Abstract
Faeces-mediated transmission of Trypanosoma cruzi (the aetiological agent of Chagas disease) by triatomine insects is extremely inefficient. Still, the parasite emerges frequently, and has infected millions of people and domestic animals. We synthesize here the results of field and laboratory studies of T. cruzi transmission conducted in and around Arequipa, Peru. We document the repeated occurrence of large colonies of triatomine bugs (more than 1000) with very high infection prevalence (more than 85%). By inoculating guinea pigs, an important reservoir of T. cruzi in Peru, and feeding triatomine bugs on them weekly, we demonstrate that, while most animals quickly control parasitaemia, a subset of animals remains highly infectious to vectors for many months. However, we argue that the presence of these persistently infectious hosts is insufficient to explain the observed prevalence of T. cruzi in vector colonies. We posit that seasonal rains, leading to a fluctuation in the price of guinea pig food (alfalfa), leading to annual guinea pig roasts, leading to a concentration of vectors on a small subpopulation of animals maintained for reproduction, can propel T. cruzi through vector colonies and create a considerable force of infection for a pathogen whose transmission might otherwise fizzle out.
Keywords: bottleneck, guinea pigs, Triatoma infestans, Trypanosoma cruzi, Chagas disease
1. Introduction
Chagas disease affects millions of people across Latin America. The transmission of the aetiological agent, Trypanosoma cruzi, to mammalian hosts via contact with the faeces of infected triatomine insects was described over 100 years ago [1]. While the parasite can be acquired orally through the ingestion of insects or foodstuffs contaminated with insect faeces, congenitally, or through blood transfusions, vector-borne transmission is responsible for most cases [2]. Vector-borne transmission occurs when contaminated triatomine faeces enter the host through the bite site or mucous membranes. This mode of transmission is very inefficient—for transmission to be successful, not only must the insect defecate on the host during or shortly after feeding, but the host must also inadvertently scratch the parasite through the bite site or otherwise bring it into contact with a mucous membrane [3,4]. Previous works have estimated that more than 1400 bites from infected triatomines are necessary, on average, to cause one new T. cruzi infection in guinea pigs [5], while more than 1700 bites are needed to infect one human [4].
The inefficiency of transmission from vectors to hosts leads to an as yet unresolved paradox in models of T. cruzi transmission. Although T. cruzi is extremely difficult to contract, in many communities, the infection prevalence in humans exceeds 40% [6–9]. Such prevalence could potentially rise if a large number of infectious vectors were in close contact with a host population. But how could the number of infected vectors reach such levels when it is so difficult for hosts to become infected?
Here, we describe our detection of highly infected colonies of the triatomine vector Triatoma infestans, often with parasite prevalence reaching 80%, through a cross-sectional survey conducted around the city of Arequipa, Peru (figure 1). All of the highly infected colonies were collected from pens of domestic guinea pigs. Guinea pigs are commonly raised for human consumption in the region. In the following sections, we present several alternative hypotheses to explain the high T. cruzi prevalence in these insects. We explore each hypothesis through mathematical models based on Ross and MacDonald's models of Malaria transmission [10–13]. Guided by the output of these models, we perform a series of laboratory studies to assess the alternative mechanisms by which parasite infection of vectors could reach the high levels observed in our field studies.
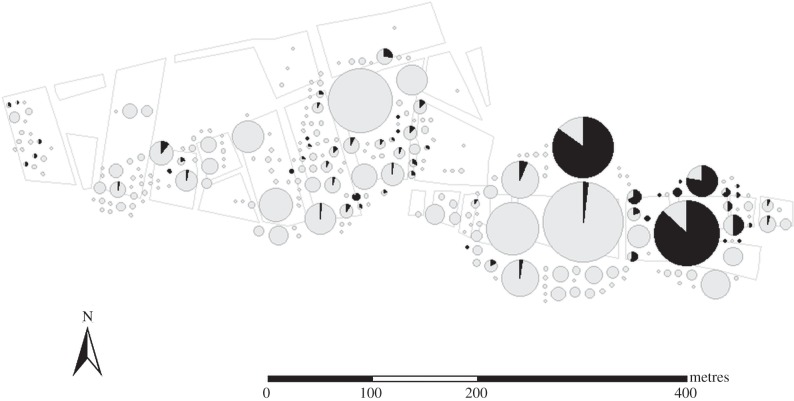
Figure 1.
Spatial distribution of T. cruzi infection status of T. infestans colonies collected from the community of Villa La Joya, Arequipa, Peru, between Aug and Nov of 2008. Vector colonies are represented by pie charts scaled by the number of vectors (ranging from 1 to 1414) collected at each site. The proportion of T. cruzi-infected triatomines in each colony is shown in black.
(a). Hypothesis I: Trypanosoma cruzi is transmitted among insects, without passing through an intermediate mammalian reservoir host, via coprophagy
One possible process that could augment the prevalence of the parasite in vector colonies is direct transmission among insects without the need for an intermediate mammalian host. The ingestion of faeces, known as coprophagy, is one mechanism by which such horizontal transmission could occur. Triatoma infestans relies on symbiotic bacteria in its gut to digest bloodmeals [14,15]. The insects are not born with these symbionts; rather, they acquire them by ingesting faeces of older bugs [16]. An isolated observation [17] raised the possibility that T. infestans also could acquire T. cruzi by this route, though the frequency of such infections seemed low. Still, the efficiency of transmission via coprophagy might be strain dependent, and a particular strain of T. cruzi may have adapted to coprophagic transmission. To assess the potential of coprophagic transmission on transmission dynamics, we modify the Ross–MacDonald model and calculate the coprophagic force of infection necessary to produce the observed parasite prevalence in vector populations. We then conduct an experiment to test whether such values of coprophagic force of infection might occur.
