Abstract
Apex predators structure ecosystems through lethal and non-lethal interactions with prey, and their global decline is causing loss of ecological function. Behavioural changes of prey are some of the most rapid responses to predator decline and may act as an early indicator of cascading effects. The Tasmanian devil (Sarcophilus harrisii), an apex predator, is undergoing progressive and extensive population decline, of more than 90% in long-diseased areas, caused by a novel disease. Time since local disease outbreak correlates with devil population declines and thus predation risk. We used hair traps and giving-up densities (GUDs) in food patches to test whether a major prey species of devils, the arboreal common brushtail possum (Trichosurus vulpecula), is responsive to the changing risk of predation when they forage on the ground. Possums spend more time on the ground, discover food patches faster and forage more to a lower GUD with increasing years since disease outbreak and greater devil population decline. Loss of top–down effects of devils with respect to predation risk was evident at 90% devil population decline, with possum behaviour indistinguishable from a devil-free island. Alternative predators may help to maintain risk-sensitive anti-predator behaviours in possums while devil populations remain low.
Keywords: giving-up densities, Tasmanian devil, brushtail possum, devil facial tumour disease, anti-predator behaviour, apex predator loss
1. Introduction
Complex lethal and non-lethal interactions between predators and prey can shape ecosystem structure. The severe and extensive depletion of apex predators worldwide has disrupted predator–prey relationships, inducing cascading effects through foodwebs. This has been demonstrated with evidence of changes in community composition [1,2], and prey population dynamics and behaviour [3–5], leading to major simplification of the Earth's ecosystems [6]. Indirect effects of predators on prey, mediated through prey behaviour to avoid the risk of predation, can be as important at a population level as the direct fitness consequences of predation [1,7]. It is necessary to study both direct and indirect risks of predation to understand the wider implications for communities of the decline in apex predator populations.
Predators create ‘landscapes of fear’ [8], moderated by habitat heterogeneity, in which prey must balance the need to forage with the need to avoid being eaten. Prey individuals finely tune their behaviour to reflect their perception of the risk of predation, including increasing vigilance, and using suboptimal habitats or activity times to avoid predators. This has consequences for prey fitness through reduced vital rates: survival, growth and reproduction [9,10]. In the absence of direct knowledge of the location, number or level of hunger of predators in the vicinity, prey use indirect cues as proxies for the level of risk of lethal encounters [11,12]. Cues include structural habitat attributes such as density of vegetation, which can provide either better visibility or concealment from predators and refuges for escape [7,11–13].
To design effective conservation strategies for ecosystem restoration, we need to understand the long-term consequences of the severe and extensive loss of larger predators. How many predators are required to maintain top–down influence on prey communities? Can loss of large predators be mitigated by the presence of other predators? If predators are lost or absent from the environment for sufficient periods of time, selective pressure to maintain expensive risk-sensitive behaviours is reduced and some specific learned and heritable anti-predator behaviours and traits may be lost [3,14]. Others are retained in species isolated from their predators for hundreds of generations [15], possibly because they are not energetically costly to maintain or because they are relevant to other types of predators.
Here, we assess the effects of the progressive decline of an apex predator on the risk-sensitive behaviour of a prey species, an opportunity provided by the disease-associated decline of the Tasmanian devil (Sarcophilus harrisii), the endemic apex mammalian predator on Australia's island state, Tasmania. Devil facial tumour disease (DFTD) is a consistently fatal transmissible cancer first detected in the mid-1990s. The ongoing spread of DFTD across the devils’ geographical range has caused severe and sustained population declines [16,17], which commence immediately following local disease outbreak. The disease has spread to most of the devils' range, reducing the overall population by about 85%. Local declines exceed 95%, although no local extinction has been recorded [16].
Widespread effects on the ecosystem are expected following the decline of the devil, and changes in the relative abundance of smaller mammalian carnivores have already been documented [18]. Behavioural and demographic changes are expected among major prey species of devils: medium-sized macropods (Bennett's wallaby, Macropus rufogriseus; Tasmanian pademelon, Thylogale billiardierii), and the common brushtail possum (Trichosurus vulpecula), although clear evidence of changes in population size has not yet been detected [19], perhaps because of the confounding influence of bottom–up factors. These species do exhibit risk-sensitive behaviour in response to predators, which may be a more sensitive indication of the impact of devil decline. When devil density is high, wallabies and pademelons forage closer to cover in forestry plantations [20], but emerge earlier before dusk and forage in open grasslands further from the forest edge [21]. Brushtail possums are a predominantly arboreal folivore, but forage extensively on the ground. On mainland Australia, brushtail possums minimize predation risk by staying close to trees [22], but travel further and visit more feeding patches in some habitat types in response to reduced densities of the introduced red fox (Vulpes vulpes) [23].
The aim of this study is to test the responsiveness of a major prey species, the brushtail possum, to the changing landscape of predation risk associated with the rapid decline of the devil as DFTD spread across Tasmania. We ask the following questions: (i) Are ground-based activity levels of possums associated with decreasing predation risk from devils? (ii) Is their risk-sensitive foraging behaviour, evaluated using the giving-up density (GUD) approach, responsive to the temporal and spatial gradient in predation risk? (iii) Does more than 90% population decline of devils in eastern Tasmania represent significant loss of top–down control of this apex predator? To answer the last question, we compare the GUD responses of possums in northeast Tasmania to those on an offshore island where possums have lived in the absence of devils for many decades [24]. We expect that the linked declines of the devil and predation risk may result in increased ground-based activity and reduction of potentially costly anti-predator behaviours for prey species.
2. Material and methods
(a). DFTD arrival times and site selection
We used the time since local DFTD outbreak to classify study sites into long-term disease (12–15 years), mid-term disease (5–8 years) and disease-free at the time of the study (figure 1). The time since DFTD outbreak provides a good proxy for the severity of local population decline, as established from longitudinal spotlighting data [19], and thus predation risk. To estimate the length of time that each site had been diseased, we extrapolated confirmed dates of first disease detection from surrounding regions and combined these with expected patterns of disease spread through the landscape [25].
