Abstract
Behavioural studies over half a century indicate that making categorical choices alters beliefs about the state of the world. People seem biased to confirm previous choices, and to suppress contradicting information. These choice-dependent biases imply a fundamental bound of human rationality. However, it remains unclear whether these effects extend to lower level decisions, and only little is known about the computational mechanisms underlying them. Building on the framework of sequential-sampling models of decision-making, we developed novel psychophysical protocols that enable us to dissect quantitatively how choices affect the way decision-makers accumulate additional noisy evidence. We find robust choice-induced biases in the accumulation of abstract numerical (experiment 1) and low-level perceptual (experiment 2) evidence. These biases deteriorate estimations of the mean value of the numerical sequence (experiment 1) and reduce the likelihood to revise decisions (experiment 2). Computational modelling reveals that choices trigger a reduction of sensitivity to subsequent evidence via multiplicative gain modulation, rather than shifting the decision variable towards the chosen alternative in an additive fashion. Our results thus show that categorical choices alter the evidence accumulation mechanism itself, rather than just its outcome, rendering the decision-maker less sensitive to new information.
Keywords: confirmation bias, perceptual decision-making, numerical averaging, changes of mind
1. Introduction
Intensive research on the neural basis of decision-making has revealed a mechanism of noisy evidence-integration towards a decision criterion [1]. This mechanism was demonstrated to be optimal in terms of achieving the fastest decision-time for a given accuracy [2–6], and to account for a wide range of behavioural [7] and neural [8–10] data. However, the process that takes place after the integrated evidence reaches the criterion is less well understood [11,12]. How does the triggering of a decision affect the way that we evaluate or accumulate additional evidence, if new information becomes available?
Consider, for example, a real-life situation whereby we deliberate which of several alternative apartments to purchase, or which political candidate to vote for, until we finally ‘make up’ our mind. On many occasions, following the decision but before its execution, there is a time window during which additional relevant information may become available (change in prices, political crises, etc.). Do we continue to accumulate evidence in the same fashion, once an initial decision has been made? A common introspection is that we are likely to give less weight to such evidence, compared with a situation in which the same evidence reaches us somewhat earlier, before we ‘made up’ our mind. While some early behavioural work indicates that people might give less weight to additional evidence after reaching a decision ([13–17]; reviewed in [18]), the mechanisms underlying these so-called commitment and confirmation-bias effects have, so far, remained elusive.
We aimed to examine and quantify the mechanisms underlying these post-decisional effects, by combining novel psychophysical protocols with computational modelling techniques. Based on indirect evidence from social psychology [13,16], we expected that observers would be less likely to take into account additional information that follows a preliminary decision as compared to a control situation entailing the presentation of identical information and similar motor responses, but without a preliminary decision.
Critically, several alternative mechanisms might give rise to such a behavioural pattern. It is possible that after deciding, the decision-maker becomes less sensitive to new-arriving information (i.e. integrates additional evidence with a reduced-gain; we label this mechanism ‘reduced-gain’). As we show in the electronic supplementary material, this hypothesis is consistent with the idea that decisions induce a transition to attractor-dynamics [19–25] which are stable and resistant to perturbations [25–27]. Alternatively, deciding may trigger a decision-consistent shift in the perceived value of the chosen alternative (we label this mechanism ‘value-shift’). For example, when deciding that a stock is worth investing in, we may overestimate its value [14]. A similar effect was also observed in a combined motion direction discrimination and continuous estimation task, in which the estimations were biased towards the direction of the decision [28]. Such value-shifts, while not reducing sensitivity to additional information, might nonetheless result in a relative underweighting of additional information (see figure 1c; electronic supplementary material, figure S3D).
Figure 1.
Experiment 1: design, predictions and overall accuracy: (a) observers were presented with eight numbers (500 ms per item), after which they made either: (i) a binary choice about the sequence's average (only-decision), (ii) a binary choice about the average, followed by an additional eight number sequence, after which they made an estimation of the mean of the entire sequence (decision-extra-evidence; DEE), or (iii) a non-decisional motor response, followed by additional eight numbers, followed by a mean-estimation of the entire sequence (no-extra-evidence; NEE); (b) the distributions from which the numbers were sampled. The first eight numbers were sampled either from the red (M = 46) or from the blue (M = 54) distribution. The additional eight numbers were independently sampled from one of the four distributions; (c) different predictions of the reduced-gain and the value-shift mechanisms (for decision <50). The divergent lines correspond to how the evaluation changes with each additional item. The reduced-gain predicts that differences (i.e. spread) between extra evidence profiles (solid and dashed lines) will diminish (red lines), while the value-shift model does not (blue lines). (d) Upper panel: observed (black bars) and predicted (blue line) accuracy in the preliminary decision, as a function of the sequence' mean absolute deviation from the decisional criterion (50) and lower panel: scatter plot of estimations (y-axis) as a function of sequence's mean (x-axis) of a single participant; error bars denote 1 s.e.m. (Online version in colour.)
To establish general principles, we conducted two complementary experiments in which observers are presented with non-stationary evidence [29] and are allowed to make preliminary decisions. The first experiment used evaluations of sequences of numerical values, which were described as monetary pay-offs (figure 1a), and the second one used perceptual evidence (figure 3a). In both experiments, we obtained a clear confirmation-bias effect: observers underweighted evidence that followed their preliminary decision. Moreover, computational analyses support the reduced-gain hypothesis according to which decisions trigger a reduction in the sensitivity of the observer to evidence that follows the decision, and are inconsistent with the idea that decisions induce a value-shift towards the chosen alternative.
Figure 3.