In addition to coprophagy, there is the possibility that T. cruzi might be transmitted from one insect to another via ‘kleptohaemodeipnonism’, which is a behaviour by which an unfed nymph pierces the exoskeleton of a fed nymph and feeds on its haemolymph [18]. A review of the role of kleptohaemodeipnonism in the transmission of T. cruzi by T. infestans concluded that it was unlikely to be of epidemiological importance [19] due to the rarity of the behaviour. Based on our observations, the behaviour is equally rare among the local T. infestans; we therefore did not investigate whether kleptohaemodeipnonism might have led to the high prevalence of T. cruzi among the vector populations we observed.
(b). Hypothesis II: a reservoir species other than guinea pigs is driving the transmission of Trypanosoma cruzi through vectors collected near guinea pig pens
Further contributing to the paradox of highly infected vector populations in guinea pig pens is the low prevalence of infection among guinea pigs in these pens. In our surveys, we found only between 2 and 5% of animals to show serological signs of infection, as assessed through enzyme-linked immunosorbent assays. Based on these results, we hypothesized that vectors may be becoming infected when feeding on another host species, perhaps other rodents drawn to the food placed in these enclosures [20]. To test this possibility, we performed molecular analysis of the bloodmeals ingested by insects collected from highly infected vector colonies.
(c). Hypothesis III: persistently parasitic hosts drive the parasite through vector populations
After first acquiring T. cruzi from a vector, mammalian hosts undergo a temporary period of parasitaemia during which circulating parasites are present in peripheral blood. During this period of parasitaemia, which is typically brief, the animals are capable of infecting other vectors. Previous studies of T. cruzi transmission in animals including guinea pigs [21], mice [22] and dogs [23] have largely been conducted in the absence of vectors, with the exception of the controlled application of insects for xenodiagnosis [24], and must therefore rely on measures of parasites in peripheral blood as proxies of infectiousness to vectors. We reasoned that such a proxy might be imperfect, and mask significant heterogeneity among hosts in their ability to infect insects. We therefore performed studies to measure the number of triatomines infected by different guinea pigs over the course of infection. We document significant heterogeneity among animals in their ability to control the parasite and identify a number of persistently infectious individuals that infected vectors long after other animals had cleared the parasite. We hypothesize that the presence of such persistently infectious guinea pigs in field populations could strongly enhance transmission and vector infection prevalence above and beyond that expected in a homogeneous host population. To test this hypothesis, we use the Ross–MacDonald model [12,13] and include heterogeneity among hosts in their duration of infectiousness. We then assess whether such hosts could drive the parasite to the high levels observed in vector colonies collected in the field.
(d). Hypothesis IV: seasonal culling of host populations drives the parasite through insect colonies by concentrating vectors upon a small number of hosts
Guinea pigs are fed mainly on alfalfa, which contains virtually all the nutrients they need for optimal growth [25]. Alfalfa is a notoriously water-dependent crop [26], and its price fluctuates strongly with water availability. In 2009, the year following our vector sampling, the price of alfalfa tripled during the months of May, June and July (figure 2). Based on focus groups and in-depth interviews conducted during the study [27], the tripling of alfalfa prices led many residents to kill most of their guinea pigs, retaining just a few in order to be able to replenish the population when doing so became economically feasible. We evaluate the effect of these host bottlenecks through a simulation based on the Ross–MacDonald model.
Figure 2.
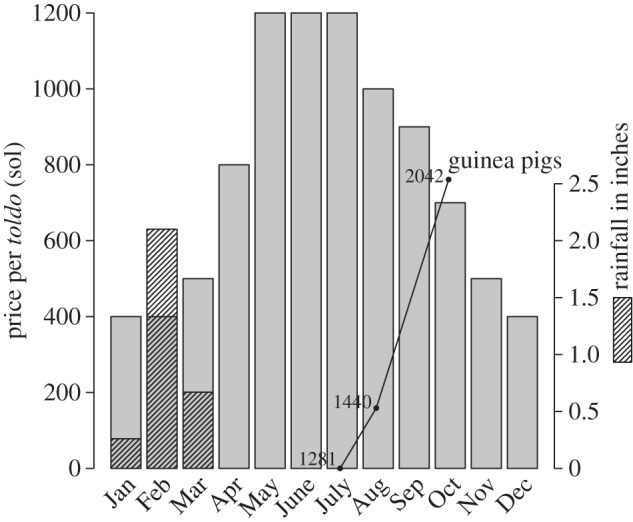
Monthly alfalfa prices in Arequipa, Peru, during the year 2009. Prices are in the Peruvian currency (the sol) and are measured per toldo (literally ‘tarp’), a local measure used for alfalfa transactions. Monthly rainfall in Arequipa for the same year (in inches) is shown in the hashed bars, with the scale provided on the right axis. The guinea pig population of the study area, estimated by surveys conducted in July, Aug and Oct of the previous year, is overlaid to demonstrate the inverse correlation with the yearly trend in the price of alfalfa. The guinea pig data are on the scale of the left axis, but vertically offset so that the population size in July lies on the axis.
