Abstract
Macroevolution, encompassing the deep-time patterns of the origins of modern biodiversity, has been discussed in many contexts. Non-Darwinian models such as macromutations have been proposed as a means of bridging seemingly large gaps in knowledge, or as a means to explain the origin of exquisitely adapted body plans. However, such gaps can be spanned by new fossil finds, and complex, integrated organisms can be shown to have evolved piecemeal. For example, the fossil record between dinosaurs and Archaeopteryx has now filled up with astonishing fossil intermediates that show how the unique plexus of avian adaptations emerged step by step over 60 Myr. New numerical approaches to morphometrics and phylogenetic comparative methods allow palaeontologists and biologists to work together on deep-time questions of evolution, to explore how diversity, morphology and function have changed through time. Patterns are more complex than sometimes expected, with frequent decoupling of species diversity and morphological diversity, pointing to the need for some new generalizations about the processes that lie behind such patterns.
Keywords: macroevolution, evolution, biodiversity, phylogenetic comparative methods, morphometrics
1. Introduction
When George Gaylord Simpson presented his model for adaptive radiations in 1944 [1], he was keenly aware of the difficulties of marrying modern and fossil data. Using examples from vertebrates, he outlined cases where the acquisition of a key adaptation, such as feathers and flight in birds, provided the stimulus for rapid diversification. Some adaptive radiations were triggered by opportunities instigated by external processes, such as a change in climate, or clearing of ecospace by a mass extinction event.
Adaptive radiations are a key element of macroevolution, encapsulating all the broad-scale, deep-time components of the expansion of species numbers, expansion of the range of habitats occupied by life and expansion of the breadth of novel adaptations both in terms of morphology and function. These are patterns of change, and a criticism of macroevolution has sometimes been that there are no models or processes, and it is simply microevolution writ large. There are in fact three models for evolutionary radiations, and these will be explored.
The most important recent advance has been the development of new tools for the investigation of macroevolution in a phylogenetic context. These tools can handle phylogenetic trees based on genomic or morphological data, and can include or exclude fossil taxa. Improvements also in the precision of geological dating, the stability of phylogenies, and care in extracting data from fossils have all helped to answer questions which a few years ago would have seemed beyond the reach of an analytical approach.
The aim of this paper is to explore key questions in macroevolution, and especially to highlight the substantial opportunity for advance at the moment, as methods and data improve massively, and as ways are found to bridge between living and extinct organisms, between biology and palaeobiology.
2. Models for macroevolutionary processes
The three models for macroevolution are broad-scale and all encompassing. They are at a different level from particular models that apply to individual radiations, for example, whether the diversification of mammals after the extinction of the dinosaurs followed an early burst, trend, Brownian motion or other model. The three process models for macroevolution are the ecospace, macromutation and developmental models.
The Simpsonian ecospace model [1,2] includes an early phase of rapid expansion, during which the new adaptation and the new ecological opportunity are tested and explored to the limit (figure 1). Then, in the early phases of the radiation, some marginal forms might die out, under selection, because they have in some way ‘overshot’ the possibilities of the new key adaptation. Available ‘adaptive space’ then causes further sorting of lineages during ‘normal’ times of evolution, after the initial explosion, and numerous initial lineages are weeded down to the really successful ones. In the ecospace model, variations in ecological opportunity control the success of major new morphologies and this produces a pattern that mimics differential introduction of innovations. There are several aspects of this model: radiations are ‘driven’ by key adaptations/innovations, whether they enter previously vacated ecospace or conquer new habitats, and they rapidly expand and overshoot, and then there is extinction/weeding out as many early lineages fail, so leaving gaps in ecospace/morphospace. Under the ecospace model, new species may emerge either into new, unexploited ecospace, or subdivide existing niches by a process of specialiazation.
Figure 1.
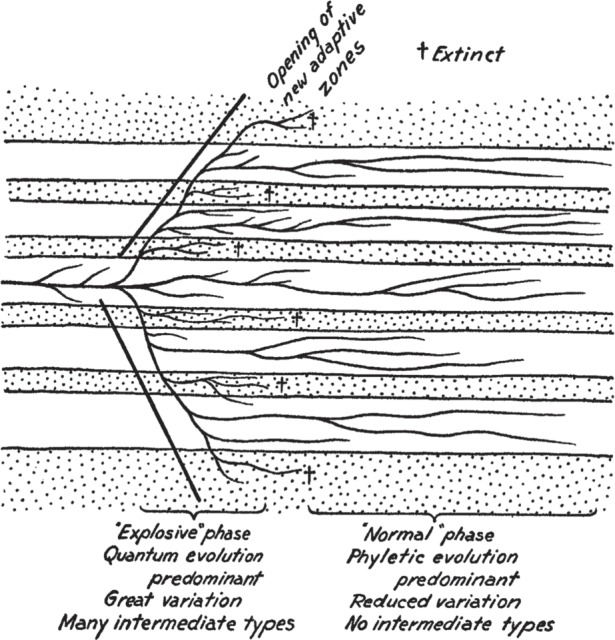
Simpson's [1] ecospace model for adaptive radiation, showing initial explosive evolution into new sectors of ecospace, followed by extinction of intermediates, and reduction of diversity to those lineages that occupy habitable ecospace (shown by blank areas), located between forbidden ecospace (stippled).
The macromutation model, much discussed in Simpson's day, and occasionally revitalized under different titles (reviewed, [3]), proposes that many clade origins were abrupt and dramatic, and produced by a genetic or genomic revolution. Such ideas of macromutations have attracted attention at times, but I assume that macroevolution is a part of Darwinian evolution, as do most others [3], and that there is probably no need for genetic or developmental revolutions. That is not to say that fundamental genomic reorganizations are not associated with major clades—indeed novelties that characterize particular clades are doubtless associated with the acquisition of unique, and perhaps stable, genomic characters such as transcription factors, their regulatory elements and post-transcriptional regulators such as micro RNAs, but these are associated with changes that characterize the clades, namely phenotypic characters, and are driven by natural selection. For example, the phenotypic characteristics of certain clades may arise through heterochrony, such as the origin of many cranial characters in birds through paedomorphosis [4], and these may be associated with particular regulatory genes. Furthermore, the origin of birds is a good example that was once cited as evidence for macromutation. Surely, it was argued, birds emerged rapidly in geological time, and they were from the start exquisitely well adapted, aerodynamically perfect organisms, and so intermediate stages could not be imagined? However, new evidence rules out the need for genetic revolutions. Palaeontologists at times favoured wholesale, macromutational changes in cases where fossils were absent: however, the phenotypic gap between dinosaurs and birds has been filled with many new fossils since 1995, and these show that the unique plexus of morphological and physiological changes that distinguish birds from dinosaurs and crocodiles, was acquired piecemeal over a span of 60 Myr, from Coelophysis to Archaeopteryx [5].
