Abstract
Animals must contend with an ever-changing environment. Social animals, especially eusocial insects such as ants and bees, rely heavily on communication for their success. However, in a changing environment, communicated information can become rapidly outdated. This is a particular problem for pheromone trail using ants, as once deposited pheromones cannot be removed. Here, we study the response of ant foragers to an environmental change. Ants were trained to one feeder location, and the feeder was then moved to a different location. We found that ants responded to an environmental change by strongly upregulating pheromone deposition immediately after experiencing the change. This may help maintain the colony's foraging flexibility, and allow multiple food locations to be exploited simultaneously. Our treatment also caused uncertainty in the foragers, by making their memories less reliable. Ants which had made an error but eventually found the food source upregulated pheromone deposition when returning to the nest. Intriguingly, ants on their way towards the food source downregulated pheromone deposition if they were going to make an error. This may suggest that individual ants can measure the reliability of their own memories and respond appropriately.
Keywords: environmental uncertainty, memory, pheromone trails, Lasius niger, metamemory, metacognition
1. Introduction
Uncertainty poses a significant problem for animals, especially when facing a changing environment. To overcome uncertainty, animals must continually gather information about their surroundings [1]. Central place foragers often have a well-developed memory [2–4]. In addition, social animals can share valuable information, such as patch productivity, safety and quality [5–7]. In many social insects, such as honeybees and ants, information transfer has become key to their ecological success. Many ants deposit pheromone trails from a food source to their nest, thereby recruiting other foragers and increasing food intake [8]. Because the chance of an ant to follow a specific pheromone trail at a bifurcation is proportional to the relative amount of pheromone on either side of the bifurcation [9,10], modulation of pheromone deposition strongly influences collective decision-making, and thus colony success [7]. Pheromone deposition is therefore modulated according to factors which impact on foraging success, such as resource quality, colony need and perceived path use [7,11,12]. However, reliance solely on trail pheromones may be risky, as accidentally depositing pheromone inappropriately could result in an erroneous information cascade [13,14], and indeed, behavioural rules are in place to avoid such events [15]. On complex paths, memorizing a route becomes more difficult, resulting in poorer-quality information and an increase in navigational errors [16].
Similarly, in a changing environment, social information can become rapidly outdated. This brings particular difficulties to ants, as pheromone trails cannot be quickly removed. Honeybees can prevent nest-mates from recruiting to a dangerous food source, when too much nectar is being retrieved, or to competing nest sites during nest-relocation, by using a stereotyped ‘stop’ signals [5,17,18]. Pharaoh's ants, Monomorium pharaonis, are reported to deposit a ‘no entry’ pheromone marker on the trail that does not lead to a food source, preventing other foragers from taking the wrong route, and it is conceivable that such a ‘no entry’ pheromone could be deployed on outdated routes [19]. However, apart from the one example in Pharaoh's ants, no other stop signals have been reported in ant organization.
An alternative method by which ant colonies could prevent redundant information from being harmful would be to upregulate the production of new information. In concrete terms, this would entail upregulating pheromone deposition in response to an environmental change.
To test this, we trained ants to a feeder location, and then changed the environment by changing the feeder location. We hypothesized that ants would respond to this change by upregulating pheromone deposition.
2. Material and methods
(a). Housing and maintenance of the ants
Colonies of the black garden ant, Lasius niger, were collected on the campus of the Ludwig-Maximilian University in Munich, and housed in plastic foraging boxes (40 × 30 × 20 cm) with a layer of plaster of Paris on the bottom. Each box contained a circular plaster nest (14 cm diameter, 2 cm high). Colonies were queenless with 1000–2000 workers and small amounts of brood. Colonies were fed three times per week with Bhatkar diet, a mixture of egg, agar, honey and vitamins [20]. While being from a queenless colony may slightly affect the behaviour of ants, this is unlikely to affect the result of this experiment, as small amounts of brood were present in the nests [21,22]. Colonies were deprived of food for 4 days prior to each trial to give high and consistent motivation for foraging and recruitment. Water was provided ad libitum.