Experiment 2: (a) illustration of the stimuli: on each trial, one of the disc's brightness level stochastically dominated the other; (b) experimental design: in the first experimental session, observers were instructed to choose the dominating disc. After making a decision, additional information was presented for at least 1.5 s. Hereafter observers could either confirm, or change their decision (t2). The same observers attended a second experimental session, in which the same streams of information were ‘re-played’, and they were asked to make a single decision; (c) observed (blue bars) and simulated (black line) accuracy of the preliminary decision in session I, as a function of response time (RT) quantiles; we find no RT differences between correct and incorrect trials. Error bars denote 1 s.e.m. (Online version in colour.)
2. Material and methods
(a). Participants
Overall, 41 participants (range 21–29 years) participated in three experiments (N1 = 25; N2 = 10; N3 = 10; different participants in each experiment). The data of four participants in experiment 1 were not analysed (because of chance performance), bringing the number of participants to 21. All participants were undergraduate students recruited through the Tel-Aviv University Psychology Department's participant pool, were naive to the purpose of the experiment and had normal, or corrected-to-normal, vision. Participants were awarded either course credit for their participation or a small financial compensation (approx. $10). Participants received a performance-dependent bonus of additional 10–20 NIS.
(b). Stimulus materials and procedure
The basic set-up of a trial in experiment 1 is depicted in figure 1a. Each trial began with a central fixation cross (200 ms) after which a sequence of 8 two-digit numbers was presented (Arabic numerals; each number displayed for 500 ms). On 50% of trials (random), following the eight-number sequence, the participants were prompted to make a binary decision about whether the sequence's average was smaller or larger than 50, received auditory error-feedback and the trial terminated. On another 25% of trials (random), at the termination of the eight-number sequence, participants were prompted to make the same binary decision, yet following the decision no feedback was delivered, and an additional eight-number sequence appeared. At the termination of the second sequence of numbers, the participants were asked to convey as accurately as possible the average value of the entire 16-number sequence, by vertically sliding a mouse-controlled bar set on a number ruler between 0 and 100 (the number corresponding to the bar's location was concurrently displayed) and pressing the left mouse button when reaching the desired number. On the remaining trials (25%, random), after observing an eight-number sequence, participants were prompted to press the space bar key, after which an additional eight-number sequence was presented. At the termination of the second number sequence, the participants were asked to estimate the average value. After completing 20 practice trials, participants underwent 360 experimental trials divided into six blocks. Each block terminated with performance-feedback on the estimations (block-average correlation) and a short, self-paced break. In order to make the distinction between trials more salient, the numbers presented on each trial were either all green or red (on black background).
To generate each sequence of eight numbers, we predefined four triangular skewed-density distributions, ranged between 10 and 90; with means of 40, 46, 54 or 60 (figure 1b). The first eight numbers were sampled from one of the two intermediate distributions (means 46 or 54) and the additional eight-number sequence was independently sampled from one out of the four distributions (random between trials). In case two identical numbers were sampled successively, the entire sequence was shuffled in order to prevent successive presentation.
The design and stimuli used in experiment 2 are depicted in figure 3a,b. Participants were presented with two brightness-flickering discs. On each trial, one disc's brightness level stochastically dominated the other (see the electronic supplementary material, for a full description of the perceptual evidence). After the participants had made their preliminary decision, the fixation-cross turned red for 1.5 s, during which the stream of evidence continued. After 1.5 s of mandatory suspension the cross turned green indicating that the participants can either confirm or change their initial decision (using the left/right keypads). After the second decision auditory error-feedback was delivered and the trial terminated. A week (±3 days) later, participants attended a second experimental session, in which the entire stream of evidence of each trial (identical stimuli) was re-presented (figure 3b; order of trials randomly shuffled). Participants were asked to decide upon the termination of the re-presented stimuli which of the discs had higher probability of being brighter. Auditory error-feedback was delivered after the decision.
All stimuli were generated using Matlab© and were presented on a gamma-corrected ViewSonic (Walnut, CA, USA) 17 inch monitor viewed at a distance of 41 cm. The screen resolution was set to 1024 × 768 pixels, and the monitor had a refresh rate of 60 Hz.
(c). Description of the models in experiment 1
In all models, xi is the number present at position i; ξi ∼ Ni(0, σ2) are stochastically independent (temporally and independent of the xi's) internal noise sampled from a normal distribution.
(i) Unbiased (null-hypothesis):
(ii) Global reduced-gain:
where ω1 = (1−ω2) and ω2 denotes the reduced-gain parameter.
(iii) Value-shift:
 |
where Δ denotes the value-shift parameter.
(iv) Selective reduced-gain:
 |
where ω1c = (1−ω2c), ω1i = (1−ω2i), ω2c denotes the reduced-gain parameter for congruent trials and ω2i for incongruent trials.
(v) Decision-induced fatigue:
where  and
and  denotes the internal noise parameter for the second interval.
denotes the internal noise parameter for the second interval.
The weights ω1 and ω2 were normalized to 1, in order to prevent systematic overestimations or underestimations, as the data did not show such a trend. The model-fitting procedures are described in the electronic supplementary material.
