Abstract
The social brain hypothesis assumes the evolution of social behaviour changes animals' ecological environments, and predicts evolutionary shifts in social structure will be associated with changes in brain investment. Most social brain models to date assume social behaviour imposes additional cognitive challenges to animals, favouring the evolution of increased brain investment. Here, we present a modification of social brain models, which we term the distributed cognition hypothesis. Distributed cognition models assume group members can rely on social communication instead of individual cognition; these models predict reduced brain investment in social species. To test this hypothesis, we compared brain investment among 29 species of wasps (Vespidae family), including solitary species and social species with a wide range of social attributes (i.e. differences in colony size, mode of colony founding and degree of queen/worker caste differentiation). We compared species means of relative size of mushroom body (MB) calyces and the antennal to optic lobe ratio, as measures of brain investment in central processing and peripheral sensory processing, respectively. In support of distributed cognition predictions, and in contrast to patterns seen among vertebrates, MB investment decreased from solitary to social species. Among social species, differences in colony founding, colony size and caste differentiation were not associated with brain investment differences. Peripheral lobe investment did not covary with social structure. These patterns suggest the strongest changes in brain investment—a reduction in central processing brain regions—accompanied the evolutionary origins of eusociality in Vespidae.
Keywords: brain investment, brain evolution, mushroom bodies, paper wasps, potter wasps
1. Introduction
We studied the neuroecology of brain investment to test predictions of social brain hypotheses, using wasps (Hymenoptera: Vespidae) as subjects. The field of neuroecology uses comparative approaches to identify environmental factors that select for evolutionary changes in brain tissue investment. Brains are compartmentalized into anatomically discrete regions with distinct cognitive functions. Because neural tissue is among the most expensive to produce and maintain metabolically, natural selection will balance cognitive processing power against tissue costs to favour an optimal investment in each brain region [1]. Investment in a given brain region (e.g. relative tissue volume) is expected to correspond to the cognitive demands placed on that region [2,3]. For example, species shifts into low-light environments (nocturnal activity, subterranean activity and cave-dwelling) can be accompanied by reductions in the size of visual-processing brain regions relative to visually oriented ancestors [4,5]. The social brain hypothesis extends the range of brain-relevant environmental factors to include social behaviour. Social brain models propose that interactions with conspecifics are an important source of cognitive challenges to animals, and generally assume increases in social complexity select for greater brain investment [6–8]. Positive correlations of brain region size and social complexity are found in diverse vertebrate taxa [7,9–11], and social brain selection has been invoked as a factor in human brain evolution [12,13]. Vertebrate social groups often comprise associations of unrelated or distantly related individuals from the same generation (excepting mole rats [14]), and considerable opportunity exists for evolutionary conflicts of interest and within-group antagonism. The need to assess and respond to intra-group conflict (including formations of alliances) can be major determinant of cognitive capacity in social vertebrates; social brain theorists refer to this as Machiavellian intelligence [15]. We propose conflict-driven effects on brain development are one class of social brain models, which we term social challenge hypotheses. Social challenge hypotheses predict positive associations between sociality or social complexity and brain investment.
However, sociality is not a unitary phenomenon. The ways societies evolve and develop differ among taxa, and the predicted effects of sociality on individual brain investment depend on how and why social groups form in a given species. The predicted direction and magnitude of brain investment with sociality is not straightforward. For example, Gronenberg & Riveros [16] developed social recognition-based models and predicted brain investment would peak at intermediate levels of sociality in ‘individual-based’ societies, and then decrease as ‘class-based’ societies evolve [17]. The capacity to recognize individual group members in small/primitive societies could favour increased brain investment relative to solitary ancestors, but group (rather than individual) recognition predominates in larger/derived class-based societies. These predictions received mixed support when tested using total brain volume and olfactory tissue investment in brains of fungus-gardening ants [17].
Here, we develop an alternative model for social brain evolution that makes different predictions, which we term the distributed cognition hypothesis. Social insect colonies typically comprise multi-generation family groups rather than co-generational associations. Strong colony-level selection, driven in part by intense between-colony (between-family) competition, can select for effective information sharing and division of labour within insect colonies. If within-group communication has supplemented (or supplanted) individual sensory assessment [18,19], social challenge hypotheses will not apply to insect societies. As an alternative, if cooperative information sharing among individuals takes precedence over within-colony conflict, selection for individual cognitive abilities can be relaxed. The general prediction of distributed cognition models is opposite to that of social challenge models: brain investment will decrease, rather than increase, with increases in sociality. Distributed cognition predictions also differ from social recognition models: individual brain investment will decrease with the advent of sociality relative to solitary ancestors. Elaborations of social complexity—increases in group size, or in the strength of division of labour within social clades—could be associated with even greater reductions in the need for individual cognition. If so, distributed cognition predicts further decreases in brain investment in more socially advanced clades.
We present the first phylogenetically informed comparative test of social brain models in social insects using social paper wasps (Polistinae) and their closest solitary relatives the potter wasps (Eumeninae—Hymenoptera: Vespidae) as subjects. In addition to the ability to compare derived social taxa with their solitary sister taxon, we took advantage of the great diversity in levels of colony complexity among social paper wasp species. Vespid species vary in colony size, mode of colony founding (by independent females or by swarms) and degree of queen/worker caste differentiation [18,20,21].