Finally, the developmental model (or ‘genomic reorganization model’) posits [6–8] a model of macroevolution in which developmental patterning has become increasingly resistant to modification, and so the potential for innovation has reduced through geological time. The proposal that genetic and developmental constraints were less restrictive at the time of the Cambrian Explosion, and then genomic regulatory networks became increasingly established and ‘hardened’ against subsequent change throughout the Phanerozoic, is not borne out by evidence from morphological disparity: major new bauplans have emerged repeatedly through post-Cambrian evolution, and within those bauplans, disparity typically expands rapidly with the origin of each new subclade. Erwin [8] and colleagues subsequently modified their view to suggest that, whereas gene regulatory networks became fixed in the Cambrian, downstream regulatory systems remained flexible to substantial changes in developmental patterns within constraints of the fundamental body plans.
The ecospace and developmental macroevolutionary models make different predictions about the evolution of life. The ecospace model says that diversification and innovation continue unabated through geological time, when environmental opportunity or novel adaptations permit. The developmental model proposes that there have been stepwise limits to the extent of morphological change. This means that much innovation would have been exhausted in the Palaeozoic, and later evolution would then have consisted of tinkering or specialization within a limited number of bauplans [8]. Such a view of long-term ‘exhaustion’ of adaptive opportunities would seem to be at odds with evidence that life has massively diversified in the past 100 Myr, expanding in terms of species numbers, disparity and functional breadth [2,9–11], but others argue that life in the sea at least reached its maximum diversity 500 Myr ago and has remained at a steady equilibrium level ever since [12,13]. These contrasting viewpoints go to the heart of a number of fundamental debates in macroevolution and palaeobiology.
3. Biodiversity and macroevolution
Biodiversity is an astonishing phenomenon. Ever since Darwin [14], and even long before, scientists and philosophers have been amazed at just how diverse life is. Current discussions among biologists have focused on estimating global biodiversity, and yet it is debated how high the figure should go above the total of the 1.7 million named species: whether 5 million, 10 million or 100 million species. Seeking to discover the actual number of living species, even to within an order of magnitude, has fascinated evolutionary biologists [15,16], and it is a theme that has profound scientific consequences, as well as socio-economic implications in terms of designing global conservation policy. In a simple world, one might expect a few thousand species; the fact that there are millions of species, and some with remarkably restricted habits and geographical distributions raises wonder among scientists and the general public: why is life so diverse?
These considerations have led to wider discussions of practical concerns, such as what is the current rate of loss of biodiversity, which kinds of species are most at risk, are certain regions or habitats more species-rich than others (and why), are there particular characters or ecologies that ensure high biodiversity, how does biodiversity recover at various scales, and how can humans mitigate the losses? Taking account of the seemingly huge scale of modern biodiversity, there are a large number of questions about causes: why is life so diverse, are some groups more species-rich than others, what are the correlates and causes of such success (in terms of species numbers), and what are the correct scales to understand origins of modern biodiversity?
The key question [17] is ‘How is biodiversity generated and maintained?’ Much work in ecology focuses on the second half of this question; my focus is on the first half, how is biodiversity generated?—or specifically—what are the triggers or drivers of success [= high biodiversity] in evolution?
Diversification, the balance between speciation and extinction, is core to half of current evolutionary theory. Darwin [14] explored two great themes in the Origin, evolution by natural selection (=microevolution) and descent with modification (=macroevolution). In arguing that all life can be traced back to a single common ancestor, he was first to show that life diversified according to a branching phylogenetic tree. Modern biodiversity then reflects that long-term pattern of branching (‘descent with modification’) and this underlies all modern studies of biogeography, ecology, behaviour, palaeobiology and physiology.
As Morlon [17, p. 508] notes, ‘Diversification is a key to understanding how biodiversity varies over geological time scales and how it is distributed across the Earth's surface, the tree of life and ecological communities … Diversification is also a primary predictor of three fundamental patterns in macroecology: the species abundance distribution, which describes how individuals are partitioned among species, the species-area relationship, which describes how species richness increases with geographical area, and the distance-decay relationship, which describes how community similarity declines with geographical distance.’
4. Methodological advances and new opportunities
Scaling issues, and the differences in data between living and extinct organisms left palaeontologists and evolutionary biologists in a conundrum because they lacked the tools to cross the living–fossil divide. Now, however, remarkable improvements in data and methods in the past 20 years are converging on a tool kit that should allow the application of acceptable analytical approaches to the study of macroevolution incorporating all data, not just living taxa and genomic trees. These methods also not only explore patterns but also allow testing of models, so addressing the process aspect.
(a). The fossil record
Knowledge of fossils worldwide has improved substantially in the past 30 years, with special focus on aspects of quality (e.g. completeness of fossil documentation, accuracy of rock dating, accuracy of recovered phylogenies). An example of a major effort to document the data in a unified manner is the Paleobiology Database (http://fossilworks.org), a community-based resource that was established in 1998, and has grown substantially since. Whereas evolutionary palaeobiologists used to use rather broad-based temporal and geographical constraints, they exercise more care now in clarifying the provenance of their fossil taxa and their nomenclature, allowing for errors in taxonomy.
(b). Time scales
At the same time, geological time determination has improved by orders of magnitude [18]. Whereas at one time, the precision of radiometric dates might have been qualified by error bars of plus or minus 5%, errors are now frequently in fractions of 1%. This, combined with close attention to the rigours of global stratigraphic correlation through the International Commission on Stratigraphy [19], has allowed questions to be answered in new ways. For example, 20 years ago, the greatest mass extinction of all time, around the Permo–Triassic boundary, was deemed to have lasted for any time up to 10 Myr [20], but it can now be refined to a duration of 180 000 years [21] or even 12 000–108 000 years [22]. The improved precision of geological dating provides a basis for more reasonable calibration of phylogenies [23] and for calculation of realistic rates of evolution.
(c). Phylogeny
Phylogenies of ever-larger size are being compiled by various means, some based on single studies of genomic data from hundreds or thousands of species, and others compiled as supertrees based on numbers of component source trees. While there is considerable debate and development of numerical methods in determining trees, using parsimony, Bayesian methods, and the like, and the mechanics of constructing supertrees are also in development, and debated, the end result of intense phylogenetic study of some clades, especially among vertebrates, since the 1970s has been a stabilization of many portions of the tree. Whereas, for example, the fundamental relationships of the mammalian orders were obscure in the 1980s [24], the discovery of Afrotheria, Laurasiatheria, Boreoeutheria, Whippomorpha and some other unexpected clades has led to increasing stability and agreement [25].