2. Material and methods
(a). Field data
Entomological surveys were conducted in the La Joya district of Arequipa, Peru, between August and November of 2008. A one-person-hour search for triatomines, including systematic examination of human and animal living spaces, was conducted in all participating households [28]. A Tetramethrin spray (Sapolio Mata Moscas©) was used to flush out insects from their hiding places. Captured T. infestans were transported to the Arequipa-based Universidad Peruana Cayetano Heredia/University of Pennsylvania zoonotic disease field laboratory. The number, life stage and sex (for adults) of the collected insects were documented by site of collection. The intestinal contents of insects were examined microscopically at 400× for the presence of T. cruzi [29,30].
(b). Experiment 1: Trypanosoma cruzi transmission via coprophagy
To estimate the rate of T. cruzi transmission via coprophagy, we placed 30 colony-reared T. infestans eggs in a small container with a single adult. We ran 10 replicate experiments, five with infected adults and five with uninfected adults as controls. Both infected and control insects were captured from field populations in Arequipa, Peru. We allowed the eggs to mature to the third instar, feeding them biweekly on uninfected chickens, and then examined their faeces microscopically for T. cruzi. Waiting until the insects reached the third instar afforded adequate time for the parasite to develop in the vector and facilitated microscopic examination for the parasite, as it is easier to extract faeces from larger insects.
(c). Experiment 2: bloodmeal identification
Using species-specific PCR reactions described in [31], we identified the host species providing the bloodmeal taken by the vectors among a sample of 36 second instar insects obtained from highly T. cruzi-infected (more than 75% prevalence) vector colonies in guinea pig pens. We selected an even number of insects that were positive and negative for T. cruzi by microscopy (18 of each group). We used second instar insects as these would have taken very few bloodmeals, reducing (but not eliminating) the possibility of identifying a bloodmeal host that was not responsible for infecting the vector. It can be difficult to identify T. cruzi by microscopy, especially in younger insects, in which the parasite would have had less time to replicate to high numbers. We therefore also performed PCR, using the methods described in [32].
(d). Experiment 3: measuring the duration of Trypanosoma cruzi parasitaemia and the infectiousness of guinea pigs to vectors over the course of infection
We infected eight guinea pigs intradermally with a local strain of T. cruzi at a concentration of 106/100 μl. Each week, we drew blood from all animals and counted parasites using a Neubauer chamber, a gridded glass slide commonly used for counting blood cells. Weekly, we exposed each animal to 20 fifth instar insects by placing a small wooden xenodiagnoses box containing the insects on the animal's abdomen. The insects were fed to repletion. The guinea pigs were not anaesthetized during the process. Forty-five days after each feeding, we performed microscopic evaluation for parasites on all vectors, allowing us to measure the probability of transmission from infected guinea pigs to vectors over the course of infection.
(i). Ross–MacDonald model, its extensions and simulation
Following the Ross–MacDonald model of malaria [10–13], we developed a deterministic model of T. cruzi transmission among hosts and vectors, using parameters from literature and our previous studies (table 1). The basic model includes a number of simplifying assumptions: the host population is assumed to be constant, homogeneous and closed to birth or migration. Hosts become susceptible to reinfection after clearing the parasite. The vector population is also homogeneous and without migration, and each vector feeds at random from the pool of available hosts [36]. We use standard notation to represent the proportion of infected hosts (X) and vectors (Y) [13]. In this parametrization, in which m is the ratio of vectors to hosts, a is the vector's biting rate, b is the transmission efficiency from vectors to hosts and 1/r is the duration of the infectious period in hosts, the change in the proportion of infected hosts is
| 2.1 |
Table 1.
Parameter values for modified Ross–MacDonald and simulation models.
| parameter | description | Ross–MacDonald model value [range in sensitivity analyses] | simulation model valuea | sources |
|---|---|---|---|---|
| a | bite rate: number of bites on host per vector per day | 1/14 [0.0625–1] | Poisson (1/14) | estimated from laboratory feeding (M.Z.L. 2015, unpublished data) |
| m | vectors per host | 100 [80–200] | 100; 500 during bottleneck periods | [33] |
| n | length of incubation period of parasite in vector (days) | 45 [10–60] | 45b | — |
| g | daily probability of vector mortality | 0.005 [0.001–0.01] | Poisson (0.005) | [34] |
| b | infected vector to susceptible host infection probability | 0.00068 [0.0005–0.001] | 0.00068b | [5] |
| c | infectious host to susceptible vector infection probability | 0.49 [0.35–1.0] | sampled from animals in experiment 3 | [35] |
| 1/r | duration of parasitaemia in host (days) | 55 [40–100] | sampled from animals in experiment 3 | [21] |
aSensitivity analyses for the stochastic simulation are shown in the electronic supplemental material, figure S6.
bWe have observed parasites to reach equilibrium densities in vectors approximately 45 days after vectors ingested an infectious blood meal. We therefore modelled vector infectiousness (b) as a logistic function, b/1 + 20e−0.20t, with infectiousness reaching a peak of 0.00068 in approximately 45 days.
Similarly, the change in the proportion of infected vectors is described by
| 2.2 |
where c is the transmission efficiency from hosts to vectors, g is the rate of vector mortality and n is the length of the parasite incubation period in the vector. The equilibrium parasite prevalence among vectors and hosts can be obtained for this version of the Ross–MacDonald model through the following equations [12,13]:
| 2.3 |
| 2.4 |
| 2.5 |
In continuous time, and when initiating these equations with a single infectious host, the prevalence increases monotonically and the equilibrium prevalence in vectors is equivalent to the maximum prevalence. The sensitivity of the maximum prevalence in vectors to each model parameter is shown in electronic supplementary material, figure S1.