(b). Experimental procedure
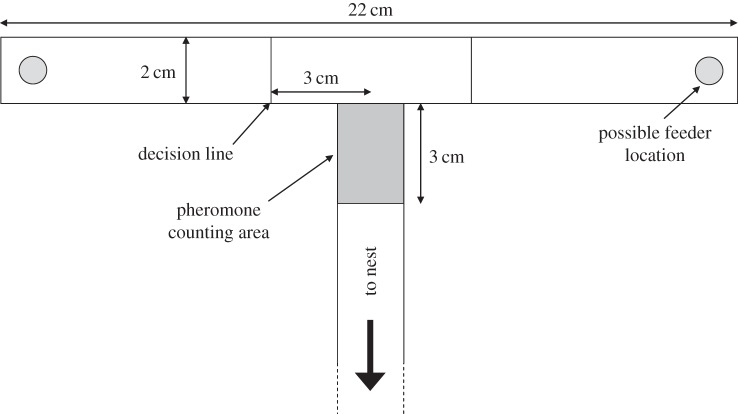
A colony was given access to a plastic T-maze (stem 15 cm long, head 22 cm, width 2 cm) via a drawbridge (figure 1). The T-maze and drawbridge were covered in printer paper overlays. Lines on the T-maze head 3 cm from the centre of the stem acted as ‘decision lines’, with ants whose antenna crossed the line being scored as having taken that direction. A bright light was placed to the right of the T-maze to act as an obvious landmark, and the experiment was carried out in a room containing many other landmarks the ants could use to form a route memory. A drop of 1 molar sucrose (Merck KGaA, Darmstadt, Germany) solution was placed on a small plastic platform at the end of one arm of the T-maze and acted as a feeder.
Figure 1.
Experimental T-maze. Pheromone depositions were counted in the grey rectangular area. Ants were considered to have chosen one arm of the T-maze when their antennae had crossed a decision line. In every experiment, the feeder was first placed at the end of one arm of the T-maze, and after a set number of visits by the ants moved to the other arm.
The first five ants to reach the feeder were individually marked on the abdomen with a dot of acrylic paint, and all other ants were removed and replaced in the foraging box. This prevented unmarked ants from visiting the feeder, depositing pheromone or otherwise interfering with the experiment. Unmarked ants were then prevented from entering the T-maze using the drawbridge. The marked ants were allowed to perform either 1, 3, 5, 10 or 15 visits to the feeder (training phase). The location of the feeder was then switched to the other arm of the T-maze. The variation in the number of training visits was implemented in order to characterize the foraging persistence of the ants, results of which are presented elsewhere [23]. Trained ants began by searching the old feeder location, but eventually discovered the new feeder location, and then were allowed to make further return trips to the feeder (testing phase). During both the outwards (towards the feeder) and return (towards the nest) journey, the number of pheromone depositions performed by each ant was counted on the 3 cm section of the T-maze stem nearest to the T-maze head (figure 1). Pheromone deposition in L. niger is a highly stereotyped behaviour, and easily quantified by eye [7]. The arm chosen by each ant at each visit was also noted. As the presence of trail pheromone itself reduces pheromone deposition [16], the paper overlay covering the T-maze head was either removed whenever an ant walked over it (pheromone-removed treatment) or was left in place throughout the experiment (pheromone-allowed treatment). Once all the marked ants had made a correct decision (as defined by the decision lines) on at least three consecutive visits to the new feeder location, the ants were removed from the colony, and the experiment ended. Each colony was tested twice at each level of training visits, once with pheromone being removed and once with pheromone remaining in place. In half of the experiments the training visits were to the right, and on the other half to the left. This was randomly assigned. A total of 413 ants from eight colonies were tested.
(c). Statistical analysis
Statistical analyses were carried out in R v. 3.1.0 [24] using generalized linear-mixed models [25]. Following Forstmeier & Schielzeth [26], we included in the tested models only factors and interactions for which we had a priori reasons for including.