3. Results
(a). Decision effects on value-evaluations: reduced-gain to additional evidence
In the first experiment (N = 21), we examined how decisions affect the overall value that observers assign to a sequence of numbers. Previous research has indicated that human subjects can accurately integrate numerical values presented at a fast rate [30–32]. We asked observers to evaluate rapid sequences of two-digit numbers, representing payoffs of a certain slot machine (500 ms per number; randomly sampled from two out of four skewed distributions with different means; see Material and methods and figure 1b). On 50% of the trials, observers made a binary choice about the average value (higher or lower than 50) of eight successive numbers, and the trial terminated. These trials were introduced in order to ensure that the observers would give priority to the decision. On other trials (25%), the observers still made a binary decision, following which an additional stream of eight numbers was presented and the observers were asked to estimate the average value of the entire sequence (16 numbers) using an analogue scale between 0 and 100 (figure 1a). We label this condition decision-extra-evidence (DEE). The remaining 25% of trials were as the DEE trials, except that observers were asked to press the space bar key after the first eight numbers, instead of indicating a binary choice. We label this condition, no-decision extra-evidence (NEE). All trials were randomly intermixed, and subjects did not know which condition was presented until a response was prompted or the trial terminated (see Material and methods). Contrasting the two conditions (DEE/NEE) allows one to distinguish between the different mechanisms that can give rise to a confirmation-bias effect. While ‘reduced-gain’ predicts that the reduced sensitivity to the extra-evidence will result in diminished discrimination between different profiles of additional information, the ‘value-shift’ hypothesis predicts a change in the estimated values in the direction of the decision, without a diminished discrimination between additional values (figure 1c; electronic supplementary material, figure S3).
Observers were able to make accurate decisions (accuracy = 65.7%; t20 = 12.99; p < 0.0001; as compared to chance level (50%)), and their accuracy increased with the absolute deviation of the sequence's mean from the decisional-criterion (repeated measures ANOVA F20,100 = 11.43; p < 0.0001; figure 1d, black error bars). Further, the final evaluations were well correlated with the actual sequence average (M = 0.49; black circles, figure 1d, lower panel; see the electronic supplementary material for data analysis), indicating that the observers were able to accurately make binary decisions about the sequences as well as to estimate its average value on a continuous scale.
The main effect of interest was the difference in the evaluations given under the DEE and the NEE conditions to otherwise identical value sequences. We use several complementary measures in order to quantify the difference between DEE and NEE conditions and distinguish between the alternative mechanisms discussed above. All these measures indicate a clear effect of the preliminary decision on the final evaluation (figure 2). First, the accuracy of the evaluations (Pearson correlation of evaluations with the sequences' actual means) is lower for the DEE condition as compared to the NEE condition (t20 = −2.4; p = 0.026; figure 2a), suggesting that the decision reduces the efficiency of the subsequent-information processing. Second, we analysed the numerical difference between the evaluations made in trials in which the average additional information is higher than 50 to those made in trials where it is lower than 50 (‘spread’). This measure reflects the difference in estimations that are due to extra evidence. We find that in the DEE condition the spread is lower than in the NEE condition (t20 = −3.79; p = 0.001; figure 2b; this observation is present in 18 of the 21 participants). This suggests that in agreement with the predictions of the reduced-gain hypothesis, but not with the value-shift hypothesis, the participants exhibit a diminished sensitivity to the additional information, after making a decision. The diminished spread in the DEE condition is not predicted by the value-shift mechanism, since value-shift does not influence the processing of extra-evidence (figure 1c; electronic supplementary material, figure S3b). Third, in the DEE condition, participants are less accurate when estimating the average of sequences in which the mean additional information was incongruent to the decision as opposed to sequences, in which the additional information was congruent (t20 = −2.72; p = 0.013; figure 2c; the same pattern is observed using correlation; Corr_Cong = 0.6; Corr_Incong = 0.18; t20 = −7.36; p < 0.0005). This pattern is not predicted by the value-shift hypothesis (see the electronic supplementary material, figure S3c). Forth, when regressing the observers' evaluations on the mean of the first eight-numbers (interval 1) and of the additional eight-numbers (interval 2) from each trial, we find a highly significant interaction between the conditions (DEE, NEE) and the regression coefficients (F1,20 = 12; p = 0.002; figure 2d; see the electronic supplementary material, figure S1 for the full time-course regressions). Post hoc comparisons reveal that in the DEE condition, observers are more influenced by information from the first than the second interval (t20 = 4.43; p < 0.001), while weights do not differ between intervals in the NEE condition (t20 = 0.78; p = 0.44). Taken together, these results demonstrate that human observers lose sensitivity to subsequent information after making a decision.
Figure 2.
Decisions produce reduced-gain to extra evidence: data and model predictions (data are shown in black bars; simulations of the global gain-reduction model are shown in blue-dashed lines): (a) accuracy of estimations (Pearson correlation between estimations and sequences' mean) is lower in the decision (DEE) condition as compared to the no-decision condition (NEE); (b) spread (difference between average estimations of sequences in which the mean of the additional numbers are higher than 50 and lower than 50) in the DEE condition is lower than in the NEE condition; (c) accuracy (absolute deviations) in the DEE condition is higher for congruent as compared to incongruent trials; (d) regression weights in the DEE (blue bars) and NEE conditions (red bars). Error bars denote 1 s.e.m. (Online version in colour.)
An alternative account of these results (reduced-gain in the DEE condition as compared to the NEE condition) is that some participants assume that the evidence of the second stream is not providing any new information and thus stop integrating the information following the preliminary decision and base their evaluations on the first stream alone. If few participants were to rely on such a strategy, the group data could show reduced-gain, as an averaging artefact. To test this possibility, we tested whether any of the participants ignore the extra-evidence in the DEE condition. The results confirmed that all participants exhibit a certain degree of sensitivity to the additional information, as reflected by (i) a positive (greater than 0) spread in the DEE condition in 21 out of 21 observers; (ii) a significant (p < 0.05) regression coefficient for the additional mean (X2) in 20 out of 21 participants (p = 0.08 for the additional participant); and (iii) all participants (21 out of 21) have made final evaluations that were opposite to their preliminary decision on a substantial fraction of trials (M = 32%; s.d. = 8%; the minimal observed fraction was 16%); 72% of these ‘changes of mind’ (M = 23%; s.d. = 7%) occurred on trials in which the mean of the additional information was incongruent with the participants' preliminary decision. These three observations demonstrate that all participants had processed the additional information to a certain (but reduced) extent, and did not assume that the mean of the additional numbers is the same as the preliminary mean.