(d). Disparity and morphometrics
Whereas species diversity can readily be quantified, morphology has been harder to document. Either discrete or continuous characters may be used as a basis for measurement of disparity. Continuous characters are often derived from landmark measurements taken from drawings or photographs of whole organisms or parts of organisms (e.g. vertebrate skulls in lateral view; leaf shapes in dorsal view). Discrete characters may be presence/absence characters or cladistic characters that record the acquisition of novelties. Statistical protocols for recording and analysing such data are well established [26–30]. The initial measurements are processed to establish intertaxon distances, which can then be subjected to multivariate treatment (e.g. principal coordinates analysis). The multivariate analyses permit visualization of taxa in a morphospace, so the position and range of morphological variation may be compared between pre-determined clusters of species, representing different subclades, time bins or geographical areas. Disparity is summarized by a variety of indices that capture the range and variance of shape variation, and so allow the analyst to track changing disparity through time.
(e). Phylogenetic comparative methods
Phylogenetic comparative methods (PCM) were proposed [31,32] as a means to correct for phylogenetic bias in comparative work in biology, and have since been developed as tools that explore the evolution of characters across trees to identify diversification shifts, evolutionary rates, and models of evolution [33–38]. Most of the currently available methods work through the coding environment R, and so can integrate with each other to explore particular datasets.
5. Key questions in macroevolution
Questions about origins and nature are commonplace concerns of all citizens. Macroevolution incorporates questions that fall into the research domains of biodiversity and global change, two themes of key scientific and socio-economic significance. It would perhaps surprise many enquiring non-scientists that we cannot say how diverse life is today, how diverse it was in the past, what key principles determine patterns of biodiversity, why some groups are more diverse than others, and how successful groups become successful. A number of key current and future questions and research themes may be identified.
(i) Why are some groups successful and others are not? Classic comparisons compare sister clades because both originated at the same time and both have been through the same vicissitudes of Earth history. For example, birds and crocodiles are sister clades that diverged 240–250 Ma: today there are 10 000 species of birds, and only 23 species of crocodiles. Other such sister-clade comparisons with such orders-of-magnitude differences in current biodiversity include Cyclostomata–Gnathostomata (jawless versus jawed fishes), Rhynchocephalia–Squamata (sphenodontians versus lizards), Holostei versus Neopterygii (sturgeons and paddle fish versus derived bony fishes) or Amiidae versus Teleostei (bowfins versus teleosts) and Monotremata versus Theria (monotremes versus marsupials + placentals). There are so many examples of such imbalance that these are not unusual cases from the end of a spectrum in which most splits are equal; it truly seems that sister clades of ‘successful’ clades may survive and yet barely expand beyond a handful of species.
(ii) What drives large-scale evolution? Is the driver for the diversification of life internal (e.g. biological adaptation) or external (e.g. environmental change)? Does evolutionary success depend on innovation or chance, or both? This is part of the broader debate about whether evolution is dominated by external drivers (e.g. climate, temperature, atmospheric composition, sea level, topography) or internal drivers (e.g. competition, predation), sometimes characterized as the Court Jester versus Red Queen models [2,10]. The question can be approached in two ways regarding external environmental changes. First, time series of external drivers and patterns of diversity and disparity can be modelled for specific cases, and goodness of fit (explanatory power) assessed. Second, the effects of particular major crises, such as mass extinctions and associated environmental shocks, as well as smaller events, such as the Palaeocene-Eocene Thermal Maximum and the Neogene ice ages can be explored for their one-off effects on particular clades.
(iii) How do clades diversify when triggered by key adaptations or by extinction events? How do clades expand—early burst or gradual? Some clades diversified in the aftermath of mass extinctions (e.g. placental mammals and neognath birds after the Cretaceous-Palaeogene (K-Pg) event 66 Ma; archosaurs, neopterygians and bivalves after the Permo–Triassic mass extinction 252 Ma). Others diversified in ‘normal’ times, when there had been no such crisis (e.g. lizards, snakes, passerine birds). Are there differences in evolutionary mode? The dominant evolutionary models can be explored across large phylogenies that include clades which diversified under both kinds of regimes [39], as well as evidence for rates of change, the scale and timing of diversification shifts and the importance of perceived key adaptations.
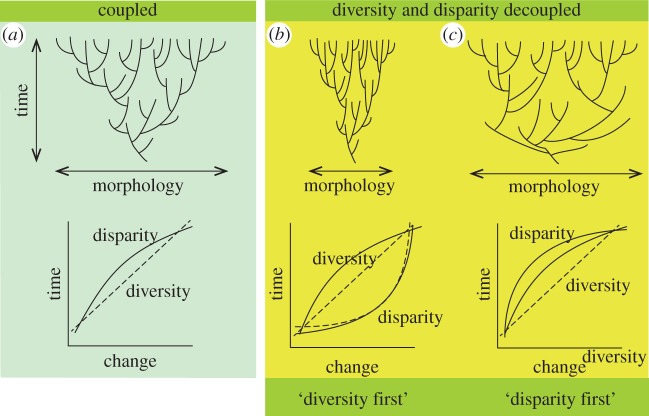
(iv) Are diversity and disparity decoupled? In other words, does evolution drive species to explore the outer limits of available morphospace early, and then morphospace is filled by ever more specialized species, or do morphospace occupation and diversity expand in tandem (figure 2)? This kind of study has been meta-analytical so far [8,34,41], meaning a summation of ‘random’ case studies, but it would be worth exploring instances across a single large phylogeny to determine the proportion of clades characterized by one or other model. If it turns out that the disparity-first model is general, this would require some re-thinking of common assumptions about adaptive radiations.
(v) Are diversifications and extinctions selective? Much has been written about the selectivity of extinctions and of recovery times [2,7,28,39,42]. Most work up to now has been based on the compilation of case studies, and these have suggested some general ‘rules’, such as that large animals are most vulnerable to extinction; restricted diets and narrow geographical ranges are strong contributors to risk; small insect-eaters are the most probable animals to lead the recovery after a mass extinction. On the other hand, clade geometry seems to play little role, with no evidence that long-lived clades are more or less likely to survive than newly emerged clades, except in the sense that long-lived clades often have wider geographical distribution than short-lived clades [43].