As the pheromone deposition behaviour of ants heading towards the food source (outgoing) and ants returning to the nest is known to be very different [15,16,27], we analysed the behaviour of outgoing and returning ants separately. As multiple data points were collected from each individual and multiple ants were tested per colony, ant and colony identity were added as random effects, with ant identity nested inside colony identity. Binomial data (pheromone deposited or not) were modelled using a binomial distribution and logit link function. Count data (number of pheromone depositions for ants, which deposited pheromone at least once) were modelled using a Poisson distribution using a log link function.
The following model formulae were used, and each model was run twice; once for outgoing and once for returning ants.
To test whether an environmental change elicits a change in pheromone deposition:
 |
With ‘pheromone.removed?’, ‘visit’ and ‘directly.after.switch’ as fixed effects.
As Czaczkes et al. [16] found that ants which had made an error while outgoing deposited more pheromone when returning, we also tested for this effect. Furthermore, in an initial survey of the data, it seemed that ants which were about to make an error decreased their pheromone deposition. We also tested explicitly for this effect. The model formulae used were as follows:
 |
 |
With ‘just.made.an.error?’, ‘will.make.an.error.this.visit?’ and ‘directly.after.switch’ as fixed effects.
Finally, to explicitly disentangle the effects of making an error and of experiencing an environmental change, we performed post hoc pairwise comparisons between the four possible combinations of error/no error and change/no change. Likewise, to explicitly disentangle the effort of making an error and travel direction, we performed post hoc pairwise comparisons between the four possible combinations of error/no error and travelling outwards/returning to nest.
3. Results
(a). Response of ant to an environmental change
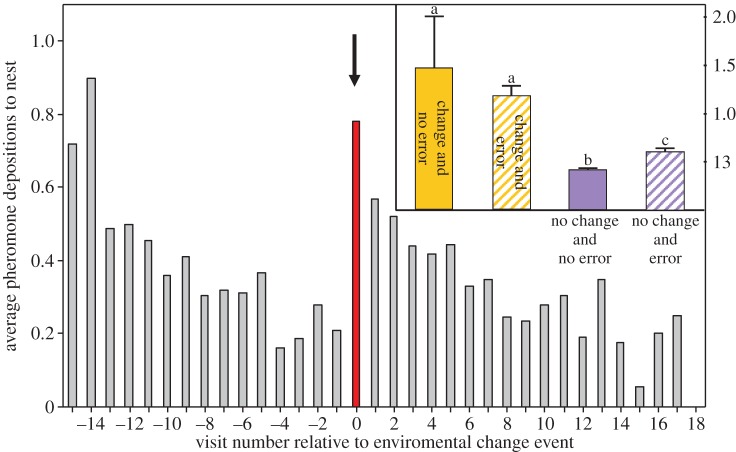
Immediately following a change in feeder locations, trained ants that had found a changed feeder position dramatically increased the number of pheromone depositions on their way back to the colony. The proportion (Z = 7.61, p < 0.001) and the number (Z = 5.53, p < 0.001) of depositions increased (main figure in figure 2). This was true of both ants which had correctly followed their memory to the old (now empty) feeder location, and those ants which had mistakenly taken the wrong path to the new (now rewarding) feeder location (figure 2 inset).
Figure 2.
Main figure: mean pheromone depositions of ants returning from the food source to the nest, by the visit number relative to the food source location change. Note that pheromone deposition is upregulated immediately after experiencing the change event. For clarity, this figure shows data only from experiments in which ants made 15 training visits to the original feeder location before the food source location was changed. See the electronic supplementary material figure S1 for similar figures from all change treatments. Inset: mean pheromone depositions of returning ants by whether or not they had experienced an environmental change event, and whether or not they had made a branch choice error before finally locating the food. This figure shows data from both ants which did and did not deposit pheromone. See the electronic supplementary material, S1 for two similar figures (figures S2 and S3) for deposition probability of ants and deposition intensity of depositing ants. Electronic supplementary material, S1 also contains all statistical results for the post hoc pairwise analyses. (Online version in colour.)