We used computational modelling to tease apart the different potential mechanisms mediating the effects of decision commitment on the evaluation of subsequent evidence (see Material and methods for a full description of the models and the electronic supplementary material for fitting procedures): (i) an unbiased (null-hypothesis) model, whereby all samples of information receive identical weight; (ii) a global reduction in gain for additional information; (iii) a decision-induced value-shift, in which the chosen alternative's value is shifted in the direction of the decision; and (iv) a variant of the gain-reduction, in which gain of additional evidence is selectively reduced only for evidence that is inconsistent with the preliminary decision (we label this model ‘selective-gain-reduction’). All the models were fitted to the observed decisions and evaluations using the maximal likelihood method (see Material and methods). We obtained, per each model, best fitting parameters and maximal likelihood estimations. Comparison of maximal likelihoods between models support the selective gain-reduction model (congruent_gain = 96%; incongruent_gain = 78%), followed closely by the global gain-reduction model (gain = 80%), whereas the other models (unbiased and value-shift) fall far behind. Bayesian information criterion comparisons, which penalize for the number of free-parameters, favour the global gain-reduction model (see the electronic supplementary material, table S1). Both the global reduction and the selective reduction models predict all the behavioural effects described above (see lines in figure 2a–d and electronic supplementary material, figure S2a–d), in contrast to the null-hypothesis and value-shift models (for the latter see the electronic supplementary material, figure S3).
In addition, we tested a hybrid, model which combines both global reduction and value-shift. This model's parameters converged onto the parameters of the original global reduction model (i.e. the value-shift parameter was zero). Finally, while the evaluations of the averages in the NEE condition were temporally unbiased (see the electronic supplementary material, figure S1, red line), since previous studies have reported recency-biased integration of numerical values in decision tasks [32], we have tested two variants of the reduced-gain model, which include leak (λ)—a decaying contribution of earlier items to the overall estimation. We find that in both model-variants λ = 0.04 (a decay per 500 ms item) in the DEE condition, suggesting that evaluations were weakly recency-biased (see the electronic supplementary material for models' description and results). Importantly, however, we find the same reduced-gain parameter (ω2 = 0.4), demonstrating the robustness of the reduced-gain result. Another prediction of the reduction in gain models, but not of the value-shift model, is that the ratio of the sensitivity between the DEE and NEE conditions (Spread_DEE/Spread_NEE) correlates with the ratio of accuracy between these conditions (Correlation_DEE/Correlation_NEE)—participants who show a stronger decrement in sensitivity for additional information, also show a stronger impairment in accuracy. This prediction is corroborated by the behavioural data (R = 0.69; see the electronic supplementary material, figure S4). Thus, the computational analysis provides support for the global reduction in gain model, closely followed by the selective gain model, suggesting that decisions trigger a reduction in sensitivity to additional information.
(b). Change of mind paradigm: reduced-gain leads to preference for early decision
In our second experiment (N = 10), we set out to test for the effects of decision-commitment in a paradigm that allows the observers to explicitly report changes of mind. Here we aimed at studying the common situation of making decisions between competing options whose values vary in time, and in which, a while after indicating our choice, we are asked to confirm or change it. During this ‘suspended’ time-window, additional evidence contradicting our preliminary choice may become available.
To create a laboratory analogue of this situation, we presented observers with two discs of fluctuating brightness [33,34] (figure 3a). On each trial, one side's brightness level stochastically dominating the other (sides counterbalanced; see Material and methods and the electronic supplementary material for details). In the first experimental session, observers were instructed to choose, when ready (free-response protocol), the disc that they believed to have the higher probability of being brighter. After making a decision, the fixation-cross turned red for 1.5 s and during this time-period additional information was presented (the perceptual stream continued). After 1.5 s of mandatory response suspension, the fixation cross turned green indicating that observers could either confirm, or change their initial decision (information was continuously displayed until the final decision). Approximately a week later, the same observers attended a second experimental session, in which the exact same streams of information were ‘re-played’ to them, and they were asked to make a single decision (which disc had an overall higher level of brightness) upon the termination of the complete stream on each trial (figure 3b).