(vi) Is diversification diversity-dependent, at clade level and at global scale? In other words, do clades (or all of life) reach limits of space or food that slow down their rate of expansion (by elevating extinction rates, or reducing speciation rates)? Models of evolution commonly incorporate density-dependent factors [44–48], and yet it is not clear that these provide the best models in macroevolution [10,49–51]. Certainly, small-scale studies involve density-dependent processes because there is usually a limiting factor (e.g. food or shelter), but such assumptions may not apply at regional or global scales where species may originate into empty ecospace (i.e. not by supplanting a pre-existing species) and where increased diversity itself provides opportunities for further increases in diversity [52,53]. In the end, most evidence supports some diversity-dependence, especially in damping down origination rates, but diversity rarely, if ever, reaches the asymptote, so there may be truth in both viewpoints [54]. Nonetheless, this has been a debate that has rumbled along since the days of Darwin and Wallace, and it has implications at all hierarchical scales from local ecology to deep-time global history of biodiversity.
(vii) Is evolution hierarchical, having once been faster than it is today (developmental model) or are opportunities and innovations as buoyant now as they were deep in time (ecological model)? This debate is fundamental to our understanding of the trajectory of biodiversity expansion, and to our understanding of genomic/developmental flexibility. The developmental model [6–8], as noted earlier, implies stagnation or an exhaustion of potential to adapt and evolve, and so would seem to be at odds with the apparently continuous diversification of life since the Palaeozoic. This, and the debate about the role of diversity-dependent processes (question vi), also speaks to the hierarchical structure of biodiversity, and whether there are real differences between processes acting at different levels in the hierarchy, or whether evolution occurs simply at the level of individuals or species, and these have passive consequences at higher levels.
Figure 2.
The null expectation is that diversity and disparity are coupled (a), but most palaeontological examples suggest they are decoupled (b,c) and that the disparity-first model is most common (c). Based on [40]. (Online version in colour.)
6. Examples of palaeontological, macroevolutionary studies
In terms of the origins of modern biodiversity, there are many case studies in phylogeography that focus on clade origins and dispersals in the past 10 000 years, since the end of the last Ice Age [53,55]. However, a deeper-time focus is appropriate in accounting for large-scale, worldwide patterns.
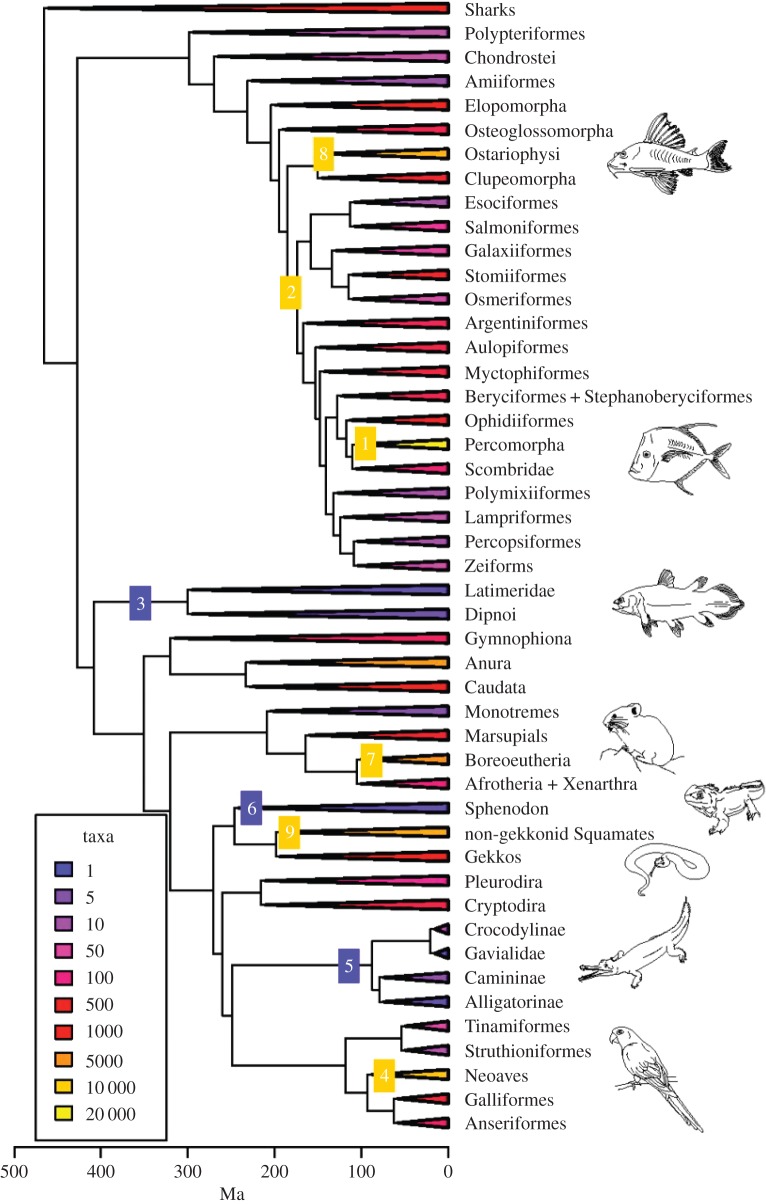
In one example, Alfaro et al. [33] found that the modern biodiversity of all 60 000 species of vertebrates could be reduced to a manageable analytical question: what is special about the six hyper-diverse clades (Ostariophysi, Euteleostei, Percomorpha, non-gekkonid Squamata, Neoaves and Boreoeutheria) that comprise 85% of those 60 000 living species (figure 3)? In the end, this might resolve into some clear answers, because the origin and main diversification burst of each of these clades could well be tied to a particular key innovation (i.e. a morphological or physiological character that allowed all members of the clade to occupy new ecospace) or to a particular external trigger, such as the extinction of a precursor competitor group or the opening of a new habitat or mode of life mediated by climatic or other physical environmental change.
Figure 3.
The evolution of key vertebrate clades, showing diversification shifts. Clades are collapsed to 47 representative stem lineages and coloured by extant species diversity. Clades with unusual diversification rates are denoted with numbers; yellow and blue squares denote diverse and impoverished clades, respectively, compared with background rates of evolution. The impoverished clades (numbers 3, 6, 5; sarcopterygian fishes, rhynchocephalians, crocodiles) are classic ‘living fossils’, whereas the speciose clades (numbers 8, 2, 1, 7, 9, 4) shows expansions of diversity clustered in the time from 150 to 100 Ma. From [33]. (Online version in colour.)