(ii). Extension to include coprophagy
We modify the base Ross–MacDonald model to include acquisition of T. cruzi by uninfected insects via ingestion of contaminated faeces. The rate of change of vector prevalence becomes
| 2.6 |
where the parameter δ represents the daily force of coprophagic infection. We run this model over a range of values of δ.
(iii). Stochastic simulation of Ross–MacDonald model with host bottlenecks
To more easily consider the effects of fluctuations in host populations, we developed a stochastic simulation based on the Ross–MacDonald model (model details, figure and code are in the electronic supplementary material). We simulated the expected prevalence of host and vector infection in a community of 10 hosts and 1000 vectors, using parameters from the literature and from data collected in our laboratory studies in Peru (table 1). We then introduced a bottleneck in the host population to mimic the seasonal reduction in guinea pig populations. We evaluate the effects of bottlenecks of varying lengths (14, 30, 60, 90 and 180 days). We assume, following Cohen & Gürtler [37], that the size of the vector population is a function of the size of the host population from the previous season; the number of vectors in the colony therefore does not decrease significantly until after the bottleneck period. In a sensitivity analysis, we explore the effect of a ‘vector bottleneck’ in which the triatomine population declines concurrently with the guinea pig bottleneck. We also explore different host and vector population sizes both before and during the bottlenecks.
3. Results
(a). Entomological survey
Triatomines infected with T. cruzi were found throughout the survey area (figure 1). Fourteen large colonies (more than 100 insects, excluding eggs and first instar nymphs) were collected, and 10 of these colonies included insects infected with T. cruzi (electronic supplementary material). The prevalence of the parasite in these large colonies was strongly bimodal. In two of the largest colonies, 544 of 639 (85.1%) and 628 of 723 (86.9%) insects were infected. Both of these colonies were collected from guinea pig pens. A third, smaller colony that exhibited very high parasite prevalence (128 of 165, 77.6%) was also collected from a guinea pig pen. The prevalence of the next most infected colony, by contrast, was only 6.4% (15 infected insects out of 234). This bimodal pattern of T. cruzi prevalence among vectors in large colonies is consistent across dozens of other large colonies examined over the course of the control campaign in Arequipa (data provided in the electronic supplementary material, table S1).
(b). Hypothesis I: Trypanosoma cruzi is transmitted among insects, without passing through an intermediate mammalian reservoir host, via coprophagy
Our model shows that relatively small daily rates of coprophagic transmission can result in high infection prevalence. As the force of coprophagic transmission increases, the infection prevalence approaches an asymptote whose value is related to the probability that the vector will survive the parasite's incubation period. For the parameters we considered (described in table 1), the equilibrium prevalence in vectors does not exceed Y = 86% even if coprophagy is very frequent. Because of the asymptotic shape of the curve, a value of 0.08 for δ would generate an equilibrium prevalence of 80% while a value of 0.46 would be necessary to generate a prevalence of 85% (electronic supplementary material).
In the experiment, which measured the actual rate of infection transmission via coprophagy, 150 triatomine eggs (across five replicate experiments) were exposed to T. cruzi in the faeces of adult insects. A total of 119 individuals survived to the third instar. None of these insects acquired T. cruzi through coprophagy. The actual rate of coprophagic transmission, even if not zero, is clearly far below the rate required to produce a high infection prevalence in our model. We conclude that coprophagy does not explain the presence of highly infected field vector colonies.
(c). Hypothesis II: a reservoir species other than guinea pigs is driving the transmission of Trypanosoma cruzi through vectors collected near guinea pig pens
Guinea pigs represented the vast majority (34/36) of bloodmeals identified from second instar insects in highly infected vector colonies found around guinea pig pens (electronic supplementary material, table S2). DNA of the blood meal from two insects was not amplified by any of the primers used in the assay. It is therefore unlikely that non-guinea pig reservoir species are producing a significant degree of infection in the observed vectors. All of the insects in which T. cruzi were detected by microscopy were confirmed by PCR; interestingly, PCR was also positive for the majority (13/18) of insects in which the parasite was not identified by microscopy, suggesting the prevalence of infection among insects in these guinea pig pens was even greater than 85%.
(d). Hypothesis III: persistently infectious hosts drive the parasite through vector populations
Some guinea pigs retained the ability to infect insects for many months (figure 3; electronic supplementary material, figure S2). The data from experiment 3 provided estimates of the probability of T. cruzi transmission from guinea pig hosts to vectors over the course of infection, which we incorporated into a stochastic Ross–MacDonald model. Results of the simulations suggest persistently infectious guinea pigs had little effect on the maximum prevalence of T. cruzi infection in vector colonies (electronic supplementary material, figure S3). This finding was initially surprising, but became more intuitive when considering the very short lifespan of guinea pigs raised for consumption—most are eaten at around four months of age. The relevance of prolonged infectiousness in any one guinea pig is limited by its brief lifespan. However, the presence of persistently infectious guinea pigs did cause T. cruzi infection to persist longer in the simulated vector populations. In the absence of such animals, infection in vectors persisted for a median of 357 days. The median persistence time rose to 442 days, an increase of almost three months, in the extreme situation in which all guinea pigs in an enclosure were persistently infectious.
Figure 3.