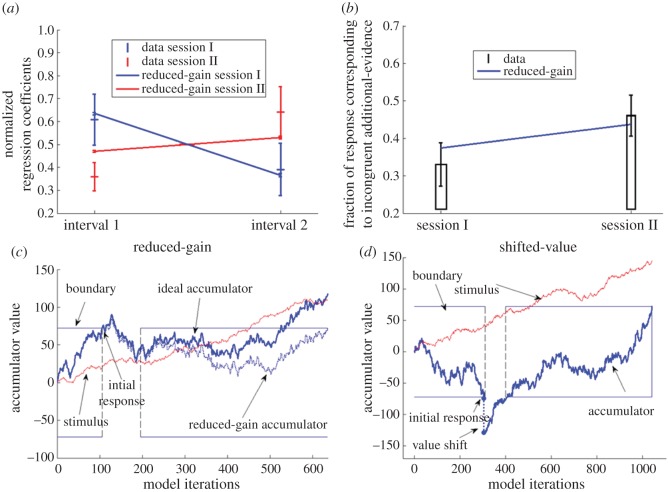
In the first experimental session (free-response), observers' accuracy in the preliminary decision was constant across their response-times (RT) quantiles (figure 3c), indicating that they integrated the perceptual evidence to a decisional boundary [35], rather than relying on idiosyncratic strategies, such an internal timer, which would have predicted an increasing accuracy with time (more evidence till timer runs out). In order to quantify the influence of the preliminary decision on the integration of additional information, we ran a logistic regression for participants’ final choice, using as predictors the perceptual evidence that was presented until the preliminary decision (interval 1) and the evidence following it (interval 2), separately for the two sessions. We find a significant interaction between (non-normalized) regression weights in the two sessions (repeated measures ANOVA of interval X session; F1,9 = 20.12; p = 0.001; figure 4a). Post hoc comparisons reveal that in the first experimental session, in which participants made a preliminary decision, more weight is ascribed to the information that preceded the decision as compared to the same information in session II, in which participants did not make a preliminary decision (t9 = 2.7; p = 0.025). Similarly, weight ascribed to additional information in session I, was lower than in session II (t9 = −2.93; p = 0.017), suggesting that the preliminary decision caused a reduction in the weight assigned to information that followed the decision, as compared to no-decision control.
Figure 4.
Experiment 2 data fits and illustration of the competing alternative mechanisms. (a) Observed and simulated normalized logistic regression weights for sessions I and II (blue and red, respectively). Data (bars) shows a crossover between sessions, this pattern is captured by the reduced-gain model (solid lines); (b) observed (bars) and simulated (blue line) fraction of trials in which observers responded according to additional incongruent information for session I and II. Data shows a reduced proportion of these trials in session I as compared to session II. This pattern is captured by the reduced-gain model; (c) illustration of the reduced-gain drift-diffusion model (dashed blue line), in which sensitivity to additional information is reduced following a decision; (d) illustration of the value-shift drift-diffusion model, in which the accumulator value is shifted (dashed line) following the preliminary decision. Error bars denote 1 s.e.m. (Online version in colour.)
We finally conducted a control experiment (experiment 3; N = 10) to test the possibility that the change in temporal weights is due to an order effect (participants having done the no-preliminary decision task in a second session). We presented each participant with the exact perceptual stream that was presented to one of the experiment 2 participants, in session II. We found that the regression-coefficients are very similar to those observed in experiment 2 session II (indicating recency; see the electronic supplementary material, figure S5) and are significantly different from the weights obtained in session I (F1,18 = 5.23; p = 0.035; see the electronic supplementary material).
As a complementary measure, we compared the fraction of trials in which participants made a final choice corresponding to the recent information, when this information was incongruent with the preliminary information (i.e. trials in which the additional information supports the response opposite to the preliminary choice; [13,16]). We found that when making a preliminary decision, participants' final decision is less likely to be in accordance with the incongruent information as compared to situations where the same information is presented, yet no preliminary decision is made (t9 = −4.1; p = 0.003; figure 4b). These results indicate that preliminary decisions affect the processing of subsequent evidence.
To distinguish between potential mechanisms underlying this effect of the first decision, we compared four models, corresponding to the models in experiment 1: (i) an unbiased (null-hypothesis) drift-diffusion model (DDM) with a leak parameter (i.e. Ornstein–Uhlenbeck diffusion; leak was added in order to account for recency in the no-decision condition; [7,36]); (ii) a global reduction in DDM-gain, in which following a preliminary decision the integrated samples are scaled-down/reduced (figure 4c); (iii) a DDM value-shift, in which the accumulator's value is shifted towards the direction of the preliminary decision (figure 4d); and (iv) a selective reduction in gain of additional evidence, whereby sensitivity is reduced only for information that is incongruent with the preliminary decision.
As the models are identical in their processing of the pre-decision evidence, we first fitted all models to the correct and incorrect RT distributions of the preliminary decision and obtained an estimate of the models' three common parameters (internal noise = 4.3; boundary = 107; leak = 0.0005). With these parameters, the model accounts well for the accuracy within each RT quantile (black line in figure 3c; see the electronic supplementary material, figure S6 for the observed and fitted RT distribution). Next, in order to identify the post-decision model-variant and its parameters, we fitted each of the models to the slope of the regression coefficients for the pre- and post-decision evidence observed in the first experimental session (figure 4a, blue line), under the constraint of meeting the observed per cent of trials in which observers changed their choice (7% ± 1 s.e.; parameters that predicted a large deviation from the observed fraction of changes of mind were not considered). We further assessed the model's predictions for the slope of the regression-weights in session II and for the fraction of trials in which observers responded according to additional incongruent information (generalizability criterion [37,38]). Consistent with the conclusion from the computational modelling of the data of experiment 1, we find that the global reduced-gain model (gain = 0.6) and the selective reduced-gain model (congruent_gain = 1.1; incongruent_gain = 0.7) are the best fitting models that are able to predict the observed data (lines in figure 4a,b; see the electronic supplementary material, figure S7 for the selective reduced-gain fit). Conversely, the value-shift model was unable to account for these effects (shift = 20; see the electronic supplementary material, figure S8).
4. Discussion
The results of the two experiments, combined with the computational analysis, point to a clear effect of decision commitment on the evaluation of, or weight given to, subsequent evidence, which generalizes across domains (perceptual and numerical) and task contingencies (categorical decisions and continuous evaluations). We find that after participants made a preliminary decision, they are less influenced by the subsequent evidence (figures 2d,4a) as compared to situations, in which the same information is presented without making a preliminary decision. This effect leads to a deteriorated accuracy (figures 2a,4b) and its magnitude predicts the reduction in accuracy (electronic supplementary material, figure S4).