Several recent studies address the macroevolution of the origin of birds [56–59], and they all come to the same conclusion, that there were continued, elevated rates of evolution for 60 Myr along the stem of theropod evolution towards Archaeopteryx, and that all the classic ‘bird’ characters were acquired piecemeal over that long span of time. Before the Chinese Jurassic–Cretaceous feathered dinosaurs and birds had been reported, Archaeopteryx seemed to emerge fully fledged as a bird 150 Ma. Therefore, it was easy to imagine that birds had evolved fast, and that perhaps the unique assemblage of adaptations for flight could only have emerged as a functioning package. Such considerations encouraged some evolutionists to speculate about sudden genetic revolutions, and of course provided creationists with material for their mockery. Now, the facts speak against a sudden revolution in the origin of birds. Most of the enumerated characters had emerged in stepwise fashion from the Late Triassic onwards. Indeed, the fossils show a remarkable phase of experimentation in flight styles, with some gliding dinosaurs such as Microraptor even experimenting with four-winged flight.
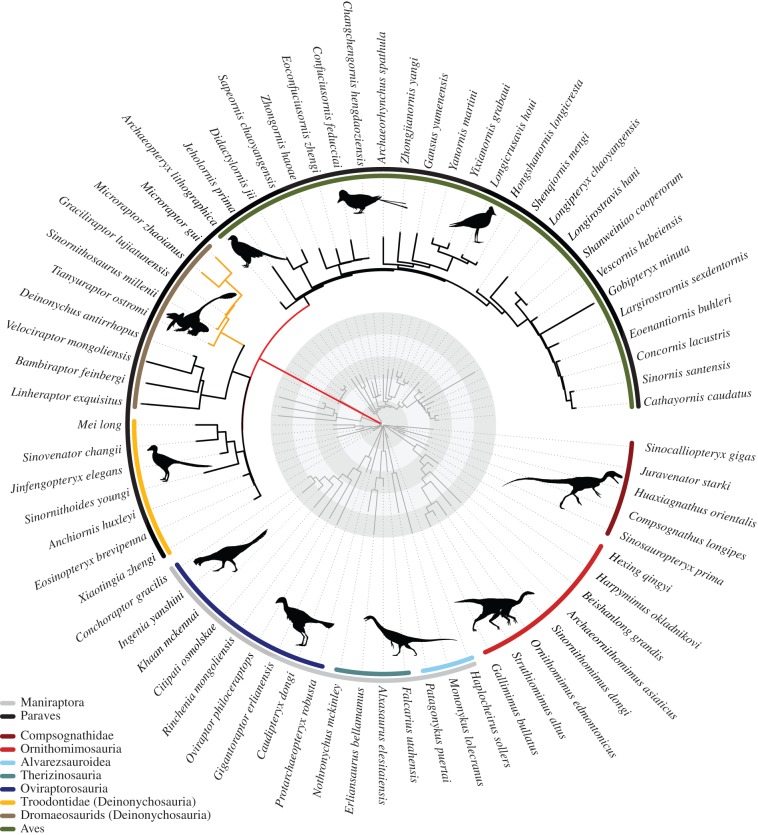
These studies of bird origins [56–59] used different datasets, different phylogenies, and different analytical techniques, and yet they converged on the same result. As an example, Puttick et al. [56] showed that miniaturization and wing expansion, critical anatomical requirements to be a bird, arose some 10 Myr before Archaeopteryx among the wider clade Paraves (figure 4), and that the rate of change was 160 times the normal evolutionary rate, suggesting a rapid, adaptive switch that enabled the diversification and success of this clade of tiny, possibly tree-climbing and gliding dinosaurs. Their analysis, like two others [58,59] was conducted on a phylogenetic tree that had been dated independently of the phylogenetic tree search, whereas Lee et al. [57] ran a tip-dating method that established the favoured tree and its time calibration as a single calculation. Other differences were in terms of the characters used for macroevolutionary analysis, whether body size alone [57,58], body size and wing size together [56], or a broad suite of cladistic characters [59]. The agreement on the main results in these studies, despite their different materials and methods, suggests that the conclusions are robust to the choice of data and methods.
Figure 4.
Rates of femur and forelimb evolution in theropod dinosaurs and early birds. Branch lengths are scaled, with the red branch leading from the centre to the Paraves indicating a 200-fold, and the yellow branches (upper left) to Microraptorinae indicating an eightfold, increase in rate of evolution relative to the background rate. The time-calibrated phylogeny is shown in dark grey, and circular rings indicate 5 Myr time intervals from the K–Pg boundary. Silhouettes drawn by Scott Hartman, Matt Martyniuk, Emily Willoughby, Jaime Headon and Craig Dylke, or modified by T. Michael Keesey, were downloaded from http://phylopic.org. From [56]. (Online version in colour.)
In another series of studies, Ruta et al. explored the nature of clade expansion following the Permo–Triassic mass extinction (figure 5), including the radiations of archosaurs [60], anomodonts [41] and cynodonts [61]. These studies all used discrete characters from cladistic datasets to establish the extent of morphospace occupation through time and between clades. A distinction was made between diversity (species richness) and disparity (morphological variance), and the aim was to determine whether diversity and disparity are coupled or not (figure 2). In all cases, the clades diversified into ecospace emptied by the crisis in a disparity-first mode. These three studies represent clades with very different histories—for example, archosaurs (including dinosaurs) and cynodonts had been small clades before the extinction, whereas anomodonts had been a large and diverse clade, and yet the recovery patterns are similar in each case, and not distinguishable from diversifications that did not follow mass extinctions. These disparity methods have also been used in studies of Cambrian animal radiation [26,27], temnospondyl evolution [62], pterosaur subclade evolution [63] and ichthyosaur radiations during recovery from mass extinction [64].
Figure 5.