Conversely, outgoing ants, which had just experienced an environmental change on their previous visit, were less likely to deposit pheromone (Z = −2.71, p = 0.0067) even though they all did eventually relocate the feeder during the previous visit. However, of the ants that did choose to deposit pheromone, the number of pheromone depositions performed did not change (Z = 0.81, p = 0.42). Surprisingly, whether or not pheromone was removed from the stem of the maze had no effect on either deposition probability (outgoing Z = 1.43, p = 0.15, returning Z = 1.45, p = 0.15) or intensity (outgoing Z = 0.99, p = 0.32, returning Z = 0.93, p = 0.35). As reported previously [16,27], in later visits, ants were less likely to deposit pheromone (outgoing Z = −8.51, p < 0.001, returning Z = −8.02, p < 0.001), and when they did, they did so less intensely (outgoing Z = −6.49, p < 0.001, returning Z = −5.85, p < 0.001) in later visits.
(b). Response of ant to making an error
When returning to the nest after eventually finding the feeder, ants which had just made an error increased both the probability (Z = 6.40, p < 0.001) and intensity (Z = 2.03, p = 0.043) of pheromone deposition, even after any effect of environmental change had been taken into account (figure 2 inset and figure 3). Post hoc analysis revealed that the upregulation of pheromone deposition in response to an environmental change and in response to making an error are separate phenomena (see figure 2 inset and electronic supplementary material, S1 for statistical test results).
Figure 3.
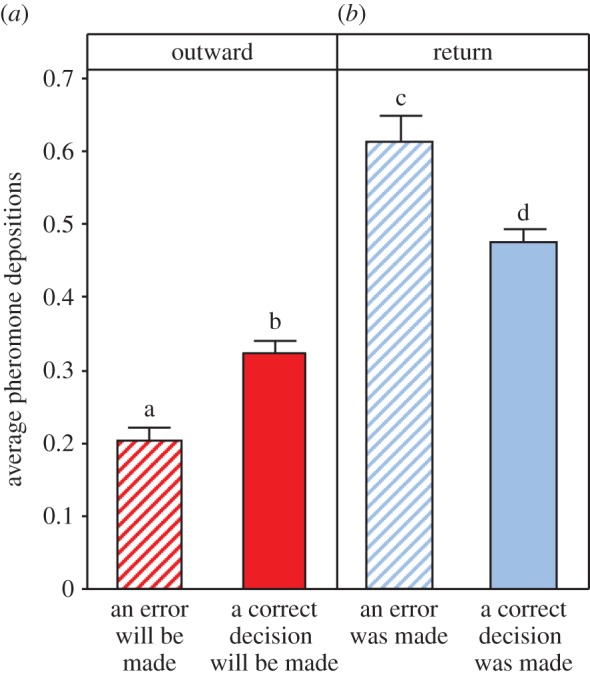
When travelling outwards towards a food source, ants deposited less pheromone if they were going to make an error at an upcoming bifurcation. When returning to the nest, ants which had made an error deposited more pheromone than ants which had made a correct decision. Different letters denote statistically significant values. These patterns are mainly driven by the proportion of ants choosing to deposit pheromone, and not by the modulation of the number of pheromone depositions by depositing ants. See the electronic supplementary material, S1 for similar figures (figures S5 and S6) in which pheromone laying probability and intensity are separated and for statistical details. Data from all visits and all treatments are pooled for clarity in this figure. (Online version in colour.)
Most surprisingly, outgoing ants which were about to make an error were significantly less likely to choose to deposit pheromone (Z = −6.19, p < 0.001, see figure 3 and electronic supplementary material, S1 and figure S4). However, of the ants that chose to deposit pheromone, the intensity of pheromone deposition was unchanged (see the electronic supplementary material, S1 and figure S5).