Previous behavioural studies have reported similar effects of decision commitment, which were interpreted to imply a cognitive dissonance or a sunk cost mechanism [14–17]. However, several different underlying information-processing mechanisms may give rise to commitment effects and it is unclear whether these phenomena are restricted to high-level motivational situations. By controlling the accumulation of the evidence before, after and in the absence of categorical decisions, we find that even in simple perceptual and numerical decisions, made in a controlled laboratory setting, observers exhibit commitment effects and confirmation bias. Future studies will need to carry out similarly controlled experiments in order to characterize post-decision bias processes, in socially relevant situations, such as evaluating a tournament contender after having already gambled on that contender.
More recent work has demonstrated that humans can revise extended actions, which can be interpreted to indicate changes of mind [39–42]. These studies, however, did not control the evidence following a decision, and thus could not quantify confirmation biases or effects. By controlling the additional streams of information, we were able to identify the dynamical mechanism that underlies the confirmation bias effects, within a sequential sampling framework. Our model comparison reveals that two models provide the best account for these effects: a model in which the preliminary decision reduces global sensitivity to subsequent information (figure 4c) and a model which postulates reduction in gain only for additional information that is incongruent with the preliminary decision. This latter variant is consistent with a recent model that assumes dynamic gain modulation during accumulation of evidence which is incongruent with the predicted mean [43]. Conversely, a model that postulates a shift of value for the chosen alternative (figure 4d) is unable to account for several of the observed phenomena in both experiments (electronic supplementary material, figures S3 and S8). Although simple, this gain-reduction mechanism may be able to account for a wide range of previously reported confirmation bias effects.
One objection that may be raised against the decision-induced global gain-reduction account is that it could reflect a non-specific reduction of arousal, or ‘fatigue’, after the initial choice. This fatigue account might entail a general detriment in information processing following the preliminary decision. Importantly, however, because decreased arousal is associated with increased neuronal variability across the cerebral cortex [44,45], this account predicts a global increase in internal noise after the preliminary decision. When fitting the data with two internal-noise parameters, one for each interval (see Computational Method, model v), we find that the internal noise is not higher in interval 2 (noise = 24) as compared to interval 1 (noise = 25)—a pattern which implies that the decision did not induce fatigue or loss of attention. Further, note that the selective gain-reduction model, which provided a fit almost as good as the global gain-reduction model, is also inconsistent with a reduction of general arousal, which would be non-selective.
Both the global and the selective gain-reduction models account for the intuition that once we reach a decision, we are less likely to respond to new evidence. The neural mechanism that underlies this reduction in sensitivity to evidence is an important topic for further investigation. One possibility is that forming a decision triggers a transition to an attractor state [26]. Accordingly, the neural circuitry that underlies the evaluation of evidence is not fixed but rather is altered at the time a decision is reached, possibly, as a result of neuromodulation [46–50]. For example, a modulatory increase in the recurrent excitation and in lateral inhibition may turn the decision state from one that is characterized by an accumulator (or drift diffusion process; [6,10]) to one characterized by attractor dynamics [20,23–25,48], in which the state is relatively insensitive to new evidence. Accordingly, the reduced gain to extra-evidence in our data may be due to the triggering of such modulations by the request to decide. In our experiment 2, for example, such decision-triggered modulations take place in session I (free-response paradigm, when the observers need to make a preliminary decision), but not in session II, until the end of the evidence is presented (interrogation paradigm). We show in the electronic supplementary material that a decision-modulated change in the decision attractor dynamics (within the leaky-competing accumulators framework) predicts reduction in sensitivity to additional information (electronic supplementary material, figures S9 and S10).
Our analyses of commitment effect in experiment 1 have focused on a time scale of seconds (i.e. sequences of eight items); a more fine-grained analysis of the relative influence of each item reveals that in the DEE condition the influence of the 11th item is reduced (see full time-course regression, electronic supplementary material, figure S1). This temporal decrease in gain could reflect decision-triggered neuromodulation (see the electronic supplementary material, figure S9b). In addition, the full time-course regression shows that the influence of the 8th item in the DEE condition is increased, indicating an enhanced weight in the accumulation process of the most recent evidence before the initial decision, and/or an enhanced memory for that most recent evidence. Future studies will be needed to investigate the fine grained time course of this effect.
While a decision-induced gain-reduction may appear disadvantageous for situations in which the decision is reversible, some degree of inertia on decisions may help to reduce indecisiveness that could lead to multiple changes of mind when faced with ambiguous evidence and to avoid time wasting [42,43]. Recent work within the framework of embodied decisions have shown that decision models in which the motor action has a stabilizing feedback input into the decision process can improve task performance, when the cost of action-changes are factored in [51]. The identification of the neural basis of a post-decision commitment mechanism, with regard to specific model-variants and its putative adaptive role, remains an important topic for future investigations.
Supplementary Material
Acknowledgements
We thank Christopher Summerfield and David Lagnado for very helpful discussions and Anne Urai for helpful comments. This research was supported by the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant no. 51/11), by a grant from the German-Israel Foundation (158/2011) and by a Leverhulme Trust Visiting Professorship to M.U., during his sabbatical at the University of Oxford (2013–2014).
Ethics
The procedures and protocols of all the experiments were approved by the ethics committee of Tel Aviv University (application 743/12). All experiments were carried out in accordance with the approved guidelines.