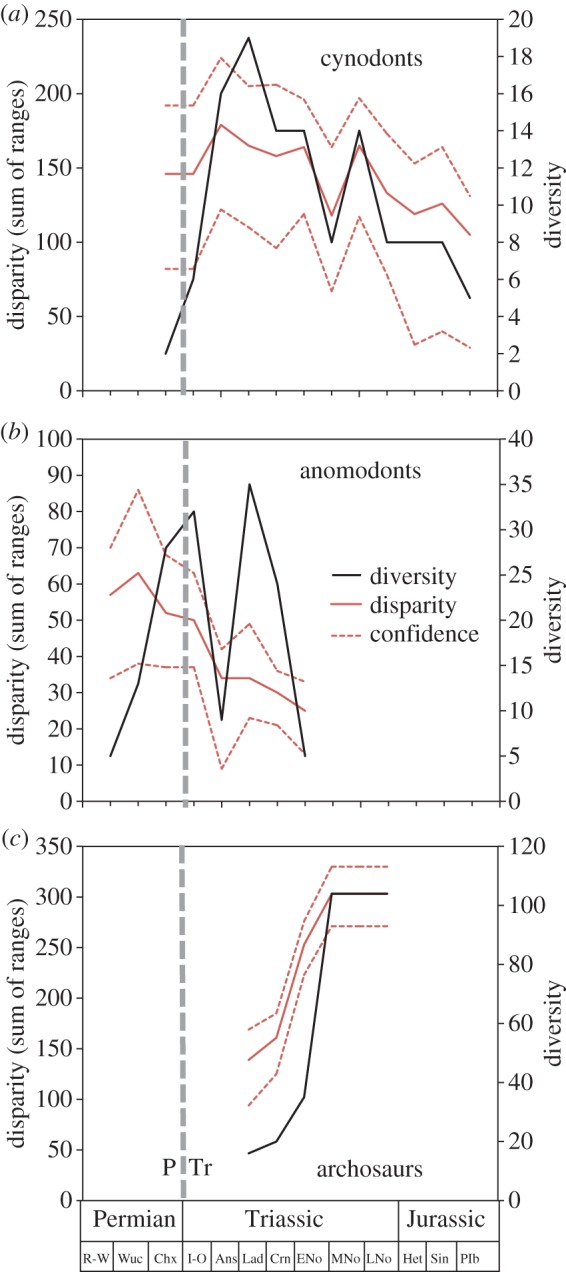
Diversity and disparity (morphological variation) in three clades that recovered after the Permo–Triassic (P–Tr) mass extinction. In each case, disparity (red, with confidence envelope marked) expands before diversity (black), although in anomodonts, this happened before the mass extinction, which generated a macroevolutionary bottleneck from which diversity recovered, but diversity never did. Ans, Anisian; Chx, Changhsingian; Crn, Carnian; ENo, early Norian; Het, Hettangian; I-O, Induan-Olenekian; Lad, Ladinian; LNo, Late Norian; MNo, middle Norian; Plb, Pilensbachian; R-W, Roadian-Wordian; Sin, Sinemurian; Wuc, Wuchaipingian. Based on data from [41,60,61]. (Online version in colour.)
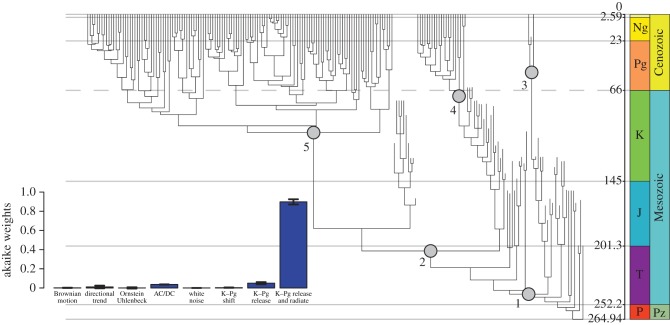
The effects of mass extinctions can be studied through PCM also. These methods test whether a pattern of change differs sufficiently from random (Brownian motion model) to be identified as a directional trend, a stabilizing or constrained pattern (Ornstein–Uhlenbeck model), or some other selective model. In a study of the effect of the K–Pg mass extinction 66 Ma on the evolution of mammals, Slater [39] found strongest support for his ‘K–Pg release and radiate’ model (figure 6). In cases such as these, the macroevolutionist is not simply describing a pattern of explosive expansion in diversity and disparity, but also determining an evolutionary process. Here, the conclusion is that Cretaceous mammal evolution was held back by the dinosaurs, but after their extinction, mammals radiated rapidly, having been released from those constraints.
Figure 6.
The phylogeny of modern mammals, and testing for the role of the K–Pg mass extinction. The evolutionary tree shows the main clades (1, Mammalia; 2, Theria, 3, crown Monotremata; 4, crown Metatheria; 5, crown Eutheria), and the dashed grey horizontal line corresponds to the K–Pg boundary. The inset histogram shows the likelihood of different models to explain variants of the tree for living and fossil Mammalia. Based on [39]. (Online version in colour.)
There is a third component used to describe a diversifying clade, after diversity and disparity (=morphology), namely adaptation or function. In describing morphospaces, it is easy to equate form and function, but the many-to-one mapping dilemma [65] is prevalent: one structure might have multiple functions, and one function might be performed by multiple structures. In exploring the macroevolution of early fishes, Anderson et al. [66] distinguished so-called ‘functional’ characters, especially those metrics of feeding efficiency and diet, from other morphological characters, and found different patterns of change through time. This may not be a complete solution of how to explore function through time—after all, the ‘functional characters’ are simply declared to be so, and it is not clear whether these are the crucial functions that might have triggered an adaptive radiation—but it is a start, and is a means to test particular hypotheses.
The need for an integrated programme of macroecological studies was highlighted by Schluter [67, p. 181], ‘Most tests of key innovation hypotheses attempt to correlate appearance of a novelty with change in the net rate of speciation rather than with adaptive radiation, of which speciation is only part. The lack of attention to effects of novel traits on ecological and phenotypic expansion is an outstanding gap in the study of key innovations.’ In other words, little has been done until recently [17,68,69] to identify patterns of timing, shapes of clades and subclades over millions of years, and the distribution of anatomical characters, including key innovations. Now, however, many researchers have the intention to carry out such studies in an appropriate manner, and the data and tools have converged in a way that makes the work possible.
7. Future advances
The themes of clade dynamics and origins are both fascinating problems couched in sometimes elusive data, but fundamental to our understanding of the world and life. They are also currently important challenges to humanity, because of intense interest in biodiversity drivers and challenges [10,17,70,71]. All these issues have a profound human and political dimension in view of concerns about climate change and biodiversity. Understanding the origins of biodiversity can be framed as a macroevolutionary question: why are some clades more successful than others? The answers will lead to rethinking of much current evolutionary research, and a re-focus of smaller-scale, phylogeographic, genomic and conservation biological approaches to comparing species-rich and species-poor subclades. There are strong opportunities to make major theoretical advances in questions of evolutionary success, hierarchy of processes and the relative significance of biotic and abiotic factors in driving evolution.
