive mathematical model. Acta Oto-Laryngol. 103, 1–13. ( 10.3109/00016488709134691) [DOI] [PubMed] [Google Scholar]
- 27.Muller M. 1999. Size limitations in semicircular duct systems. J. Theor. Biol. 198, 405–437. ( 10.1006/jtbi.1999.0922) [DOI] [PubMed] [Google Scholar]
- 28.Billet G, Germain D, Ruf I, de Muizon C, Hautier L. 2013. The inner ear of Megatherium and the evolution of the vestibular system in sloths. J. Anat. 223, 557–567. ( 10.1111/joa.12114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekdale EG, Rowe T. 2011. Morphology and variation within the bony labyrinth in zhelestids (Mammalia, Eutheria) and other therian mammals. J. Vertebr. Paleontol. 31, 658–675. ( 10.1080/02724634.2011.557284) [DOI] [Google Scholar]
- 30.Spoor F, Bajpal S, Hussain ST, Kumar K, Thewissen JGM. 2002. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature 417, 163–166. ( 10.1038/417163a) [DOI] [PubMed] [Google Scholar]
- 31.Nowak RM, Wilson DE. 1999. Walker’s mammals of the world, 6th edn Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 32.Steppan SJ, Storz BL, Hoffmann RS. 2004. Nuclear DNA phylogeny of the squirrels (Mammalia: Rodentia) and the evolution of arboreality from c-myc and RAG1. Mol. Phylogenet. Evol. 30, 703–719. ( 10.1016/S1055-7903(03)00204-5) [DOI] [PubMed] [Google Scholar]
- 33.Huchon D, Madsen O, Sibbald MJJB, Ament K, Stanhope MJ, Catzeflis F, DeJong WW, Douzery EJP. 2002. Rodent phylogeny and a timescale for the evolution of Glires: evidence from an extensive taxon sampling using three nuclear genes. Mol. Biol. Evol. 19, 1053–1065. ( 10.1093/oxfordjournals.molbev.a004164) [DOI] [PubMed] [Google Scholar]
- 34.Adkins RM, Walton AH, Honeycutt RL. 2003. Higher-level systematics of rodents and divergence time estimates based on two congruent nuclear genes. Mol. Phyolgenet. Evol. 26, 409–420. ( 10.1016/S1055-7903(02)00304-4) [DOI] [PubMed] [Google Scholar]
- 35.Montgelard C, Forty E, Arnal V, Matthee CA. 2008. Suprafamilial relationships among rodentia and the phylogenetic effect of removing fast-evolving nucleotids in mitochondrial, exon and intron fragments. BMC Evol. Biol. 8, 1–16. ( 10.1186/1471-2148-8-321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanga-Kanfi S, Miranda H, Penn O, Pupko T, DeBry RW, Huchon D. 2009. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 9, 1–12. ( 10.1186/1471-2148-9-71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavocat RRM, Parent J-P. 1985. Phylogenetic analysis of middle ear feature in fossil and living rodents. In Evolutionary relationships among rodents: a multidisciplinary analysis. (eds Luckett WP, Hartenberger J-L.), pp. 685–713. New York, NY: Plenum Press. [Google Scholar]
- 38.Vianey-Liaud M. 1985. Possible evolutionary relationships among Eocene and lower Oligocene rodents of Asia, Europe and North America. In Evolutionary relationships among rodents: a multidisciplinary analysis (eds Luckett WP, Hartenberger J-L.), pp. 277–310. New York, NY: Plenum Press. [Google Scholar]
- 39.Michaux J, Hautier L, Simonin T, Vianey-Liaud M. 2008. Phylogeny, adaptation and mandible shape in Sciuridae (Rodentia, Mammalia). Mammalia 72, 286–296. ( 10.1515/MAMM.2008.049) [DOI] [Google Scholar]
- 40.Wilson DE, Reeder DAM. 2005. Mammal species of the world. A taxonomic and geographic reference, 3rd edn Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 41.Thorington RW, Darrow K, Betts ADK. 1997. Comparative myology of the forelimb of squirrels (Sciuridae). J. Morphol. 234, 155–182. () [DOI] [PubMed] [Google Scholar]
- 42.Hopkins SSB. 2008. Phylogeny and evolutionary history of the Aplodontoidea (Mammalia: Rodentia). Zool. J. Linn. Soc. Lond. 153, 769–838. ( 10.1111/j.1096-3642.2011.00757.x) [DOI] [Google Scholar]