Data accessibility
The data and research materials supporting the results in the article are available at http://dx.doi.org/10.5061/dryad.40f6v.
Authors' contributions
Z.Z.B, N.B, T.D and M.U designed experiments 1 and 3; Z.Z.B and N.B developed and ran experiments 1 and 3 and analysed the results; K.T and M.U designed experiment 2; Z.Z.B, N.B ran and analysed experiment 2. All authors developed the models; Z.Z.B, N.B and R.M analysed and fitted the models. Z.Z.B, N.B, T.D and M.U wrote the manuscript; R.M and K.T contributed valuable comments.
Competing interests
We declare we have no competing interests.
References
- 1.Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574. ( 10.1146/annurev.neuro.29.051605.113038) [DOI] [PubMed] [Google Scholar]
- 2.Cassey TC, Evens DR, Bogacz R, Marshall JA, Ludwig CJ. 2013. Adaptive sampling of information in perceptual decision-making. PLoS ONE 8, e78993 ( 10.1371/journal.pone.0078993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drugowitsch J, Moreno-Bote R, Churchland AK, Shadlen MN, Pouget A. 2012. The cost of accumulating evidence in perceptual decision making. J. Neurosci. 32, 3612–3628. ( 10.1523/JNEUROSCI.4010-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran R. 2014. Optimal decision making in heterogeneous and biased environments. Psychon. Bull. Rev. 22, 38–53 ( 10.3758/s13423-014-0669-3) [DOI] [PubMed] [Google Scholar]
- 5.Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. 2006. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 113, 700–765. ( 10.1037/0033-295X.113.4.700) [DOI] [PubMed] [Google Scholar]
- 6.Gold JI, Shadlen MN. 2001. Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 5, 10–16. ( 10.1016/S1364-6613(00)01567-9) [DOI] [PubMed] [Google Scholar]
- 7.Ratcliff R, McKoon G. 2008. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 20, 873–922. ( 10.1162/neco.2008.12-06-420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami M, Vicente MI, Costa GM, Mainen ZF. 2014. Neural antecedents of self-initiated actions in secondary motor cortex. Nat. Neurosci. 17, 1574–1582. ( 10.1038/nn.3826) [DOI] [PubMed] [Google Scholar]
- 9.Huk AC, Shadlen MN. 2005. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J. Neurosci. 25, 10 420–10 436. ( 10.1523/JNEUROSCI.4684-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. 2003. A role for neural integrators in perceptual decision making. Cerebral Cortex 13, 1257–1269. ( 10.1093/cercor/bhg097) [DOI] [PubMed] [Google Scholar]
- 11.Moran R, Teodorescu AR, Usher M. 2014. Post choice information integration as a causal determinant of confidence: novel data and a computational account. Cogn. Psychol. 78, 99–147. ( 10.1016/j.cogpsych.2015.01.002) [DOI] [PubMed] [Google Scholar]
- 12.Simen P. 2012. Evidence accumulator or decision threshold: which cortical mechanism are we observing? Front. Psychol. 3, 183 ( 10.3389/fpsyg.2012.00183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arkes HR, Blumer C. 1985. The psychology of sunk cost. Organ. Behav. Hum. Decis. Process. 35, 124–140. ( 10.1016/0749-5978(85)90049-4) [DOI] [Google Scholar]
- 14.Brehm JW. 1956. Postdecision changes in the desirability of alternatives. J. Abnorm. Social Psychol. 52, 384–389. ( 10.1037/h0041006) [DOI] [PubMed] [Google Scholar]
- 15.Festinger L. 1962. A theory of cognitive dissonance. Stanford, CA: Stanford University Press. [Google Scholar]
- 16.Staw BM. 1976. Knee-deep in the big muddy: a study of escalating commitment to a chosen course of action. Organ. Behav. Hum. Perform. 16, 27–44. ( 10.1016/0030-5073(76)90005-2) [DOI] [Google Scholar]
- 17.Knox RE, Inkster JA. 1968. Postdecision dissonance at post time. J. Pers. Soc. Psychol. 8, 319–323. ( 10.1037/h0025528) [DOI] [PubMed] [Google Scholar]
- 18.Nickerson RS. 1998. Confirmation bias: a ubiquitous phenomenon in many guises. Rev. Gen. Psychol. 2, 175–220. ( 10.1037/1089-2680.2.2.175) [DOI] [Google Scholar]
- 19.Liu F, Wang X-J. 2008. A common cortical circuit mechanism for perceptual categorical discrimination and veridical judgment. PLoS Comput. Biol. 4, e1000253 ( 10.1371/journal.pcbi.1000253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usher M, McClelland JL. 2001. The time course of perceptual choice: the leaky, competing accumulator model. Psychol. Rev. 108, 550–592. ( 10.1037/0033-295X.108.3.550) [DOI] [PubMed] [Google Scholar]
- 21.Tsetsos K, Gao J, McClelland JL, Usher M. 2012. Using time-varying evidence to test models of decision dynamics: bounded diffusion vs. the leaky competing accumulator model. Front. Neurosci. 6, 79 ( 10.3389/fnins.2012.00079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogacz R, Usher M, Zhang J, McClelland JL. 2007. Extending a biologically inspired model of choice: multi-alternatives, nonlinearity and value-based multidimensional choice. Phil. Trans. R. Soc. B 362, 1655–1670. ( 10.1098/rstb.2007.2059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdonck S, Tuerlinckx F. 2014. The ising decision maker: a binary stochastic network for choice response time. Psychol. Rev. 121, 422–462. ( 10.1037/a0037012) [DOI] [PubMed] [Google Scholar]
- 24.Wang X-J. 2002. Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36, 955–968. ( 10.1016/S0896-6273(02)01092-9) [DOI] [PubMed] [Google Scholar]