4. Discussion
We found that ants strongly upregulate pheromone deposition in response to an environmental change. This might also be seen as ants upregulating pheromone deposition on finding a new, unexpected, food source. Decision-making systems based on positive feedback loops, such as the ants’ pheromone-based recruitment, are very adept at reaching collective decisions, but are susceptible to becoming trapped in initial decisions [28–30]. This is because pheromone trails leading to initially chosen options become so strong as to outcompete any incipient trails to new options. Even if a better resource is found, the strong recruitment of a few ants cannot produce a trail strong enough to compete with the established trail. Likewise, algorithms based on positive feedback and ant colony optimization (ACO) can also suffer from early convergence and becoming trapped in local optima [31], as early paths strongly constrain future exploration of the available options by the algorithm. By implementing a rule upregulating pheromone deposition after an environmental change, colonies and ACO algorithms may be able to respond more rapidly to environmental changes, by facilitating the breaking out from previous decisions.
However, this response is seemingly only open to systems composed of agents with a memory. Only by comparing the previous situation to the current one can an environmental change be noted. However, an alternative behaviour, which does not require a memory, would be for ants to upregulate pheromone when returning on an unmarked path, and in fact, pheromone deposition of returning ants is higher on unmarked paths [7,15,32]. Decision-making in biological systems is characterized by multiple redundant or complementary mechanisms, and it is thus perhaps not surprising to find two separate mechanisms, which seem to fulfil the same role. A further alternative to upregulating recruitment in the face of environmental change would be to downregulate recruitment to the old food location. Indeed, honeybees can employ a piping signal to stop recruitment to dangerous food sources, and to prevent decision-making deadlocks [5,17,18]. We might expect honeybees, which have an excellent memory, to also show an upregulation in recruitment when faced with an environmental change. Likewise, it may be fruitful to search for a downregulation signal in ant recruitment systems. In honeybees, downregulation might be more straightforward, as individual dancing bees may be targeted. As ants deposit a pheromone trail and then leave, other mechanisms, such as perhaps a ‘no entry’ signal [19], might need to be employed.
Another potential benefit of responding to a changing environment by upregulating pheromone deposition would be in maintaining the pheromone trail as an ‘external memory’ [8]. Positive-feedback-based systems tend to converge on a single decision—a process termed ‘symmetry breaking’ [30,33]. By upregulating recruitment to new or underused resources such convergence may be avoided. Computer scientists tellingly have implemented rules strengthening underused parts of a network, specifically to prevent early convergence [34]. Other mechanisms for coping with environmental change include reducing the intensity of recruitment, or using more linear positive feedback mechanisms, such as individual recruitment [29,35–37].
The apparent response of ants to making an error by upregulating pheromone deposition on the return journey has been described previously [16]. Intriguingly, we also found that outgoing ants which went on to make an error deposited less pheromone. This seems to imply that the ants can judge the quality of their own memories, and respond accordingly: if the ant does not trust its own memory, it deposits less pheromone. The ability to assess the quality of a memory (known as metamemory judgement, which is an aspect of metacognition) is a highly advanced cognitive ability, which has previously only be demonstrated in mammals and some birds [38–41]. Social insects, such as ants and honeybees, are often found to have impressive cognitive abilities, such as making generalizations (e.g. something being similar or different to something) and learning abstract relationships between stimuli [42–45]. Nonetheless, it is hard to believe that such tiny-brained animals are capable of such an advanced cognitive feat. However, a recent study showed that honeybees are capable of selectively ‘opting out’ of difficult choices [46], although the authors stopped short of definitively claiming that metacognition was being used. Their study could not disentangle metacognition from simple association of the more difficult stimulus with the ‘opt-out’ decision. While our study avoids this particular pitfall and other problems which plague demonstrations of metacognition [47–49] (see the electronic supplementary material, S1), it was not designed specifically to address this question. Thus, one could conceive of several alternative explanations for our findings, which do not invoke metacognition. For example, it is possible that a third factor which we did not control, e.g. age, may predict both pheromone deposition and learning accuracy (although the opposite pattern found for returning ants speaks against this example). Individual variation between ants may also have caused the correlation we report. We therefore do not claim a definitive demonstration of metacognition in ants. Nonetheless, our findings, alongside similar results from honeybees [46], are suggestive of metacognitive abilities in social insects. As Smith [40,47], one of the founders of the field of metacognition, warns: we must also be careful that over-stringent demands for demonstrations of cognitive abilities do not cause us to ‘throw the baby out with the bathwater’.