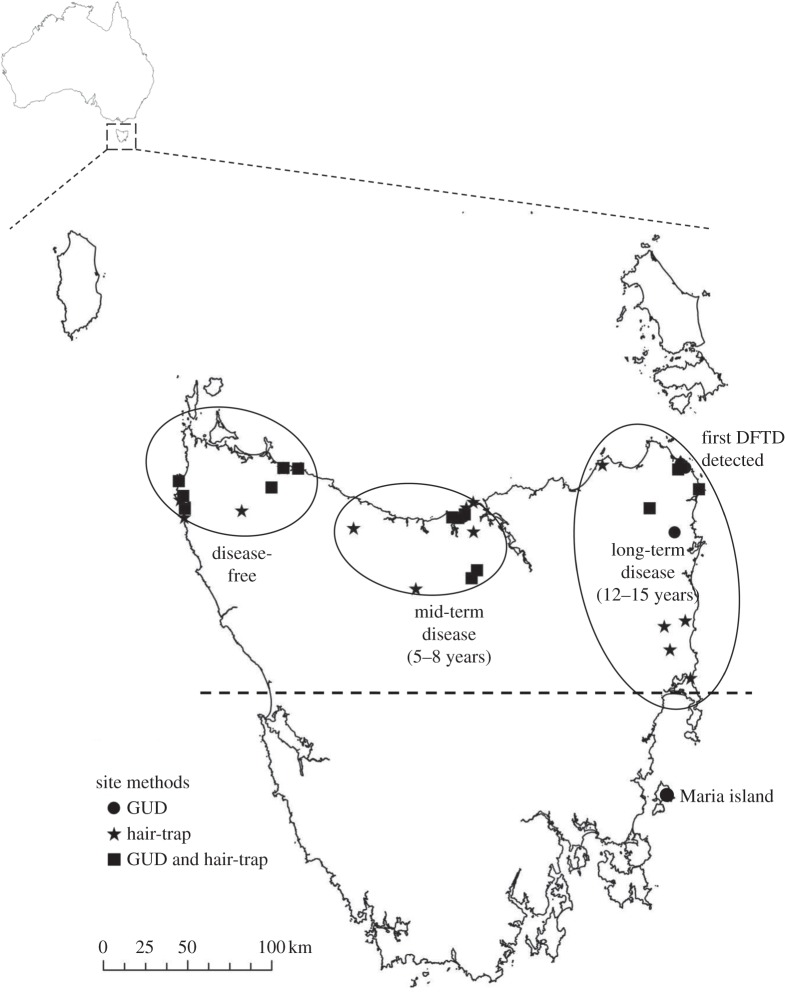
Figure 1.
Field site locations for the GUD experiment and hair traps in Tasmania, Australia. Circles enclose the sites within each of the three Tasmanian devil predation risk regions: low (long-term disease), medium (mid-term disease) and high (disease-free). Sites on mainland Tasmania were kept to the northern half of the island to reduce environmental variability, represented by the dashed line.
We selected 30 sites on mainland Tasmania and six sites on Maria Island, 4 km off the east coast of Tasmania, using ArcGIS (v. 9.2). The sites represented the full spectrum of the predation risk gradient, from the earliest records of DFTD in the northeast to not yet diseased in the northwest (figure 1). Sites were located on public land (State Forest or National Park). We selected sites below 650 m, with mean precipitation between 1100 and 1600 mm per year (Bureau of Meteorology data). To avoid north–south temperature gradients, we restricted sites to northern Tasmania. Equal numbers of dry eucalypt forest sites and coastal woodland sites with open grassland/scrub were selected in each of the three disease outbreak regions: long-term disease, mid-term disease and disease-free. We conducted experiments in two different vegetation types to assess, first, if activity of prey and predator varied with habitat structure and resource availability and, second, whether attributes at a microhabitat scale affected anti-predator behavioural responses. Brushtail possum densities are known to vary with habitat type and disturbance [26,27]. Preliminary camera and hair-trap surveys showed that both habitat types supported high densities of possums. Sites were chosen where there would be minimal impact of culling, which occurs in Tasmania under crop protection permits, and significantly affects possum abundance [28].
(b). Index of terrestrial possum and devil activity
To obtain an index of ground-based activity of devil and possum populations across the gradient of DFTD arrival times, we conducted a snapshot survey at 24 of the selected sites on mainland Tasmania, using four dry eucalypt and four coastal woodland scrub sites in each of the three disease outbreak regions (figure 1). Twenty hair tubes, consisting of a PVC pipe with double-sided tape, and 20 commercially available hair funnels (Faunatech, Melbourne) were placed at each site; 960 hair traps in total. Half of each hair-trap type were baited with carnivore bait (dried liver soaked in muttonbird oil) and half with herbivore bait (rolled oats, peanut butter and walnut oil). We placed one each of the herbivore and carnivore traps, using a mixture of tubes and funnels, at 100 m intervals on alternating sides of a 2 km track. Traps were secured to logs or trees at ground level and inspected after three nights for the presence of hairs. In the laboratory, we matched the samples to species using features of the hair medulla and cross-sectional shape [29]. We derived activity index estimates from the total number of hair traps at a site that had possum or devil hair present.
(c). Giving-up densities
GUDs have been used to measure the temporal and spatial use of habitat by diverse terrestrial prey species in relation to predation risk [11,12,13,23]. This approach tests the decisions of the forager as it makes trade-offs between the nutritional reward gained from foraging in the patch with the costs of perceived predation risk, holding all other costs equal [30,31]. The method captures the aggregated effect of many behaviours and foraging preferences in a single measure [31] and it reduces the risk of bias arising in observational studies, which commonly focus on a single behaviour. GUDs can be used to understand the way prey species use the landscape and respond to environmental disruptions at multiple scales [13]. We also use this experimental procedure to determine whether possums find food patches faster when predation risk is lower, as a measure of the extent of their ground-foraging activity. Possums are morphologically adapted for arboreal activity and use trees to escape from predators such as devils, which are much less adept climbers. Differences in the GUD value, which is measured as the amount of food remaining in an artificial food patch at the end of the experimental period, reflect the differences in the risk of predation between patches [31].