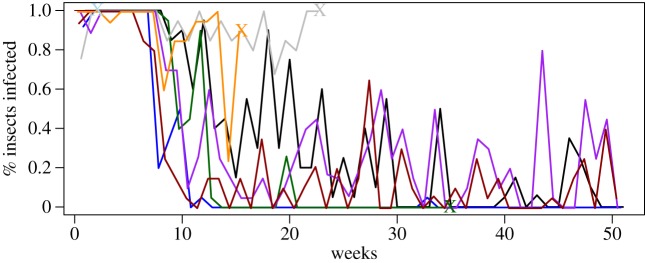
Observed variability in the percentage of triatomines infected (out of sets of 20 exposed insects) after feeding on eight experimentally infected guinea pigs weekly over the course of a year. Guinea pigs were initially inoculated with 106/100 μl of a local strain of T. cruzi. (Online version in colour.)
(e). Hypothesis IV: host bottlenecks increase vector infection prevalence
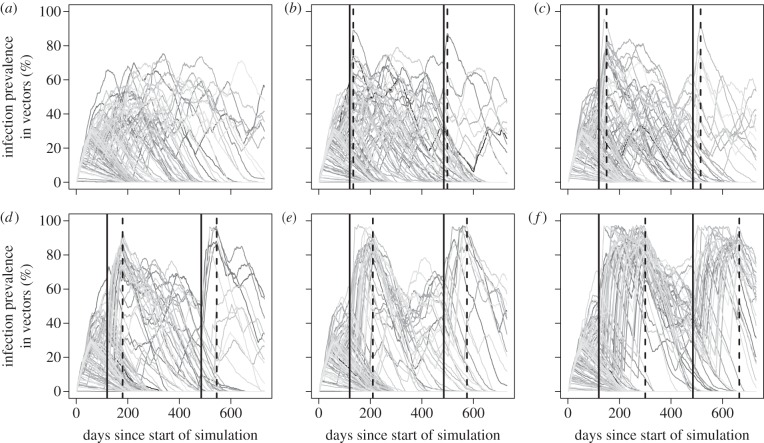
Including host population crashes or ‘bottlenecks' in our stochastic simulations produced several vector populations with upwards of 85% T. cruzi prevalence (figure 4; electronic supplementary material, figure S5). During each bottleneck, a starting host population of 10 guinea pigs declined to only two members for a period of time (while the number of vectors was held constant at 1000). Host population crashes that lasted for as little as two weeks were able to push vector infection prevalence to more than 85%, although the probability of reaching such a high prevalence increased when the bottleneck length was at least two months. As the sizes of the host populations within and outside the bottleneck period are varied, the resultant pattern of infection among vectors also shifts, such that the more dramatic the bottleneck, the more bimodal the peak infection rates in the vector population (electronic supplementary material, figure S4). Results of additional analyses that consider more detailed vector population dynamics, including bottlenecks in vector populations following the bottlenecks in their host populations, are presented in the electronic supplementary material, figure S5b. In the event of a bottleneck in vector populations, 85% prevalence of T. cruzi infection can still occur, but much less frequently. Additional sensitivity analyses to simulation parameters are shown in electronic supplementary material, figure S6.
Figure 4.
Simulated time course of T. cruzi in colonies of T. infestans. Simulations tracked 1000 vectors and a host population of 10 guinea pigs that declined to two hosts during bottleneck periods. A total of 1000 replicate simulations were run for each bottleneck length. (a) No bottleneck. (b–f) Bottleneck durations of 14, 30, 60, 90 and 180 days, respectively. Solid vertical lines indicate the start of the bottleneck, and dashed vertical lines indicate the end.
(f). Synthesis
Our findings allow us to discard several mechanisms we hypothesized might explain the observed high prevalence of T. cruzi in vector populations. Vector infection does not appear to be due to reservoir hosts other than guinea pigs. Although mathematical models suggest that coprophagy could produce high prevalences in insect populations, horizontal transmission of T. cruzi between vectors via faeces ingestion was not observed in our experiments. While the presence of persistently infectious hosts does not produce the observed prevalence of T. cruzi, these animals may play a role in maintaining the parasite in vector populations over longer time scales. Bottlenecks in host populations, even relatively brief ones, can lead to high levels of T. cruzi infection among triatomine vectors.
4. Discussion
Fluctuations in host populations can have outsized effects on transmission of vector-borne pathogens [38]. Even a very unlikely and unstable transmission cycle can propel a pathogen to high frequencies in vector populations, and, in turn, create significant risk to animals and humans, when hosts fluctuate in numbers. We document precisely this scenario, in which guinea pig population bottlenecks lead to a high prevalence of T. cruzi in triatomine populations, and ultimately intensify the risk of Chagas disease in households. Outside of the Andes, the role of guinea pigs (and rodents in general) in domestic T. cruzi transmission is probably minor [39] (though see [40]). But the dynamics we describe—including the concentration of vectors on a limited number of host animals—are common. In small households, domestic animal populations are rarely stable. The unpredictable birth and death of these animals creates fluctuations in the ratio of the numbers of vectors feeding on any given host, a key parameter in the Ross–MacDonald model. On the scale of a household, with only a handful of hosts, these fluctuations are strong enough to set off epidemics. The effect of the change in the ratio between vectors and hosts on pathogen prevalence is likely to be especially pronounced for triatomines and other vectors that are relatively sessile.