Acknowledgements
I thank Phil Donoghue for encouraging, and insightful remarks, and advice from the referees, and editors Norman MacLeod and Innes Cuthill.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 2.Stanley SM. 1979. Macroevolution; pattern and process. San Francisco, CA: WH Freeman. [Google Scholar]
- 3.Futuyma DJ. 2015. Can modern evolutionary theory explain macroevolution? In Macroevolution (eds Serrelli E, Gontier N.), pp. 29–85. Basel, Germany: Springer. [Google Scholar]
- 4.Bhullar B-A, Marugán-Lobán J, Racimo F, Bever GS, Rowe TB, Norell MA, Abzhanov A. 2012. Birds have paedomorphic dinosaur skulls. Nature 487, 223–226. ( 10.1038/nature11146) [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Zhou ZH, Dudley R, Mackem S, Chuong CM, Erickson GM, Varricchio DJ. 2014. An integrative approach to understanding bird origins. Sciecne 346, 1253293 ( 10.1126/science.1253293) [DOI] [PubMed] [Google Scholar]
- 6.Valentine JW. 1995. Why no new phyla after the Cambrian? Genome and ecospace hypotheses revisited. Palaios 10, 190–194. ( 10.2307/3515182) [DOI] [Google Scholar]
- 7.Valentine JW. 2004. On the origin of phyla. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Erwin DH. 2007. Disparity: morphological pattern and developmental context. Palaeontology 50, 57–73. ( 10.1111/j.1475-4983.2006.00614.x) [DOI] [Google Scholar]
- 9.Benton MJ. 1995. Diversification and extinction in the history of life. Science 268, 52–58. ( 10.1126/science.7701342) [DOI] [PubMed] [Google Scholar]
- 10.Benton MJ. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732. ( 10.1126/science.1157719) [DOI] [PubMed] [Google Scholar]
- 11.Stanley SM. 2007. An analysis of the history of marine animal diversity. Paleobiology 33, 1–55. ( 10.1666/06020.1) [DOI] [Google Scholar]
- 12.Raup DM. 1972. Taxonomic diversity during the Phanerozoic. Science 177, 1065–1071. ( 10.1126/science.177.4054.1065) [DOI] [PubMed] [Google Scholar]
- 13.Alroy J. 2010. The shifting balance of diversity among major marine animal groups. Science, 329, 1191–1194. ( 10.1126/science.1189910) [DOI] [PubMed] [Google Scholar]
- 14.Darwin C. 1859. On the origin of species. London, UK: John Murray. [Google Scholar]
- 15.Wilson EO. 1992. The diversity of life. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.May RM. 1997. The dimensions of life on Earth. In Nature and human society (ed. Raven PH.), pp. 30–45. Washington, DC: National Academy Press. [Google Scholar]
- 17.Morlon H. 2014. Phylogenetic approaches for studying diversification. Ecol. Lett. 17, 508–525. ( 10.1111/ele.12251) [DOI] [PubMed] [Google Scholar]
- 18.Erwin DH. 2006. Dates and rates: temporal resolution in the deep time stratigraphic record. Ann. Rev. Earth Planet. Sci. 34, 569–590. ( 10.1146/annurev.earth.34.031405.125141) [DOI] [Google Scholar]
- 19.Gradstein FM, Ogg JG, Schmitz MD, Ogg GM. (eds). 2012. The geologic time scale 2012. Boston, MA: Elsevier. [Google Scholar]
- 20.Erwin DH. 1993. The great Paleozoic crisis. New York, NY: Columbia University Press. [Google Scholar]
- 21.Shen SZ, et al. 2011. Calibrating the end Permian mass extinction. Science 334, 1367–1372. ( 10.1126/science.1213454) [DOI] [PubMed] [Google Scholar]
- 22.Burgess SD, Bowring S, Shen SZ. 2014. High-precision timeline for Earth's most severe extinction. Proc. Natl Acad. Sci. USA 111, 3316–3321. ( 10.1073/pnas.1317692111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton MJ, Donoghue PCJ. 2007. Paleontological evidence to date the tree of life. Mol. Biol. Evol. 24, 26–53. ( 10.1093/molbev/msl150) [DOI] [PubMed] [Google Scholar]
- 24.Novacek MJ, Wyss AR. 1986. Higher-level relationships of the recent eutherian orders: morphological evidence. Cladistics 2, 257–287. ( 10.1111/j.1096-0031.1986.tb00463.x) [DOI] [PubMed] [Google Scholar]
- 25.Dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PCJ, Yang ZH. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B 279, 3491–3500. ( 10.1098/rspb.2012.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills MA, Briggs DEG, Fortey RA. 1994. Disparity as an evolutionary index: a comparison of Cambrian and recent arthropods. Palaeobiology 20, 93–130. [Google Scholar]
- 27.Wills MA. 1998. Cambrian and recent disparity: the picture from priapulids. Paleobiology 24, 177–199. [Google Scholar]
- 28.Foote M. 1997. The evolution of morphological diversity. Ann. Rev. Ecol. Syst. 28, 129–152. ( 10.1146/annurev.ecolsys.28.1.129) [DOI] [Google Scholar]
- 29.Wagner PJ. 1997. Patterns of morphologic diversification among Rostroconchia. Paleobiology 23, 115–150. [Google Scholar]
- 30.Ruta M, Benton MJ. 2008. Calibrated diversity, tree topology and the mother of mass extinctions: the lesson of temnospondyls. Palaeontology 51, 1261–1288. ( 10.1111/j.1475-4983.2008.00808.x) [DOI] [Google Scholar]
- 31.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 33.Alfaro ME, Santini F, Brock C, Alamillo H, Dornburg A, Rabosky DL, Carnevale G, Harmon LJ. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 196, 13 410–13 414. ( 10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadler TJ. 2011. Mammalian phylogeny reveals recent diversification rate shifts. Proc. Natl Acad. Sci. USA 108, 6187–6192. ( 10.1073/pnas.1016876108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas GH, Freckleton RP. 2012. MOTOT: models of trait macroevolution on trees. Methods Ecol. Evol. 3, 145–151. ( 10.1111/j.2041-210X.2011.00132.x) [DOI] [Google Scholar]
- 36.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 37.Harmon LJ, Weir J, Brock C, Glor R, Challenger W. 2008. Geiger: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 38.Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 ( 10.1371/journal.pone.0089543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slater GJ. 2013. Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the Cretaceous–Palaeogene boundary. Methods Ecol. Evol. 4, 734–744. ( 10.1111/2041-210X.12084) [DOI] [Google Scholar]