We conducted the GUD experiment at 18 of the selected sites across the predation risk spectrum on mainland Tasmania and six sites on Maria Island, with three dry eucalypt forest and three coastal woodland scrub sites in each of the three disease outbreak regions and on Maria Island. Each site comprised 10 stations at least 100 m apart. At each station, we placed artificial food patches in environments that we categorized as ‘safe’ (at the base of an escape tree that a possum could climb to evade predators), and ‘risky’ (in open ground 5–12 m from any escape tree—limited by the proximity of neighbouring escape trees). We defined an escape tree as having a minimum of 10 cm in diameter at breast height [23], and where possible we selected trees that had evidence of possum use (e.g. scats or scratches). The distance to the nearest escape tree measured the potential ‘safety’ or ‘riskiness' of the food patch.
For a food patch, we filled a 4 litre round plastic container with a substrate of 2.5 litres of medium-sized (approx. 1.5 cm diameter) smooth river pebbles. We provisioned each tray with 100 sultanas mixed into the substrate. The container was fitted with a lid that had a 10 cm diameter hole cut into it, allowing either a head or a paw into the container but not both. This design allowed the possums to sort through the substrate but inhibited their ability to empty the container of pebbles and therefore food. We chose sultanas as the food-type because they are highly attractive to possums [23] and retain their integrity when wet, making them easy to identify and count. We considered counting to be more accurate than weighing, as dirt and moisture could affect the weight. To determine the species responsible for foraging in the food patch on a particular night, we placed double-sided adhesive tape around the edge of the container and identified the species of the hair collected. We also placed motion-activated cameras (Scout Guard SG 550) on a subset of the food patches to confirm that the species identified from hair were accurate. We deployed the feeding patches for four consecutive nights. Each morning, we recorded whether the food patch had been visited and collected and replaced the adhesive tape. If there was evidence of visitation, we counted the sultanas remaining and replenished the food patch with 100 fresh sultanas. The camera memory cards were collected at the end of each experiment. Once feeding patches were found by possums, they were invariably visited on subsequent nights.
Foraging by non-target species was limited by the design of the containers and substrate. Rarely did non-target species take more than 15% of the food and never more than 25%. Non-target species detected included Tasmanian pademelon, Bennetts wallaby, Southern brown bandicoot (Isoodon obesulus) and Rattus spp. The weight of the substrate and space within the container reduced small mammal activity and the lid prevented wallaby foraging, as observed from camera footage and assessed during an initial pilot study on feeding patch design. Where there was evidence of non-target species and no possum activity, data were discarded.
We conducted habitat surveys at each food patch to assess whether microhabitat variables were important predictors of an individual's anti-predator behaviour response. Microhabitat has been documented in previous studies to affect prey foraging behaviour in the presence of predators [7,13,23]. We recorded the following microhabitat variables in a 5 m radius of the food patch that could influence possums' foraging behaviour and predator detection: percentage open ground cover (percentage of the ground layer that is open or free of vegetation less than 50 cm in height); percentage shrub cover (50 cm to 2 m in height); and percentage tree canopy cover. Vegetative cover to 50 cm is likely to be most relevant to possums, whose height when erect is no more than 50 cm.
(d). Statistical analysis
(i). Activity patterns
To assess the effects of devils on possum activity pattern, we used generalized linear models (GLMs) with the number of hair-traps positive for possum hair for each site as the response variable. Predictor variables were the number of traps with devil hair (as a measure of local devil predation pressure during the study season), the year since DFTD arrival (as a measure of the extent of decline of devil populations and predation risk through time) and vegetation type (dry eucalypt forest or coastal woodland scrub). All combinations of predictor variables were modelled, giving a total of eight candidate models including the null model. We applied a negative binomial error structure with log-link function to account for over-dispersion in the data. To assess the support for alternative models, we used the weights (wi) derived from small sample corrected Akaike information criteria (AICc), where the weight provides the relative support for each model [32]. All models were fitted using R (R Development Core Team 2011, v. 2.11.0).
(ii). Time taken to find food patches
We applied survival analysis, using a Cox proportional hazards model [33], to data on the time taken for possums to find individual feeding patches across the predation risk gradient on mainland Tasmania. The data were right censored as not all of the food patches had been found by possums when the experiment concluded after four nights. For visualization purposes, we divided the sites into three DFTD arrival regions of long-term disease, mid-term disease and disease-free (figure 1). For the Cox model, we used a continuous variable of years since DFTD arrival as the measure of predation risk, in addition to the three microhabitat variables as for the GUDs analysis. The best model was chosen using AICc weights.
(iii). Giving-up densities
To assess the anti-predator behaviour of possums from their GUDs across the predation risk gradient on mainland Tasmania, we used generalized linear mixed models (GLMMs) with Gaussian error structure and identity link function (library ‘lme4’ in R v. 2.11.0). Stations (pairs of food patches) within sites and nights within each individual food patch were included as random effects. Stations were removed from the data where there was no evidence that possums had visited the patch, or where a definitive species identification could not be made. The relationship between the total amount of food (number of sultanas) remaining in a food patch, which represents the average GUD for the one or more possums that foraged at this patch, was modelled against the following predictor variables: the level of predation risk represented by the proxy of the number of years a site had been diseased, the three microhabitat variables, vegetation type and the measure of relative riskiness of the food patch represented by the distance in metres to an escape tree. We included interaction terms between distance to escape tree and both years diseased and vegetation type, because the importance of distance to safety for possums may vary with the density of the local devil population and with the availability of escape trees, the latter influenced by vegetation type. All combinations of predictor variables including interaction terms were modelled, together with the null model. Correlation between variables was assessed prior to inclusion in the models, using Pearson's correlation coefficient; no correlations were greater than 0.50.
To evaluate the extent to which devils retain ecological functionality in the long-term diseased sites, we compared those six sites with all sites on Maria Island. We used GLMM models in an analysis identical to that above except for the replacement of years since disease arrival with a dummy variable representing the presence (mainland sites; 1) or absence (Maria Island; 0) of any predation risk from devils.