The proximate causes of the bottlenecks in domestic guinea pig populations in southern Peru are clear. In the months of June, July and August, a series of local, regional and national holidays are celebrated in Arequipa with cuyadas—large guinea pig cookouts. During these holidays, guinea pigs in the region have an expected lifespan similar to that of turkeys in the United States in November. While these festivals are the proximate cause of the decrease in guinea pig populations, the reason these festivals are celebrated with the consumption of guinea pigs rather than other animals is probably economic: the price of alfalfa rises sharply prior to this season (figure 2) and it becomes economically counterproductive to maintain large guinea pig populations. The ultimate causes of the host fluctuations are related to water and climate. Arequipa is in an arid region, and the city and surrounding agricultural lands both rely on rains and snowmelt at higher altitudes. While the price of alfalfa may be driven by many factors, including demand from the cattle industry, it ultimately tracks the seasonal availability of water.
The forces that lead to bottlenecks in domestic guinea pig populations in Peru are becoming aggravated. The Andean glaciers, the source of the water for the region, are retreating [41], while human populations and farming activities continue to rise. It is possible that these forces in concert will make guinea pig husbandry economically unfeasible—a change that would be likely to decrease T. cruzi transmission in the region, but would also deprive a large population of an important source of protein. Alternatively, a transition from guinea pig to chicken husbandry may occur. Chickens do not carry T. cruzi, but may support large vector populations. Fluctuations in chicken populations may then cause vectors to seek alternative hosts, potentially becoming concentrated around a competent mammalian reservoir of T. cruzi [37], leading to dynamics of parasite transmission through vector colonies similar to those we describe here.
Our modelling is intentionally limited in scope. We focus only on explaining the high rates of vector infection seen in a few large colonies, and do not address the large variations in T. cruzi prevalence between households. Interestingly, surrounding highly infected vector colonies, we found equally large colonies in which very few (less than 7%) insects harboured the parasite. One explanation for this finding is that, by chance, in some households only uninfected guinea pigs survive the seasonal culling. Despite the host bottleneck, vector infection prevalence would remain low in these guinea pig enclosures. Alternatively, it is possible that some large vector colonies become highly infected, possibly through the bottleneck dynamics we describe, and insects from these colonies then migrate to nearby large colonies where the parasite had not yet emerged. Another limitation of our study is that our models are sensitive to the parameter describing the probability of transmission from an infected vector to a susceptible host (electronic supplementary material, figure S1). This transmission efficiency, which we assume to be very low, is extremely difficult to measure directly. Instead, its value has been estimated using formulae that combine separate and possibly erroneous estimates of the incidence of new T. cruzi infections in hosts: the biting frequency of vectors and the prevalence of T. cruzi in vectors [4,5]. If the estimates of this parameter turn out to be wrong, then our conclusions regarding T. cruzi transmission would need to be re-evaluated.
We observed heterogeneity of infectiousness among guinea pigs experimentally infected with T. cruzi. While our models suggest that the presence of persistently infectious guinea pigs alone is insufficient to drive the parasite to the levels observed in field vector colonies, such animals may be very important in the introduction of the parasite or the maintenance of the parasite in a metapopulation. Prolonged infectiousness is not limited to guinea pigs. Whenever xenodiagnoses are used to assess T. cruzi infection status, results are typically heterogeneous across individuals of the same species [42]. The same is true for humans—the early clinical trials for Chagas disease treatments were assessed with xenodiagnoses, and some individuals in the control group remained infectious to insects for an extended period of time [43,44].
It is unclear why some individuals are persistently infectious while others control the parasite. Trypanosomes in general are famous for their complex mechanisms of host immune evasion [45]; the stochastic nature of evasion mechanisms could lead to heterogeneity in parasitaemia among some individuals. Co-infection with additional parasites might also decrease some individuals' ability to control T. cruzi [46]. Genetic differences may partially explain elevated or prolonged parasitaemia [47], a finding that may be particularly relevant to domestic guinea pigs.
Our findings suggest that T. cruzi transmission in households could be diminished by preventing bottlenecks in host populations. The ecology of T. cruzi transmission is highly variable; the cast of vectors and hosts of the parasite changes from habitat to habitat. For the specific case of transmission by T. infestans in southern Peru, infections could potentially be decreased through economic measures to stabilize the price of alfalfa, thereby mitigating situations in which large populations of insects are concentrated on a small number of infected guinea pigs. Control measures that are more direct and easier to implement are already in place across much of southern Peru [48]; attempts to control host fluctuations would be more relevant to areas difficult to reach with traditional methods. By identifying and addressing the drivers of host population bottlenecks in other areas affected by Chagas disease, the conditions that permit the emergence or re-emergence of the parasite might be eliminated, at least in some contexts.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Members of the Chagas Disease Working Group in Arequipa who contributed and facilitated this work include Jesus Pinto Caballero, Maritza Calderon, Lily Chou Chu, Fernando Malaga, Andy Catacora, Karina Oppe, Juan Cornejo del Carpio, Edith Malaga, Danitza Pamo, Vitaliano Cama, Malwina Niemierko, Caryn Bern and Robert Gilman. We gratefully acknowledge the invaluable contributions of the Ministerio de Salud del Perú (MINSA), the Dirección General de Salud de las Personas (DGSP), the Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxenicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), the Dirección General de Salud Ambiental (DIGESA), the Gobierno Regional de Arequipa, the Gerencia Regional de Salud de Arequipa (GRSA), the Pan American Health Organization (PAHO/OPS) and the Canadian International Development Agency (CIDA).
Ethics
The Institutional Animal Care and Use Committee (IACUC) of Universidad Peruana Cayetano Heredia reviewed and approved the animal-handling protocol for this study (no. 57822).
Authors' contributions
All authors contributed to the design, conduct and interpretation of one or more of the studies reported herein.
Competing interests
The authors have no competing interests.