Ants and social insects, in general, have a wide variety of elegant behavioural rules, which allow them to reach adaptive collective decisions in an ever-changing environment. The combination of individual cognitive abilities, such as a memory, alongside the ability to communicate information, allows for added complexity [8,23]. Whether or not they also possess metacognitive abilities, social insects demonstrate an impressive array of behavioural adaptations. While we have learned a great deal about social insect organization, and applied some of what we have learned, there are clearly many more aspects left to apply, and much more to learn about how social insects make decisions.
Supplementary Material
Acknowledgements
Many thanks to Carolin Iglhaut for help with data collection, and to Balint Marko and several anonymous reviewers for comments on an earlier versions of this manuscript.
Ethics
No licences or permits were required for this research, and no ethical approval was required.
Data accessibility
The raw data from these experiments is accessible via Dryad http://dx.doi.org/10.5061/dryad.mr73c.
Authors' contributions
T.J.C. conceived and designed the study, collected and analysed the empirical data, and wrote the manuscript; J.H. co-coordinated the project and critically revised the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
T.J.C. was supported by an Alexander von Humboldt postdoctoral fellowship. J.H. received no funding for work on this project.
References
- 1.Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. ( 10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 2.Collett TS, Collett M. 2002. Memory use in insect visual navigation. Nat. Rev. Neurosci. 3, 542–552. ( 10.1038/nrn872) [DOI] [PubMed] [Google Scholar]
- 3.Wallraff HG. 2005. Avian navigation: pigeon homing as a paradigm. Berlin, Germany: Springer. [Google Scholar]
- 4.Collett M, Chittka L, Collett TS. 2013. Spatial memory in insect navigation. Curr. Biol. 23, R789–R800. ( 10.1016/j.cub.2013.07.020) [DOI] [PubMed] [Google Scholar]
- 5.Nieh J. 2010. A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr. Biol. 20, 310–315. ( 10.1016/j.cub.2009.12.060) [DOI] [PubMed] [Google Scholar]
- 6.Valone TJ. 1996. Food-associated calls as public information about patch quality. Oikos 77, 153–157. ( 10.2307/3545595) [DOI] [Google Scholar]
- 7.Beckers R, Deneubourg JL, Goss S. 1993. Modulation of trail laying in the ant Lasius niger (Hymenoptera: Formicidae) and its role in the collective selection of a food source. J. Insect Behav. 6, 751–759. ( 10.1007/BF01201674) [DOI] [Google Scholar]
- 8.Czaczkes TJ, Grüter C, Ratnieks FLW. 2015. Trail pheromones: an integrative view of their role in colony organisation. Annu. Rev. Entomol. 60, 30.1–30.19. ( 10.1146/annurev-ento-010814-020627) [DOI] [PubMed] [Google Scholar]
- 9.Hangartner W. 1967. Spezifität und Inaktivierung des Spurpheromons von Lasius fuliginosus Latr. und Orientierung der Arbeiterinnen im Duftfeld. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 57, 103–136. ( 10.1007/BF00303068) [DOI] [Google Scholar]
- 10.von Thienen W., Metzler D, Choe D-H, Witte V. 2014. Pheromone communication in ants: a detailed analysis of concentration-dependent decisions in three species. Behav. Ecol. Sociobiol. 68, 1–17. ( 10.1007/s00265-014-1770-3)24436508 [DOI] [Google Scholar]
- 11.Mailleux A-C. 2006. Starvation drives a threshold triggering communication. J. Exp. Biol. 209, 4224–4229. ( 10.1242/jeb.02461) [DOI] [PubMed] [Google Scholar]