3. Results
(a). Terrestrial possum and devil activity
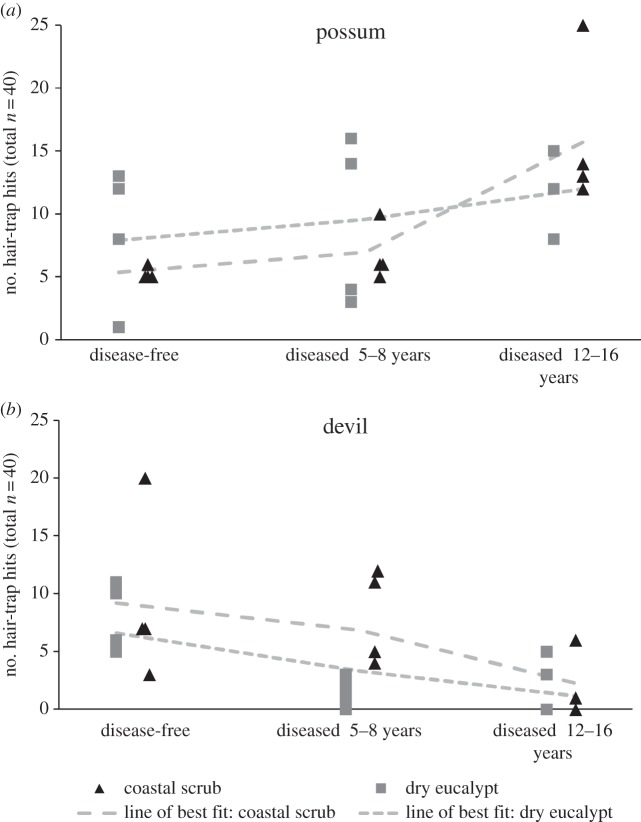
The effects of DFTD on devil populations were dramatic (figure 2) with few or no devil hairs detected at most sites where DFTD had been present for more than 8 years. This is in accordance with longitudinal data showing severe and significant declines within a few years of DFTD arrival [16,17]. Possum activity varied considerably between sites and possums maintained populations in all areas of the state even in the presence of high devil populations (figure 2). The number of hair traps in which possum hair was found indicated that there was higher ground-based possum activity with increasing number of years since DFTD arrival and subsequent devil decline. The model containing this single variable ranked highest, carrying 60% of the weight in the candidate model set, and its relative importance was 95% (table 1). The second- and third-ranked models contained the length of time diseased and either devil activity or vegetation type, respectively. The relative importance of both of these variables was low at 22% and 20%, respectively.
Figure 2.
(a,b) Numbers of hair traps per site in which Tasmanian devil or brushtail possum hair was recorded, representing an index of animal activity, in two vegetation types (dry eucalypt forest and coastal woodland scrub) across the three DFTD outbreak regions, corresponding to different levels of predation risk from devils. Dashed lines represent the lines of best fit derived from the negative binomial GLM model predictors for each vegetation type.
Table 1.
Model outputs from GLM analysis assessing factors affecting the activity index of brushtail possums measured through hair-trap hit rates. Table shows the model rank, change in AICc (ΔAICc) from top model, model weights (wi), number of model parameters (k) and model co-efficient estimates ±s.e. for each explanatory variable.
| model rank | ΔAICc | wi | k | intercept | years diseased | devil abundance | vegetation type |
|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 0.60 | 3 | 1.85 ± 0.15 | 0.05 ± 0.02 | ||
| 2 3 4 5 (null) | 2.66 2.72 5.55 6.66 | 0.16 0.15 0.04 0.02 | 4 4 5 2 | 1.74 ± 0.26 1.81 ± 0.18 1.65 ± 0.31 2.26 ± 0.11 | 0.06 ± 0.02 0.05 ± 0.020.06 ± 0.02 | 0.01 ± 0.020.02 ± 0.03 | 0.08 ± 0.190.11 ± 0.20 |
| relative importance of variable | 95 | 22 | 20 | ||||
(b). Time to find food patches
Possums found food patches much sooner in areas where DFTD had been present the longest (figure 3), with years since DFTD arrival significantly and positively associated with the time it took for this to occur (relative importance of parameter 100%; table 2). For every year since DFTD arrival, there was an estimated 4.5% increased chance of a food patch being found. Possums discovered food patches earlier in areas with a higher proportion of open ground (relative importance 100%), and in coastal woodland scrub. There was some evidence to suggest that tree canopy cover and shrub cover may have influenced whether possums found food patches. The weight of the models containing these variables, which indicates the relative importance of the variables, was 55% and 33%, respectively (table 2).
Figure 3.
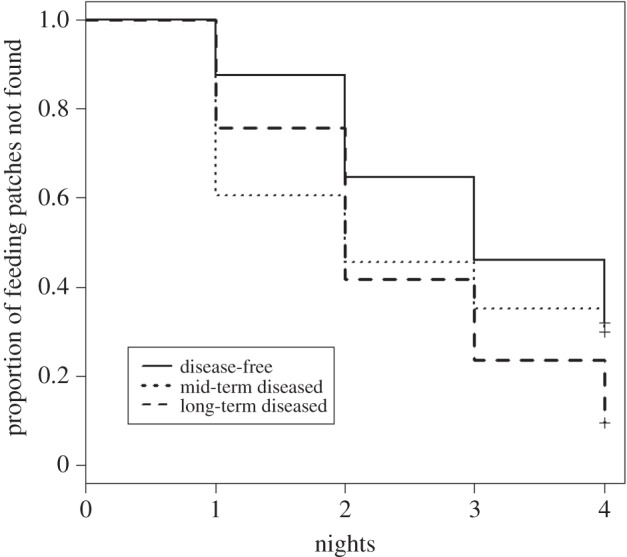
Kaplan–Meier estimates of the survival function for the proportion of food patches not found by brushtail possums over the four nights they were deployed in different DFTD outbreak regions.
Table 2.