- 43.Huchon D, Chevret P, Jordan U, Kilpatrick CW, Ranwez V, Jenkins PD, Brosius J, Schmitz J. 2007. Multiple molecular evidences for a living mammalian fossil. Proc. Natl Acad. Sci. USA 104, 7495–7499. ( 10.1073/pnas.0701289104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tullberg T. 1899. Ueber das System der Nagethiere-eine phylogenetische Studie. Upsala, MN: Akademische Buchdruckerei Berling. [Google Scholar]
- 45.Simpson GG. 1945. The principles of classification and a classification of mammals. Bull. Am. Mus. Nat. Hist. 85, 1–367. [Google Scholar]
- 46.Bugge J. 1985. Systematic value of the carotid arterial pattern in rodents. New York, NY: Plenum Press. [Google Scholar]
- 47.Maier W, Klingler P, Ruf I. 2002. Ontogeny of the medial masseter muscle, pseudo-myomorphy, and the systematic position of the Gliridae (Rodentia, Mammalia). J. Mammal. Evol. 9, 253–269. ( 10.1023/A:1023979212759) [DOI] [Google Scholar]
- 48.Samuels JX, Van Valkenburgh BV. 2008. Skeletal indicators of locomotor adaptations in living and extinct rodents. J. Morphol. 269, 1387–1411. ( 10.1002/jmor.10662) [DOI] [PubMed] [Google Scholar]
- 49.Ekdale EG. 2010. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anat. Rec. 293, 1896–1912. ( 10.1002/ar.21234) [DOI] [PubMed] [Google Scholar]
- 50.Garland T, Dickerman AW. 1993. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292. ( 10.1093/sysbio.42.3.265) [DOI] [Google Scholar]
- 51.Mason MJ. 2001. Middle ear structures in fossorial mammals: a comparison with non-fossorial species. J. Zool. 255, 467–486. ( 10.1644/07-mamm-a-401.1) [DOI] [Google Scholar]
- 52.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 53.Pagel M. 1994. Detecting correlated evolutions on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 54.Bloomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1554/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 55.Paradis E. 2012. Analysis of phylogenetics and evolution with R, 2nd edn New York, NY: Springer. [Google Scholar]
- 56.Maddison WP, Maddison DR. 2015. Mesquite: a modular system for evolutionary analysis, v. 3.02 See http://mesquiteproject.org.
- 57.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org). [Google Scholar]
- 58.Paradis E, Claude J, Strimmer K. 2004. APE: analysis of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 59.Hackethon R, et al. 2014. Phylobase: Base package for phylogenetic structures and comparative dare See http://Phylobase.r-forge.r-project.org/.
- 60.Murrell P. 2005. R graphics. London, UK: Chapman & Hall/CRCPress. [Google Scholar]
- 61.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.0069.x) [DOI] [Google Scholar]
- 62.Jones GM, Spells KE. 1962. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc. R. Soc. Lond. B 157, 403–419. ( 10.1098/rspb.1963.0019) [DOI] [PubMed] [Google Scholar]
- 63.Yang A, Hullar TE. 2007. Relationship of semicircular canal size to vestibular- nerve afferent sensitivity in mammals. J. Neurophysiol. 98, 3197–3205. ( 10.1152/jn.00798.2007) [DOI] [PubMed] [Google Scholar]
- 64.Jameson EW, Mead RA. 1964. Seasonal changes in body fat, water and basic weight in Citellus lateralis, Eutamias speciosus and E. amoenus. J. Mammal. 45, 359–365. ( 10.2307/1377407) [DOI] [Google Scholar]
- 65.Armitage KB, Downhower JF, Svendsen GE. 1976. Seasonal changes in weigths of marmots. Am. Midl. Nat. 96, 36–51. ( 10.2307/2424566) [DOI] [Google Scholar]
- 66.Linn I, Key G. 1996. Use of space by the African striped ground squirrel Xerus erythropus. Mamm. Rev. 26, 9–26. ( 10.1111/j.1365-2907.1996.tb00144.x) [DOI] [Google Scholar]
- 67.Herron MD, Waterman JM. 2004. Mammalian species: Xerus erythropus. Am. Soc. Mammal. 748, 1–4. ( 10.1644/748) [DOI] [Google Scholar]
- 68.Scheibe JS, Paskins KE, Ferdous S, Birdsill D. 2007. Kinematics and functional morphology of leaping and branch use in Glaucomys sabrinus. J. Mammal. 88, 850–861. ( 10.1644/06-mamm-s-331r1.1) [DOI] [Google Scholar]