- 12.Czaczkes TJ, Grüter C, Ratnieks FLW. 2013. Negative feedback in ants: crowding results in less trail pheromone deposition. J. R. Soc. Interface 10, 20121009 ( 10.1098/rsif.2012.1009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikhchandani S, Hirshleifer D, Welch I. 1992. A theory of fads, fashion, custom, and cultural change as informational cascades. J. Polit. Econ. 100, 992–1026. ( 10.1086/261849) [DOI] [Google Scholar]
- 14.Schneirla TC. 1944. A unique case of circular milling in ants, considered in relation to trail following and the general problem of orientation. Am. Mus. Novit. 1254, 1–26. [Google Scholar]
- 15.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. 2011. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 7, 521–524. ( 10.1098/rsbl.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czaczkes TJ, Grüter C, Ratnieks FLW. 2013. Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. J. Exp. Biol. 216, 188–197. ( 10.1242/jeb.076570) [DOI] [PubMed] [Google Scholar]
- 17.Nieh JC. 1993. The stop signal of honey bees: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56. ( 10.1007/BF00164346) [DOI] [Google Scholar]
- 18.Seeley TD, Visscher PK, Schlegel T, Hogan PM, Franks NR, Marshall JAR. 2011. Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 335, 108–111. ( 10.1126/science.1210361) [DOI] [PubMed] [Google Scholar]
- 19.Robinson EJH, Jackson DE, Holcombe M, Ratnieks FLW. 2005. Insect communication: ‘no entry’ signal in ant foraging. Nature 438, 442 ( 10.1038/438442a) [DOI] [PubMed] [Google Scholar]
- 20.Bhatkar A, Whitcomb WH. 1970. Artificial diet for rearing various species of ants. Fla. Entomol. 53, 229–232. ( 10.2307/3493193) [DOI] [Google Scholar]
- 21.Herbers J, Choiniere E. 1996. Foraging behaviour and colony structure in ants. Anim. Behav. 51, 141–153. ( 10.1006/anbe.1996.0012) [DOI] [Google Scholar]
- 22.Portha S, Deneubourg J-L, Detrain C. 2004. How food type and brood influence foraging decisions of Lasius niger scouts. Anim. Behav. 68, 115–122. ( 10.1016/j.anbehav.2003.10.016) [DOI] [Google Scholar]
- 23.Czaczkes TJ, Czaczkes B, Iglhaut C, Heinze J. (in press) Composite collective decision making. Proc. R. Soc. B 282, 20142723 ( 10.1098/rspb.2014.2723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 25.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H. 2014. lme4: linear mixed-effects models using Eigen and S4. See http://cran.r-project.org/web/packages/lme4/index.html. [Google Scholar]
- 26.Forstmeier W, Schielzeth H. 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. ( 10.1007/s00265-010-1038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckers R, Deneubourg J, Goss S. 1992. Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insect. Soc. 39, 59–71. ( 10.1007/BF01240531) [DOI] [Google Scholar]
- 28.Goss S, Aron S, Deneubourg JL, Pasteels JM. 1989. Self-organized shortcuts in the Argentine ant. Naturwissenschaften 76, 579–581. ( 10.1007/BF00462870) [DOI] [Google Scholar]
- 29.Beckers R, Deneubourg JL, Goss S, Pasteels JM. 1990. Collective decision making through food recruitment. Insect. Soc. 37, 258–267. ( 10.1007/BF02224053) [DOI] [Google Scholar]
- 30.Sumpter DJT, Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280. ( 10.1006/anbe.2003.2224) [DOI] [Google Scholar]
- 31.Dorigo M, Stützle T. 2004. Ant colony optimization. Cambridge, MA: MIT Press. [Google Scholar]