The set of Cox proportional hazards models within ΔAICc of 2.0 of the top model for the number of nights for a brushtail possum to find food patches across a predation risk gradient. Table shows the model rank, change in AICc (ΔAICc), model weights (wi) and model co-efficient estimates ±s.e. for each explanatory variable. The relative importance of variables denotes the sum of weights of all candidate models containing the variable. Not all models are shown.
| model rank | ΔAICc | wi | vegetation type (dry) | open ground cover | years since DFTD arrival | tree canopy cover 5 m | shrub cover |
|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 0.27 | −0.310 ± 0.127 | 0.015 ± 0.003 | 0.044 ± 0.010 | 0.003 ± 0.002 | |
| 2 | 0.25 | 0.24 | −0.325 ± 0.126 | 0.015 ± 0.003 | 0.041 ± 0.010 | ||
| 3 | 1.75 | 0.11 | −0.316 ± 0.127 | 0.016 ± 0.004 | 0.044 ± 0.010 | 0.002 ± 0.003 | |
| 4 | 1.81 | 0.11 | −0.304 ± 0.127 | 0.015 ± 0.004 | 0.045 ± 0.010 | 0.003 ± 0.002 | 0.002 ± 0.003 |
| 5 (null) | 42 | 0.00 | |||||
| relative importance of variable | 92 | 100 | 100 | 55 | 33 | ||
(c). Giving-up densities
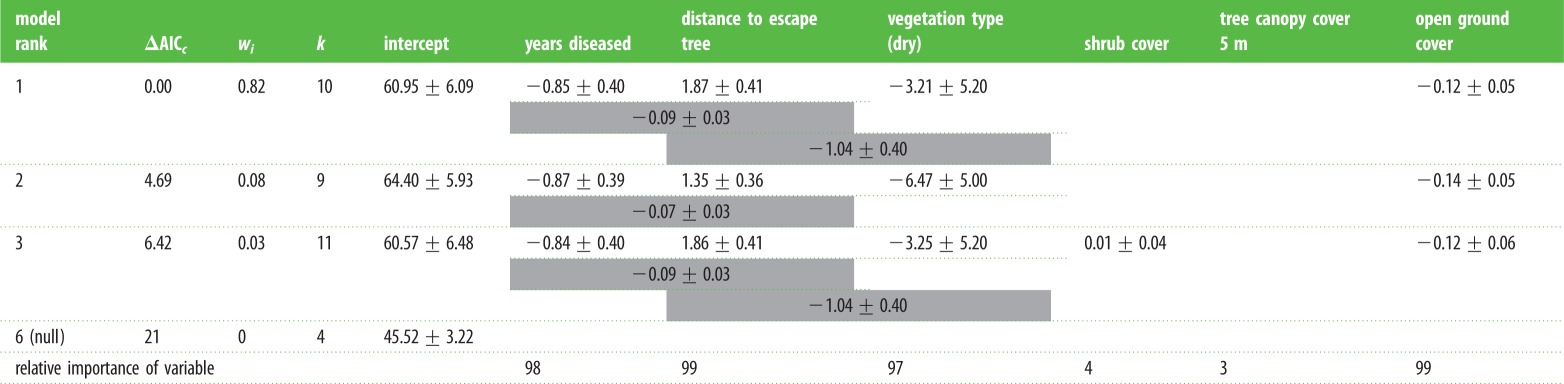
The most parsimonious model explaining the amount of food remaining in artificial food patches received 82% of the weight in the candidate model set (table 3). It included four main effects: percentage of open ground, distance to escape tree, years diseased and vegetation type. There were two interaction terms: distance from escape tree became less important as the number of years since DFTD arrival increased; and distance to escape tree was more important in coastal woodland scrub than in dry eucalypt forest (table 3; electronic supplementary material, figure S4). The major difference in GUDs between ‘risky’ and ‘safe’ occurs in the disease-free area, with the other sites with lower devil density being much more similar (electronic supplementary material, figure S4). In addition, the more open the ground layer surrounding a food patch, the lower the GUDs, suggesting that possums perceive feeding patches in open ground to be safer and to have a lower cost. Shrub cover and tree canopy cover did not play a significant role in explaining the GUDs for possums.
Table 3.
Most parsimonious GLMM models for the number of sultanas remaining in a feeding patch. Table shows the model rank, change in AICc (ΔAICc) from the top model, model weights (wi), number of model parameters (k) and parameter estimates ±s.e. for each explanatory variable included in the models, together with interactions (indicated by shaded cells between adjacent explanatory variables). The relative importance of the variable denotes the sum of weights of all candidate models containing the variable. Not all models are shown.
 |
The ability of possums to distinguish between very low devil densities at long-diseased sites in the northeast of Tasmania and the long-term absence of devils on Maria Island was equivocal. There were 14 models in the candidate set, all of them with low weight (weight 0.09–0.03; electronic supplementary material, table S1). The interaction between distance to escape tree and vegetation type was the most important variable (top model; relative importance of the main effect parameters 42% and 39%, respectively). Models including tree canopy cover had a combined weight of 40% and shrub cover of 28%. The presence (northeast mainland Tasmania) or absence (Maria Island) of devils had a relative importance of 29% among all model weights and indicated that GUDs are higher on Maria Island. The models comparing the offshore island sites and the low devil presence sites gave almost no importance to open ground cover (14% of model weights), whereas it was important when comparing sites where some devils were present (98% of model weights).
4. Discussion
Our results indicate rapid change in risk-sensitive foraging behaviours in the brushtail possum, in a direct and dynamic response to the recent and ongoing decline of their major mammalian predator, the Tasmanian devil. As devil abundance declined, possums increased their terrestrial activity, foraging for longer and further from refuge. Severe population decline of the predator resulted in loss of effective top–down control in the foodweb, at least with respect to risk-sensitive habitat use and foraging behaviour in a major prey species; we found no differences in ground-foraging behaviour in possums between northeast Tasmania, where devils had experienced at least 90% population decline, and Maria Island, which at the time was devil-free. Our results reinforce assertions of the rapidity of behavioural change following loss of an apex predator [3,34]; with complete relaxation of predator-specific risk-sensitive behaviours over large regions within a 10-year period. Possums responded differently to microhabitats on mainland and island sites, indicating flexible risk-sensitive behaviours targeted to different types of predators.