- 40.Benton MJ, Forth J, Langer MC. 2014. Models for the rise of the dinosaurs. Curr. Biol. 24, R87–R95. ( 10.1016/j.cub.2013.11.063) [DOI] [PubMed] [Google Scholar]
- 41.Ruta M, Angielczyk KD, Fröbisch J, Benton MJ. 2013. Decoupling of morphological disparity and taxic diversity during the adaptive radiation of anomodont therapsids. Proc. R. Soc. B 280, 20131071 ( 10.1098/rspb.2013.1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes M, Gerber S, Wills MA. 2013. Clades reach highest morphological disparity early in their evolution. Proc. Natl Acad. Sci. USA 110, 13 875–13 879. ( 10.1073/pnas.1302642110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foote M, Crampton JS, Beu AG, Cooper RA. 2008. On the bidirectional relationship between geographic range and taxonomic duration. Paleobiology 34, 421–433. ( 10.1666/08023.1) [DOI] [Google Scholar]
- 44.Macarthur RH, Wilson EO. 1963. An equilibrium theory of insular zoogeography. Evolution 17, 373–387. ( 10.2307/2407089) [DOI] [Google Scholar]
- 45.Sepkoski JJ. 1984. A kinetic model of Phanerozoic taxonomic diversity. Paleobiology 10, 246–267. [Google Scholar]
- 46.Etienne RS, Haegeman B, Stadler T, Aze T, Pearson PN, Purvis A, Phillimore AB. 2011. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc. R. Soc. B 279, 1300–1309. ( 10.1098/rspb.2011.1439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 48.Rabosky DL. 2013. Diversity-dependence, ecological speciation, and the role of competition in macro-evolution. Ann. Rev. Ecol. Evol. Syst. 44, 481–502. ( 10.1146/annurev-ecolsys-110512-135800) [DOI] [Google Scholar]
- 49.Benton MJ. 1987. Progress and competition in macroevolution. Biol. Rev. 62, 305–338. ( 10.1111/j.1469-185X.1987.tb00666.x) [DOI] [Google Scholar]
- 50.Ezard THG, Aze T, Pearson PN, Purvis A. 2011. Interplay between changing climate and species' ecology drives macroevolutionary dynamics. Science 332, 349–351. ( 10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 51.Moen D, Morlon H. 2014. Why does diversification slow down? Trends Ecol. Evol. 29, 190–197. ( 10.1016/j.tree.2014.01.010) [DOI] [PubMed] [Google Scholar]
- 52.Emerson BC, Kolm N. 2005. Species diversity can drive speciation. Nature 434, 1015–1017. ( 10.1038/nature03450) [DOI] [PubMed] [Google Scholar]
- 53.Benton MJ, Emerson BC. 2007. How did life become so diverse? The dynamics of diversification according to the fossil record and molecular phylogenetics. Palaeontology 50, 23–40. ( 10.1111/j.1475-4983.2006.00612.x) [DOI] [Google Scholar]
- 54.Cornell HV. 2013. Is regional species diversity bounded or unbounded? Biol. Rev. 88, 140–165. ( 10.1111/j.1469-185X.2012.00245.x) [DOI] [PubMed] [Google Scholar]
- 55.Lemey P, Rambaut A, Welch JJ, Suchard MA. 2010. Phylogeography takes a relaxed random walk in continuous space and time. Mol. Biol. Evol. 27, 1877–1885. ( 10.1093/molbev/msq067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puttick MN, Thomas GH, Benton MJ. 2014. High rates of evolution preceded the origin of birds. Evolution 68, 1497–1510. ( 10.1111/evo.12363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MSY, Cau A, Naish D, Dyke GJ. 2014. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345, 562–566. ( 10.1126/science.1252243) [DOI] [PubMed] [Google Scholar]
- 58.Benson RBJ, Campione NE, Carrano MT, Mannion PD, Sullivan C, Upchurch P, Evans DCV. 2014. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 ( 10.1371/journal.pbio.1001853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brusatte SL, Lloyd GT, Wang SC, Norell MA. 2014. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr. Biol. 24, 2386–2392. ( 10.1016/j.cub.2014.08.034) [DOI] [PubMed] [Google Scholar]
- 60.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488. ( 10.1126/science.1161833) [DOI] [PubMed] [Google Scholar]
- 61.Ruta M, Botha-Brink J, Mitchell SA, Benton MJ. 2013. The radiation of cynodonts and the ground plan of mammalian morphological diversity. Proc. R. Soc. B 280, 20131865 ( 10.1098/rspb.2013.1865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruta M, Wagner PJ, Coates MI. 2006. Evolutionary patterns in early tetrapods. I. Rapid initial diversification followed by decrease in rates of character change. Proc. R. Soc. B 273, 2107–2111. ( 10.1098/rspb.2006.3577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prentice KC, Ruta M, Benton MJ. 2011. Evolution of morphological disparity in pterosaurs. J. Syst. Palaeontol. 9, 337–353. ( 10.1080/14772019.2011.565081) [DOI] [Google Scholar]
- 64.Thorne PM, Ruta M, Benton MJ. 2011. Resetting the evolution of marine reptiles at the Triassic-Jurassic boundary. Proc. Natl Acad. Sci. USA 108, 8339–8344. ( 10.1073/pnas.1018959108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alfaro ME, Bolnick DI, Wainwright PC. 2005. Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am. Nat. 165, E140–E154. ( 10.1086/429564) [DOI] [PubMed] [Google Scholar]
- 66.Anderson PSL, Friedman M, Brazeau MD, Rayfield EJ. 2011. Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature 476, 206–209. ( 10.1038/nature10207) [DOI] [PubMed] [Google Scholar]
- 67.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Rabosky DL, Alfaro ME. 2010. Evolutionary bangs and whimpers: methodological advances and conceptual frameworks for studying exceptional diversification. Syst. Biol. 59, 615–618. ( 10.1093/sysbio/syq061) [DOI] [PubMed] [Google Scholar]
- 69.Pennell MW, Harmon LJ. 2013. An integrative view of phylogenetic comparative methods: connections to population genetics, community ecology, and paleobiology. Ann. NY Acad. Sci. 1289, 90–105. ( 10.1111/nyas.12157) [DOI] [PubMed] [Google Scholar]
- 70.Gavrilets S, Losos JB. 2009. Adaptive radiation: contrasting theory with data. Science 323, 732–737. ( 10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- 71.Davies TJ, et al. 2008. Phylogenetic trees and the future of mammalian biodiversity. Proc. Natl Acad. Sci. USA 105, 11 556–11 563. ( 10.1073/pnas.0801917105) [DOI] [PMC free article] [PubMed] [Google Scholar]