- 25.Deco G, Rolls ET, Romo R. 2009. Stochastic dynamics as a principle of brain function. Prog. Neurobiol. 88, 1–16. ( 10.1016/j.pneurobio.2009.01.006) [DOI] [PubMed] [Google Scholar]
- 26.Amit DJ. 1992. Modeling brain function: the world of attractor neural networks. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Wang X-J. 2008. Decision making in recurrent neuronal circuits. Neuron 60, 215–234. ( 10.1016/j.neuron.2008.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jazayeri M, Movshon JA. 2007. A new perceptual illusion reveals mechanisms of sensory decoding. Nature 446, 912–915. ( 10.1038/nature05739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behrens TE, Woolrich MW, Walton ME, Rushworth MF. 2007. Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221. ( 10.1038/nn1954) [DOI] [PubMed] [Google Scholar]
- 30.Brezis N, Bronfman ZZ, Usher M. In press Adaptive spontaneous transitions between two mechanisms of numerical averaging. Sci. Rep. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malmi RA, Samson DJ. 1983. Intuitive averaging of categorized numerical stimuli. J. Verbal Learn. Verbal Behav. 22, 547–559. ( 10.1016/S0022-5371(83)90337-7) [DOI] [Google Scholar]
- 32.Tsetsos K, Chater N, Usher M. 2012. Salience driven value integration explains decision biases and preference reversal. Proc. Natl Acad. Sci. USA 109, 9659–9664. ( 10.1073/pnas.1119569109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossmy O, Moran R, Pfeffer T, Tsetsos K, Usher M, Donner TH. 2013. The timescale of perceptual evidence integration can be adapted to the environment. Curr. Biol. 23, 981–986. ( 10.1016/j.cub.2013.04.039) [DOI] [PubMed] [Google Scholar]
- 34.Teodorescu AR, Usher M. 2013. Disentangling decision models: from independence to competition. Psychol. Rev. 120, 1–38. ( 10.1037/a0030776) [DOI] [PubMed] [Google Scholar]
- 35.Ratcliff R, Rouder JN. 1998. Modeling response times for two-choice decisions. Psychol. Sci. 9, 347–356. ( 10.1111/1467-9280.00067) [DOI] [Google Scholar]
- 36.Feng S, Holmes P, Rorie A, Newsome WT. 2009. Can monkeys choose optimally when faced with noisy stimuli and unequal rewards? PLoS Comput. Biol. 5, e1000284 ( 10.1371/journal.pcbi.1000284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busemeyer JR, Wang Y-M. 2000. Model comparisons and model selections based on generalization criterion methodology. J. Math. Psychol. 44, 171–189. ( 10.1006/jmps.1999.1282) [DOI] [PubMed] [Google Scholar]
- 38.Ahn WY, Busemeyer JR, Wagenmakers EJ, Stout JC. 2008. Comparison of decision learning models using the generalization criterion method. Cogn. Sci. 32, 1376–1402. ( 10.1080/03640210802352992) [DOI] [PubMed] [Google Scholar]
- 39.Barca L, Pezzulo G. 2012. Unfolding visual lexical decision in time. PLoS ONE 7, e35932 ( 10.1371/journal.pone.0035932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resulaj A, Kiani R, Wolpert DM, Shadlen MN. 2009. Changes of mind in decision-making. Nature 461, 263–266. ( 10.1038/nature08275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albantakis L, Deco G. 2011. Changes of mind in an attractor network of decision-making. PLoS Comput. Biol. 7, e1002086 ( 10.1371/journal.pcbi.1002086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiani R, Cueva CJ, Reppas JB, Newsome WT. 2014. Dynamics of neural population responses in prefrontal cortex indicate changes of mind on single trials. Curr. Biol. 24, 1542–1547. ( 10.1016/j.cub.2014.05.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheadle S, Wyart V, Tsetsos K, Myers N, De Gardelle V, Herce Castañón S, Summerfield C. 2014. Adaptive gain control during human perceptual choice. Neuron 81, 1429–1441. ( 10.1016/j.neuron.2014.01.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polack P-O, Friedman J, Golshani P. 2013. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339. ( 10.1038/nn.3464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. 2014. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362. ( 10.1016/j.neuron.2014.09.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultz W. 2007. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288. ( 10.1146/annurev.neuro.28.061604.135722) [DOI] [PubMed] [Google Scholar]
- 47.Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. 1999. The role of locus coeruleus in the regulation of cognitive performance. Science 283, 549–554. ( 10.1126/science.283.5401.549) [DOI] [PubMed] [Google Scholar]
- 48.Usher M, Davelaar EJ. 2002. Neuromodulation of decision and response selection. Neural Netw. 15, 635–645. ( 10.1016/S0893-6080(02)00054-0) [DOI] [PubMed] [Google Scholar]
- 49.Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. ( 10.1146/annurev.neuro.28.061604.135709) [DOI] [PubMed] [Google Scholar]
- 50.de Gee JW, Knapen T, Donner TH. 2014. Decision-related pupil dilation reflects upcoming choice and individual bias. Proc. Natl Acad. Sci. USA 111, E618–E625. ( 10.1073/pnas.1317557111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lepora NF, Pezzulo G. 2015. Embodied choice: how action influences perceptual decision making. PLOS Comp. Biol. e1004110 ( 10.1371/journal.pcbi.1004110) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and research materials supporting the results in the article are available at http://dx.doi.org/10.5061/dryad.40f6v.