- 69.Hayssen V. 2008. Patterns of body and tail length and body mass in Sciuridae. J. Mammal. 89, 852–873. ( 10.1644/07-mamm-a-217.1) [DOI] [Google Scholar]
- 70.Essner JRL. 2003. Locomotion, morphology, and habitat use in arboreal squirrels (Rodentia, Sciuridae), 134p PhD dissertation, College of Arts and Sciences of Ohio University, Athens, OH, USA. [Google Scholar]
- 71.Thorington RWJ, Ferell K. 2006. Squirrels: the animal answer guide. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 72.Roth VL, Thorington RW. 1982. Relative brain size among African squirrels. J. Mammal. 63, 168–173. ( 10.2307/1380690) [DOI] [Google Scholar]
- 73.Meier PT. 1983. Relative brain size within North American Sciuridae. J. Mammal. 64, 642–647. ( 10.2307/1380520) [DOI] [Google Scholar]
- 74.Mace GM, Eisenberg JF. 1982. Competition, niche specialization and the evolution of brain size in the genus Peromyscus. Biol. J. Linn. Soc. 17, 243–257. ( 10.1111/j.1095-8312.1982.tb02019.x) [DOI] [Google Scholar]
- 75.Nelson S, Badgley C, Zakem E. 2005. Microwear in modern squirrels in relation to diet. Palaeontol. Electron 8, 1–15. [Google Scholar]
- 76.Thorington RW, Schennum CE, Pappas LA, Pitassy D. 2005. The difficulties of identifying flying squirrels (Sciuridae: Pteromyini) in the fossil record. J. Vertebr. Paleontol. 25, 950–961. ( 10.1671/0272-4634(2005)025[0950:TDOIFS]2.0.CO;2) [DOI] [Google Scholar]
- 77.Schutz H, Jamniczky HA, Hallgrímsson B, Garland T., Jr 2014. Shape-shift: semicircular canal morphology responds to selective breeding for increased locomotor activity. Evolution 68, 3184–3198. ( 10.1111/evo.12501) [DOI] [PubMed] [Google Scholar]
- 78.Matano S, Kubo T, Niemitz C, Guenther M. 1985. Semicircular canal organ in 3 primate species and behavioral correlations. Forts. Zool. 30, 677–680. [Google Scholar]
- 79.Matano S, Kubo T, Matsunaga T, Niemitz C, Günther M. 1986. On the size of the semicircular canal organ in Tarsius bancanus. In Current perspective in primate biology (eds Taub DM, King FA.), pp. 122–129. New York, NY: Van Nostrand Reinhold. [Google Scholar]
- 80.Simpson J, Graf W. 1981. Eye-muscle geometry and compensatory eye movements in lateral-eyed and frontal-eyed animals. Ann. NY Acad. Sci. 374, 20–30. ( 10.1111/j.1749-6632.1981.tb30856.x) [DOI] [PubMed] [Google Scholar]
- 81.Frost BJ, Wylie DR, Wang YC. 1994. Perception and motor control in birds. Berlin, Germany: Springer. [Google Scholar]
- 82.Cox PG, Jeffrey N. 2007. Morphology of the mammalian vestibulo-ocular reflex: the spatial arrangment of the human fetal semicircular canals and extraocular muscles. J. Morphol. 268, 878–890. ( 10.1002/jmor.10559) [DOI] [PubMed] [Google Scholar]
- 83.Cox PG, Jeffrey N. 2008. Geometry of the semicircular canals and extraocular muscles in rodents, lagomorphs, felids and modern humans. J. Anat. 213, 583–596. ( 10.1111/j.1469-7580.2008.00983.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thorington RW, Heaney LR. 1981. Body proportions and gliding adaptations of flying squirrels (Petauristinae). J. Mammal. 62, 101–114. ( 10.2307/1380481) [DOI] [Google Scholar]
- 85.McVean A. 1999. Are the semicircular canals of the European mole, Talpa europaea, adapted to a subterranean habitat? Comp. Biochem. Phys. A 123, 173–178. ( 10.1016/S1095-6433(99)00047-1) [DOI] [PubMed] [Google Scholar]
- 86.Bugge J. 1974. The cephalic arterial system in insectivores, primates, rodents and lagomorphs, with special reference to the systematic classification. Basel, Switzerland: S. Karger. [PubMed] [Google Scholar]
- 87.Farr MRB, Mason MJ. 2008. Middle ear morphology in dormice (Rodentia: Gliridae). Mamm. Biol. 73, 330–334. ( 10.1016/j.mambio.2007.02.010) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is available from the Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.dc965.