Funding
Funding for these studies came from National Institutes of Health NIAID P50 AI074285 and 5R01 AI101229.
References
- 1.Chagas C. 1909. Nova tripanozomiaze humana: estudos sobre a morfolojia eo ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1, 159–218. ( 10.1590/S0074-02761909000200008) [DOI] [Google Scholar]
- 2.Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gürtler RE. 2007. The challenges of Chagas disease—grim outlook or glimmer of hope? PLoS Med. 4, e332 ( 10.1371/journal.pmed.0040332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovich JE, Wisnivesky-Colli C, Solarz ND, Gurtler RE. 1990. Probability of transmission of Chagas disease by Triatoma infestans (Hemiptera: Reduviidae) in an endemic area of Santiago del Estero, Argentina. Bull. World Health Organ. 68, 737–746. [PMC free article] [PubMed] [Google Scholar]
- 4.Nouvellet P, Dumonteil E, Gourbiere S. 2013. The improbable transmission of Trypanosoma cruzi to human: the missing link in the dynamics and control of Chagas disease. PLoS Negl. Trop. Dis. 7, e2505 ( 10.1371/journal.pntd.0002505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catala SS, Gorla DE, Basombrio MA. 1992. Vectorial transmission of Trypanosoma cruzi: an experimental field study with susceptible and immunized hosts. Am. J. Trop. Med. Hyg. 47, 20–26. [DOI] [PubMed] [Google Scholar]
- 6.Breniere SF, Bosseno MF, Noireau F, Yacsik N, Liegeard P, Aznar C, Hontebeyrie M. 2002. Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem. Inst. Oswaldo Cruz 97, 289–295. ( 10.1590/S0074-02762002000300002) [DOI] [PubMed] [Google Scholar]
- 7.Feliciangeli MD, Campbell-Lendrum D, Martinez C, Gonzalez D, Coleman P, Davies C. 2003. Chagas disease control in Venezuela: lessons for the Andean region and beyond. Trends Parasitol. 19, 44–49. ( 10.1016/S1471-4922(02)00013-2) [DOI] [PubMed] [Google Scholar]
- 8.Samuels AM, et al. 2013. Epidemiology of and impact of insecticide spraying on Chagas disease in communities in the Bolivian Chaco. PLoS Negl. Trop. Dis. 7, e2358 ( 10.1371/journal.pntd.0002358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pless M, Juranek D, Kozarsky P, Steurer F, Tapia G, Bermudez H. 1992. The epidemiology of Chagas’ disease in a hyperendemic area of Cochabamba, Bolivia: a clinical study including electrocardiography, seroreactivity to Trypanosoma cruzi, xenodiagnosis, and domiciliary triatomine distribution. Am. J. Trop. Med. Hyg. 47, 539–546. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. 1910. The prevention of malaria. New York, NY: Dutton. [Google Scholar]
- 11.Macdonald G. 1957. The epidemiology and control of malaria. London, UK: Oxford University Press. [Google Scholar]
- 12.Smith DL, McKenzie FE. 2004. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 3, 13 ( 10.1186/1475-2875-3-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DL, McKenzie FE, Snow RW, Hay SI. 2007. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 5, e42 ( 10.1371/journal.pbio.0050042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias E. 1934. Estudos sobre o Schizotrypanum cruzi. Mem. Inst. Oswaldo Cruz 28, 1–110. ( 10.1590/S0074-02761934000100001) [DOI] [Google Scholar]
- 15.Duncan J. 1926. On a bactericidal principle present in the alimentary canal of insects and arachnids. Parasitology 18, 238–252. ( 10.1017/S0031182000005205) [DOI] [Google Scholar]
- 16.Beard C, Dotson E, Pennington P, Eichler S, Cordon-Rosales C, Durvasula R. 2001. Bacterial symbiosis and paratransgenic control of vector-borne Chagas disease. Int. J. Parasitol. 31, 621–627. ( 10.1016/S0020-7519(01)00165-5) [DOI] [PubMed] [Google Scholar]
- 17.Schaub GA. 1988. Direct transmission of Trypanosoma cruzi between vectors of Chagas’ disease. Acta Trop. 45, 11–19. [PubMed] [Google Scholar]
- 18.Ryckman RE. 1951. Recent observations of cannibalism in Triatoma (Hemiptera: Reduviidae). J. Parasitol. 37, 433–434. ( 10.2307/3273249) [DOI] [PubMed] [Google Scholar]
- 19.Phillips N. 1960. Experimental studies on the quantitative transmission of Trypanosoma cruzi: aspects of the rearing, maintenance and testing of vector material, and of the origin and course of infection in the vector. Ann.trop.Med.Parasit.5. 4, 397–414. [DOI] [PubMed] [Google Scholar]
- 20.Ayaqui R, Córdova E. 1990. Infección natural de roedores sinantrópicos por Trypanosoma cruzi Chagas. Acta Med. Agustina 1, 66–70. [Google Scholar]
- 21.Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velásquez DE, Sterling CR, Martin D, Bern C. 2011. Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am. J. Pathol. 179, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarleton R, Koller B, Latour A, Postan M. 1992. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356, 338–340. ( 10.1038/356338a0) [DOI] [PubMed] [Google Scholar]
- 23.Guedes PMDM, Veloso VM, Tafuri WL, Galvão LMDC, Carneiro CM, Lana MD, Chiari E, Ataide Soares K, Bahia MT. 2002. The dog as model for chemotherapy of the Chagas’ disease. Acta Trop. 84, 9–17. ( 10.1016/S0001-706X(02)00139-0) [DOI] [PubMed] [Google Scholar]
- 24.Machado EMDM, et al. 2001. A study of experimental reinfection by Trypanosoma cruzi in dogs. Am. J. Trop. Med. Hyg. 65, 958–965. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez RC. 2002. Crianza y comercialización de cuyes: granja y negocios. Lima, Peru: Ripalme. [Google Scholar]
- 26.British Columbia Ministry of Agriculture, Food and Fisheries 2001. Crop coefficients for use in irrigation scheduling. See http://www.agf.gov.bc.ca/resmgmt/publist/500Series/577100-5.pdf.