This study surveyed sites with varied DFTD arrival times to investigate the impact on brushtail possums of time since devil decline commenced. This substitution of space for time was unavoidable, given that we wanted to investigate the effects of devil decline over a timescale of at least a decade. A range of longitudinal information on devil and possum numbers available from other sources provides confidence that this substitution of space for time does not introduce major confounding effects. Analysis of more than 20 years of animal counts collected during spotlighting surveys along 10 km stretches of road, shows that densities of devils from 1985 to 1995, before DFTD had been recorded, were similar (0.4–0.6/10 km) in each of three regions we studied [19]. Numbers declined dramatically following disease arrival, but no such decline was evident in the disease-free northwest [19].
The higher levels of terrestrial activity of possums in areas with lower predation risk, evident from the hair-trap surveys and the earlier detection of food patches, may represent an increase in ground-foraging behaviour (microhabitat use), changes in vegetation use (macrohabitat use), greater population abundance or combinations of all three. While this evidence, corroborated by spotlight data [19], provides consistent results, it does not shed light on whether the underlying change is behavioural or demographic. The spotlighting data indicated consistently lower numbers of possums in northwest Tasmania, which is not diseased and still has high devil densities, and a slight increase in possum numbers in the long-time diseased northeast now with few devils, although possum numbers were also strongly affected by bottom–up factors such as rainfall [19].
Possum GUDs provide evidence of risk-responsive changes in behaviour irrespective of changes in population density. Possums foraged food patches to a lower GUD further from refuge in response to disease-induced devil decline, reflecting favourable trade-offs between food harvest rate and reduced predation risk. For many vertebrates, physical characteristics of the environment significantly affect their perceived level of risk and they will modify their use of space accordingly [7,12,13]. The negative interaction between foraging distance to an escape tree and time since DFTD outbreak shows that as predation risk from devils is reduced, possums are much less sensitive to the distance at which they will forage from refuge. Possums prefer open ground cover that offers good line of sight when foraging on the ground. Similar behaviour has been observed in other medium-sized mammals, including free-ranging domestic goats [35], where low, dense vegetation can reduce landscape surveillance and predator detection [13,35]. This contrasts with many small terrestrial prey species such as rodents having higher GUDs in open habitats, which represent higher risk of predation from visual predators such as owls and cats [30,36,37].
The somewhat equivocal inclusion of the presence of devils in the large set of candidate models to distinguish possum GUDs between the northeast Tasmanian mainland and Maria Island suggests that low devil density no longer has a substantial effect on possum behaviour. Maria Island lacked devils probably since the end of the last glacial period 10 000 years BP. Possums were introduced 50 years ago from mainland Tasmanian populations that coexisted with devils. These results reinforce the rapidity of complete relaxation of predator-specific risk-sensitive behaviours when the apex predator disappears.
Loss of top–down control with respect to devils influencing possum behaviour may be a forewarning of significant ecological changes in Tasmania, as devil populations are likely to remain at very low levels for decades. Over longer timeframes, behaviour is linked to fitness, through survival and reproductive output, and can lead to a demographic response and increased abundance [38]. Indeed, indirect effects of predators on prey behaviour can comprise a substantial proportion of the total effect of a predator on prey [1,7]. In the presence of grey wolves (Canis lupus), elk (Cervus elaphus) alter behavioural responses that are associated with decreased reproduction and reduced population size [39]. At least in the wolf/elk study system, indirect effects of wolf presence on elk fitness were explained by the nutritional costs associated with less optimal foraging opportunities [40].
Any positive changes to the population dynamics and behaviour of herbivore prey species from the disruption of predator–prey relationships have the potential to initiate a trophic cascade [3,41]. Initially, this would occur through increases in herbivore populations, with ramifications for vegetation communities and other biotic and abiotic elements (e.g. [42]). The substantial culling of brushtail possums and other native herbivores across Tasmania for grazing protection and forestry may limit the scale of trophic cascades resulting from increases in herbivores.
Anti-predator behaviours can be energetically demanding and therefore costly to maintain for an organism [7,30]; however, in the absence of predators, some traits can be retained for hundreds of generations [15]. If a predator is functionally absent from ecosystems for a long time, the presence of other predators can help to maintain general anti-predator behaviours even if risk-sensitive behaviours specific to a predator species that has declined are lost [3]. Evidence for this in Tasmanian ecosystems is in the different responses of possums to microhabitat between northeast Tasmania and Maria Island, which have different suites of predators. In addition to the devil, the Tasmanian mainland has both mammalian and avian mesopredators of possums: the largely nocturnal spotted-tailed quoll (Dasyurus maculatus) and feral cat (Felis catus) [43,44], nocturnal masked owl (Tyto novaehollandiae) (which prey on juvenile possums) and the diurnal/crepuscular wedge-tailed eagle (Aquila audax). Maria Island has these avian predators and feral cats but no quolls and no devils at the time of the study. On the Tasmanian mainland, possums prefer open ground cover when foraging on the ground, which may allow early detection of terrestrial predators by impeding the predators' ability to conceal themselves on approach. Brushtail populations on mainland Australia also prefer open ground cover at low and medium terrestrial predator densities, but not when predation risk is high [22,23]. On Maria Island, where avian predators predominate, possums show a preference not for open ground cover but for higher tree canopy cover, which is likely to offer the best protection from avian predators. Prey species can respond rapidly to changes in predation risk from different types of predators that provide different risk landscapes, whereby behavioural decisions that reduce risk from one predator increase risk from another [45,46]. Gerbils (Gerbillus sp.), for example, avoid open ground in the presence of owls [37], but when faced with the combined risk of predation from owls and vipers, increased their exposure to owls [46].
The importance of distance to an escape tree is most likely a reflection of possums' being highly arboreal and their best chance of escaping any predator is to climb a tree. The distance to an escape tree was important, and more so when devil density was high, and irrespective of the predator suite, demonstrated here and from studies on mainland Australia [22,23]. Habitat type also influenced distance to an escape tree. It became less important in the more structurally complex habitat, dry eucalypt forest, which may offer multiple modes of escape.