- 32.Czaczkes TJ, Grüter C, Jones SM, Ratnieks FLW. 2012. Uncovering the complexity of ant foraging trails. Commun. Integr. Biol. 5, 78–80. ( 10.4161/cib.18209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanan MC, Dornhaus A, Jones EI, Waser A, Bronstein JL. 2012. The trail less travelled: individual decision-making and its effect on group behavior. PLoS ONE 7, e47976 ( 10.1371/journal.pone.0047976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong KY, See PC. 2009. A new minimum pheromone threshold strategy (MPTS) for max–min ant system. Appl. Soft Comput. 9, 882–888. ( 10.1016/j.asoc.2008.11.011) [DOI] [Google Scholar]
- 35.Czaczkes TJ. 2014. How to not get stuck: negative feedback due to crowding maintains flexibility in ant foraging. J. Theor. Biol. 360, 172–180. ( 10.1016/j.jtbi.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 36.De Biseau JC, Deneubourg JL, Pasteels JM. 1991. Collective flexibility during mass recruitment in the ant Myrmica sabuleti (Hymenoptera: Formicidae). Psyche J. Entomol. 98, 323–336. ( 10.1155/1991/38402) [DOI] [Google Scholar]
- 37.Beekman M, Dussutour A. 2009. How to tell your mates: costs and benefits of different recruitment mechanisms. In Food exploitation by social insects: ecological, behavioral, and theoretical approaches (eds Jarau S, Hrncir M.), pp. 115–134. Boca Raton, FL: CRC Press. [Google Scholar]
- 38.Sutton JE, Shettleworth SJ. 2008. Memory without awareness: pigeons do not show metamemory in delayed matching to sample. J. Exp. Psychol. Anim. Behav. Process. 34, 266–282. ( 10.1037/0097-7403.34.2.266) [DOI] [PubMed] [Google Scholar]
- 39.Fujita K. 2009. Metamemory in tufted capuchin monkeys (Cebus apella). Anim. Cogn. 12, 575–585. ( 10.1007/s10071-009-0217-0) [DOI] [PubMed] [Google Scholar]
- 40.Smith JD. 2009. The study of animal metacognition. Trends Cogn. Sci. 13, 389–396. ( 10.1016/j.tics.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 41.Goto K, Watanabe S. 2012. Large-billed crows (Corvus macrorhynchos) have retrospective but not prospective metamemory. Anim. Cogn. 15, 27–35. ( 10.1007/s10071-011-0428-z) [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan MV, Zhang SW, Zhu H. 1998. Honeybees link sights to smells. Nature 396, 637–638. ( 10.1038/25272) [DOI] [Google Scholar]
- 43.Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. 2001. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 410, 930–933. ( 10.1038/35073582) [DOI] [PubMed] [Google Scholar]
- 44.Avarguès-Weber A, Giurfa M. 2013. Conceptual learning by miniature brains. Proc. R. Soc. B 280, 20131907 ( 10.1098/rspb.2013.1907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czaczkes TJ, Schlosser L, Heinze J, Witte V. 2014. Ants use directionless odour cues to recall odour-associated locations. Behav. Ecol. Sociobiol. 68, 981–988. ( 10.1007/s00265-014-1710-2) [DOI] [Google Scholar]
- 46.Perry CJ, Barron AB. 2013. Honey bees selectively avoid difficult choices. Proc. Natl Acad. Sci. USA 110, 19 155–19 159. ( 10.1073/pnas.1314571110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JD, Couchman JJ, Beran MJ. 2012. The highs and lows of theoretical interpretation in animal-metacognition research. Phil. Trans. R. Soc. B 367, 1297–1309. ( 10.1098/rstb.2011.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crystal JD, Foote AL. 2009. Metacognition in animals. Comp. Cogn. Behav. Rev. 4, 1–16. ( 10.3819/ccbr.2009.40001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jozefowiez J, Staddon JER, Cerutti DT. 2009. Metacognition in animals: how do we know that they know? Comp. Cogn. Behav. Rev. 4, 29–39. ( 10.3819/ccbr.2009.40003) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data from these experiments is accessible via Dryad http://dx.doi.org/10.5061/dryad.mr73c.