- 27.Bayer AM, Hunter GC, Gilman RH, Del C, Juan GC, Naquira C, Bern C, Levy MZ. 2009. Chagas disease, migration and community settlement patterns in Arequipa, Peru. PLoS Negl. Trop. Dis. 3, e567 ( 10.1371/journal.pntd.0000567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy MZ, Bowman NM, Kawai V, Waller LA, del Carpio JGC, Benzaquen EC, Gilman RH, Bern C. 2006. Periurban Trypanosoma cruzi–infected Triatoma infestans, Arequipa, Peru. Emerg. Infect. Dis. 12, 1345 ( 10.3201/eid1209.051662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurtler RE, Chuit R, Cecere MC, Castanera MB, Cohen JE, Segura EL. 1998. Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages in northwest Argentina: environmental, demographic, and entomologic associations. Am. J. Trop. Med. Hyg. 59, 741–749. [DOI] [PubMed] [Google Scholar]
- 30.Delgado S, et al. 2011. A history of Chagas disease transmission, control, and re-emergence in Peri-Rural La Joya, Peru. PLoS Negl. Trop. Dis. 5, e970 ( 10.1371/journal.pntd.0000970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizarro JC, Stevens L. 2008. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS ONE 3, e3585 ( 10.1371/journal.pone.0003585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, Svoboda M. 2003. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 68, 574–582. [DOI] [PubMed] [Google Scholar]
- 33.Basombrio MA, Gorla D, Catala S, Segura MA, Mora MC, Gomez L, Nasser J. 1996. Number of vector bites determining the infection of guinea pigs with Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 91, 421–423. ( 10.1590/S0074-02761996000400006) [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Pacheco G, Esteva L, Vargas C. 2012. Control measures for Chagas disease. Math. Biosci. 237, 49–60. ( 10.1016/j.mbs.2012.03.005) [DOI] [PubMed] [Google Scholar]
- 35.Gurtler RE, Cecere MC, Castanera MB, Canale D, Lauricella MA, Chuit R, Cohen JE, Segura EL. 1996. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am. J. Trop. Med. Hyg. 55, 24–31. [PubMed] [Google Scholar]
- 36.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. 2012. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 8, e1002588 ( 10.1371/journal.ppat.1002588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen JE, Gürtler RE. 2001. Modeling household transmission of American trypanosomiasis. Science 293, 694–698. ( 10.1126/science.1060638) [DOI] [PubMed] [Google Scholar]
- 38.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS Biol. 4, e145 ( 10.1371/journal.pbio.0040145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gascon J, Bern C, Pinazo M. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115, 22–27. ( 10.1016/j.actatropica.2009.07.019) [DOI] [PubMed] [Google Scholar]
- 40.Grijalva MJ, Terán D, Dangles O. 2014. Dynamics of sylvatic Chagas disease vectors in coastal Ecuador is driven by changes in land cover. PLoS Negl. Trop. Dis. 8, e2960 ( 10.1371/journal.pntd.0002960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser B. 2012. Melting in the Andes: goodbye glaciers. Nature. 491, 180–182. ( 10.1038/491180a) [DOI] [PubMed] [Google Scholar]
- 42.Gürtler R, Cecere M, Lauricella M, Cardinal M, Kitron U, Cohen J. 2007. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology 134, 69–82. ( 10.1017/S0031182006001259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerisola J, Rohwedder R, Segura E, Del Prado C, Alvarez M, De Martini G. 1974. El xenodiagnóstico: normatización. Buenos Aires, Argentina: Instituto Nacional de Diagnóstico e Investigación de la Enfermedad de Chagas (INDIECH). [Google Scholar]
- 44.Cerisola JA, Silva N, Prata A, Schenone H, Rohwedder R. 1977. Evaluación mediante xenodiagnostico de la efectividad del nifurtimox en la infección chagásica crónica humana. Bol. Chil. Parasitol. 32, 51–62. [PubMed] [Google Scholar]
- 45.Donelson JE, Hill KL, El-Sayed N. 1998. Multiple mechanisms of immune evasion by African trypanosomes. Mol. Biochem. Parasitol. 91, 51–66. ( 10.1016/S0166-6851(97)00209-0) [DOI] [PubMed] [Google Scholar]
- 46.Lass S, Hudson PJ, Thakar J, Saric J, Harvill E, Albert R, Perkins SE. 2013. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. J. R. Soc. Interface 10, 20120588 ( 10.1098/rsif.2012.0588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrightsman R, Krassner S, Watson J. 1982. Genetic control of responses to Trypanosoma cruzi in mice: multiple genes influencing parasitemia and survival. Infect. Immun. 36, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buttenheim AM, et al. 2014. Is participation contagious? Evidence from a household vector control campaign in urban Peru. J. Epidemiol. Commun. Health 68, 103–109. ( 10.1136/jech-2013-202661) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.