Reintroduction of apex predators into ecosystems may be one way to re-establish predator–prey interactions and limit the magnitude of trophic cascades. The re-introduction of grey wolves into Yellowstone Park in North America 100 years after they were extirpated, resulted in both behavioural and density changes of herbivore populations, leading to reduced grazing pressure [42]. The expected outcome of the facial tumour epidemic in devils based on other host/pathogen interactions is evolution of reduced pathogen virulence leading to coexistence [47], a course followed by the only other naturally occurring transmissible cancer, Canine Transmissible Venereal Sarcoma [48]. Evolution of DFTD to an endemic disease that allows persistence or recovery of the devil is likely to take decades, a timescale on which evolutionary change in prey populations could occur.
The extensive loss of apex predators worldwide is having far-reaching effects linking all levels of foodwebs in often complex and unexpected ways and leading to trophic simplification [6]. The importance of anti-predator behaviour in prey may be under-appreciated as an influential driver of ecosystems. However, it can be difficult to distinguish between density- and behaviour-related effects of browsers and grazers, and the evidence for behaviourally mediated trophic cascades remains controversial [49,50]. Changes in prey behaviour in response to loss of a predator may be among the most rapid responses to such a decline and may provide an early warning and index of change. Prolonged periods of apex predator loss may compromise prey behaviour and species interactions [3] to the detriment of ecosystem dynamics and stability.
Supplementary Material
Supplementary Material
Acknowledgements
We sincerely thank the volunteers who assisted with fieldwork.
Data accessibility
All data are available on request from T.H. at tracey.hollings@unimelb.edu.au
Authors' contributions
T.H. designed study, conducted fieldwork, performed statistical analyses and wrote the manuscript; H.M. contributed to study design and statistical analysis; N.M. contributed to study design; K.K. conducted fieldwork for Maria Island; M.J. contributed to study design, statistical analyses and writing the manuscript. All authors contributed substantially to revisions.
Competing interests
We have no competing interests.
Funding
The study was funded with an Eric Guiler Tasmanian Devil Research Grant through the Save the Tasmanian Devil Appeal Australian Research Council Discovery grant DP110103069 “Keystone effects of Australia's top predators: dingoes, devils and biodiversity”.
References
- 1.Heithaus MR, Frid A, Wirsing AJ, Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. ( 10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 2.Terborgh J, et al. 2001. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926. ( 10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- 3.Berger J. 1999. Anthropogenic extinction of top carnivores and interspecific animal behaviour: implications of the rapid decoupling of a web involving wolves, bears, moose and ravens. Proc. R. Soc. Lond. B 266, 2261–2267. ( 10.1098/rspb.1999.0917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripple WJ, Beschta RL. 2006. Linking a cougar decline, trophic cascade, and catastrophic regime shift in Zion National Park. Biol. Conserv. 133, 397–408. ( 10.1016/j.biocon.2006.07.002) [DOI] [Google Scholar]
- 5.Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS. 2005. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330. ( 10.1890/04-0953) [DOI] [Google Scholar]
- 6.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 7.Lima SL. 1998. Nonlethal effects in the ecology of predator–prey interactions. BioScience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 8.Brown J, Laundré JW, Gurung M. 1999. The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 80, 385–399. ( 10.2307/1383287) [DOI] [Google Scholar]
- 9.Fuelling O, Halle S. 2004. Breeding suppression in free-ranging grey-sided voles under the influence of predator odour. Oecologia 138, 151–159. ( 10.1007/s00442-003-1417-y) [DOI] [PubMed] [Google Scholar]
- 10.Krebs CJ. 2011. Of lemmings and snowshoe hares: the ecology of northern Canada. Proc. R. Soc. B 278, 481–489. ( 10.1098/rspb.2010.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob J, Brown JS. 2000. Microhabitat use, giving-up densities and temporal activity as short- and long-term anti-predator behaviors in common voles. Oikos 91, 131–138. ( 10.1034/j.1600-0706.2000.910112.x) [DOI] [Google Scholar]
- 12.Jones M, Mandelik Y, Dayan T. 2001. Coexistence of temporally partitioned spiny mice: roles of habitat structure and foraging behaviour. Ecology 82, 2164–2176. ( 10.1890/0012-9658(2001)082[2164:COTPSM]2.0.CO;2) [DOI] [Google Scholar]
- 13.Druce DJ, Brown JS, Kerley GIH, Kotler BP, Mackey RL, Slotow ROB. 2009. Spatial and temporal scaling in habitat utilization by klipspringers (Oreotragus oreotragus) determined using giving-up densities. Austral Ecol. 34, 577–587. ( 10.1111/j.1442-9993.2009.01963.x) [DOI] [Google Scholar]
- 14.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumstein DT. 2002. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 29, 685–692. ( 10.1046/j.1365-2699.2002.00717.x) [DOI] [Google Scholar]
- 16.McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, Beeton N, Lazenby B. 2009. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology 90, 3379–3392. ( 10.1890/08-1763.1) [DOI] [PubMed] [Google Scholar]
- 17.Lachish S, Jones M, McCallum H. 2007. The impact of disease on the survival and population growth rate of the Tasmanian devil. J. Anim. Ecol. 76, 926–936. ( 10.1111/j.1365-2656.2007.01272.x) [DOI] [PubMed] [Google Scholar]
- 18.Hollings T. 2013. Ecosystem effects of disease-induced apex predator decline: the Tasmanian devil and devil facial tumour disease (DFTD). PhD thesis, University of Tasmania, Hobart, Australia. [Google Scholar]
- 19.Hollings T, Jones M, Mooney N, McCallum H. 2013. Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Conserv. Biol. 28, 63–75. ( 10.1111/cobi.12152) [DOI] [PubMed] [Google Scholar]
- 20.While GM, McArthur C. 2006. Distance from cover affects artificial food-patch depletion by macropod herbivores. Wildl. Res. 33, 565–570. ( 10.1071/WR05063) [DOI] [Google Scholar]
- 21.Nielsen AS. 2009. The potential impact of Tasmanian devil (Sarcophilus harrisii) decline on prey behaviour. Honours thesis, University of Tasmania, Hobart, Australia. [Google Scholar]
- 22.Cruz J, Sutherland DR, Anderson DP, Glen AS, Paul J, Leung LK-P. 2013. Antipredator responses of koomal (Trichosurus vulpecula hypoleucus) against introduced and native predators. Behav. Ecol. Sociobiol. 67, 1329–1338. ( 10.1007/s00265-013-1561-2) [DOI] [Google Scholar]
- 23.Pickett KN, Hik DS, Newsome AE, Pech RP. 2005. The influence of predation risk on foraging behaviour of brushtail possums in Australian woodlands. Wildl. Res. 32, 121–130. ( 10.1071/WR03098) [DOI] [Google Scholar]
- 24.Rounsevell D. 1989. Managing offshore island reserves for nature conservation in Tasmania. In Australian and New Zealand islands: nature conservation values and management (ed. Burbidge A.), pp. 157–161. Perth, Australia: Department of Conservation and Land Management. [Google Scholar]
- 25.McCallum H, Tompkins DM, Jones M, Lachish S, Marvanek S, Lazenby B. 2007. Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth 4, 318–325. ( 10.1007/s10393-007-0118-0) [DOI] [Google Scholar]
- 26.le Mar K, McArthur C. 2005. Habitat selection by common brushtail possums in a patchy eucalypt-forestry environment. Aust. Mammal. 27, 119. [Google Scholar]
- 27.Hocking GJ. 1981. The population ecology of the brush-tail possum, Trichosurus vulpecula, in Tasmania. Hobart, Australia: University of Tasmania. [Google Scholar]
- 28.Driessen MM, Hocking GJ. 1992. Review and analysis of spotlight surveys in Tasmania: 1975–1990. Scientific Report. March, 1992. Report no. 92, Department of Parks, Wildlife and Heritage, Tasmania, Australia. [Google Scholar]
- 29.Triggs B, Brunner H, Ltd EP. 2002. Hair ID: an interactive tool for identifying Australian mammalian hair. Collingwood, VA: CSIRO Publishing. [Google Scholar]
- 30.Brown JS, Kotler BP. 2004. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 7, 999–1014. ( 10.1111/j.1461-0248.2004.00661.x) [DOI] [Google Scholar]
- 31.Brown JS. 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47. ( 10.1007/BF00395696) [DOI] [Google Scholar]
- 32.Burnham KP, Anderson DR. 2002. Model selection and multimodal inference: a practical information and theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 33.Hosmer DW, Lemeshow S, May S. 2008. Applied survival analysis: regression modeling of time-to-event data, 2nd edn Hoboken, NJ: Wiley Interscience. [Google Scholar]
- 34.Reznick DN, Ghalambor CK, Crooks K. 2008. Experimental studies of evolution in guppies: a model for understanding the evolutionary consequences of predator removal in natural communities. Mol. Ecol. 17, 97–107. ( 10.1111/j.1365-294X.2007.03474.x) [DOI] [PubMed] [Google Scholar]
- 35.Shrader AM, Brown JS, Kerley GIH, Kotler BP. 2008. Do free-ranging domestic goats show ‘landscapes of fear’? Patch use in response to habitat features and predator cues. J. Arid Environ. 72, 1811–1819. ( 10.1016/j.jaridenv.2008.05.004) [DOI] [Google Scholar]
- 36.McGregor HW, Legge S, Jones ME, Johnson CN. 2014. Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 9, e109097 ( 10.1371/journal.pone.0109097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotler BP, Brown JS, Hasson O. 1991. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72, 2249–2260. ( 10.2307/1941575) [DOI] [Google Scholar]
- 38.Morris DW, Kotler BP, Brown JS, Sundararaj V, Ale SB. 2009. Behavioral indicators for conserving mammal diversity. Ann. NY Acad. Sci. 1162, 334–356. ( 10.1111/j.1749-6632.2009.04494.x) [DOI] [PubMed] [Google Scholar]
- 39.Creel S, Christianson D, Liley S, Winnie JA. 2007. Predation risk affects reproductive physiology and demography of elk. Science 315, 960 ( 10.1126/science.1135918) [DOI] [PubMed] [Google Scholar]
- 40.Creel S, Winnie JA, Christianson D. 2009. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proc. Natl Acad. Sci. USA 106, 12 388–12 393. ( 10.1073/pnas.0902235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 42.Ripple WJ, Beschta RL. 2007. Restoring Yellowstone's aspen with wolves. Biol. Conserv. 138, 514–519. ( 10.1016/j.biocon.2007.05.006) [DOI] [Google Scholar]
- 43.Molsher R, Newsome A, Dickman C. 1999. Feeding ecology and population dynamics of the feral cat (Felis catus) in relation to the availability of prey in central-eastern New South Wales. Wildl. Res. 26, 593–607. ( 10.1071/WR98058) [DOI] [Google Scholar]
- 44.Belcher CA. 1995. Diet of the Tiger Quoll Dasyurus maculatus in East Gippsland, Victoria. Wildl. Res. 22, 341–357. ( 10.1071/WR9950341) [DOI] [Google Scholar]
- 45.Korpimaki E, Koivunen V, Hakkarainen H. 1996. Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation? Behav. Ecol. 7, 30–34. ( 10.1093/beheco/7.1.30) [DOI] [Google Scholar]
- 46.Kotler BP, Blaustein L, Brown JS. 1992. Predator facilitation: the combined effects of snakes and owls on the foraging behavior of gerbils. Ann. Zool. Fennici 29, 199–206. [Google Scholar]
- 47.Dybdahl MF, Storfer A. 2003. Parasite local adaptation: red queen versus suicide king. Trends Ecol. Evol. 18, 523–530. ( 10.1016/S0169-5347(03)00223-4) [DOI] [Google Scholar]
- 48.Murchison EP. 2008. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene 27, S19–S30. ( 10.1038/onc.2009.350) [DOI] [PubMed] [Google Scholar]
- 49.Creel S, Christianson D. 2009. Wolf presence and increased willow consumption by Yellowstone elk: implications for trophic cascades. Ecology 90, 2454–2466. ( 10.1890/08-2017.1) [DOI] [PubMed] [Google Scholar]
- 50.Kauffman MJ, Brodie JF, Jules ES. 2010. Are wolves saving Yellowstone's aspen? A landscape-level test of a behaviorally mediated trophic cascade. Ecology 91, 2742–2755. ( 10.1890/09-1949.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on request from T.H. at tracey.hollings@unimelb.edu.au