We used two measures of brain investment to test for relationships with sociality. (i) Relative size of the mushroom body (MB) calyces. Mushroom bodies are paired neuropils in the forebrain of arthropods, used in multisensory integration, associative learning and spatial memory [22,23]. MB calyx volume correlates with dendrite length and branching complexity, and with the number of neuronal synapses [23–25]. Increases in paper wasp MB calyx volume are associated with complex behavioural tasks (foraging) and with dominance status [26,27]. MB calyx investment provides an arthropod analogue of cerebral investment among vertebrates [6,28]. Recent studies of brain evolution in the insect order Hymenoptera showed changes in brain architecture, such as increases in MB size and structural complexity, pre-dated the origins of sociality [29]. Previous studies included few or no social taxa and did not directly address the predictions of social brain models. (ii) The ratio of antennal lobe (olfactory processing) to optic lobe (visual processing) volumes. Riveros et al. [17] recommended the antennal to optic lobe ratio as an indicator of brain investment for social species that rely heavily on chemical (pheromonal) communication.
To analyse associations of brain structure with social behaviour, we first asked whether social and solitary Vespidae species differed in brain structure. We then tested whether further evolutionary transitions in social complexity among the social species were associated with brain structure differences. We used three indices of colony complexity in our species-level comparative analyses: (i) mature colony size (numbers of adults), (ii) mode of colony founding (independent versus swarm) and (iii) degree of queen/worker caste differentiation.
2. Material and methods
(a). Subject taxa and sampling effort
All subjects were adult females. We analysed brain architecture of 180 individual vespid wasps from 29 species in 20 genera (see electronic supplementary material for details of phylogeny). Six species in five genera were solitary-nesting potter wasps (Eumeninae). Social species were sampled from the subfamily Polistinae: two species in two genera of Old World Ropalidiini, one species each of Mischocyttarini and Polistini, and 19 species from 11 genera of Neotropical swarm-founding Epiponini [30–32]. We collected neuroanatomical data on one to 13 wasps per species (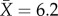 ), with one to 10 individuals from each caste (
), with one to 10 individuals from each caste (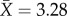 queens,
queens, 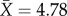 workers) for the social species. We obtained workers for all 23 social species, and queens for 18 of these species. We sampled one species per genus, except in some Eumeninae and in the speciose paper wasp genus Polybia, where we used a subgeneric phylogeny to select a maximum of two species per subgenus [31,32].
workers) for the social species. We obtained workers for all 23 social species, and queens for 18 of these species. We sampled one species per genus, except in some Eumeninae and in the speciose paper wasp genus Polybia, where we used a subgeneric phylogeny to select a maximum of two species per subgenus [31,32].
(b). Specimen collection and preparation
Wasps were field-collected into and stored in buffered aldehyde-based fixative (Prefer, Anatech Ltd) for at least two months until histological processing. See electronic supplementary material for details of collection dates and locations. All wasps were collected from nests in the field except Brachygastra, which were collected from a swarm, and Parapolybia, Ropalidia and the Eumeninae, which were collected as foragers at flowers.
(c). Determining subjects' caste
We dissected each subject's gaster (the terminal abdominal body region) in fixative. We exposed the ovaries and examined them at 10× under a binocular dissecting scope. We selected workers that had filamentous ovarioles with no visible opaque oocyte swelling, and queens with at least one fully opaque oocyte more than 2.5 times as long as broad per ovariole. All subjects were mature wasps with fully hardened, deeply coloured cuticles. We did not know the individual histories of the subjects and we assumed our haphazardly chosen samples were representative of mature females of each caste.
(d). Social complexity variables
Colony size. Colony sizes were mean numbers of adults in mature colonies; values were obtained from published records or from our own field collections [20,33–36]. Mode of founding. Social species were categorized as independent-founding (new nests initiated by a solitary queen) or swarm-founding (nests initiated socially by a group of queens and workers) based on published accounts [20,37]. Caste differentiation. We categorized the degree of queen/worker caste differentiation in each species based on published morphometric analyses and our own observations [38–41]. We used the caste-differentiation categories of Noll et al. [41] to classify the social species' degree of queen/worker caste differences as follows. No castes: continuous morphological and ovary development variation. Physiological castes: only queens show ovary development. Size castes: queens are larger but similar in shape. Morphological castes: queens are distinct in shape (body allometry). We pooled species with physiological and size castes for our analyses.
(e). Histology and neuroanatomy
We used standard histology and light photomicrography techniques to estimate the volumes of brain regions [42,43]. To summarize, we embedded wasps' head capsules in plastic resin and sectioned the entire brain on a rotary microtome. After staining the sections, we captured digital images of all brain tissues with a compound microscope. We traced the areas of targeted brain regions in images of serial sections, and then multiplied (area × section thickness) to estimate the volume of each brain region [44]. Details of these methods are given in the electronic supplementary material. We measured the brain neuropils (regions of dendritic arborization and axonal connections) and did not include the layers of neuron cell bodies that surround the brain. The MB calyx lip, collar and basal ring were pooled into a volume for MB calyx (a measure of central/integrative processing), two regions of the optic lobes (medulla and lobula) were pooled to yield optic lobe volume (peripheral visual processing), and the volume of the olfactory glomeruli yielded antennal lobe volume (peripheral chemosensory processing). We used total volume of all other brain structures—central complex, protocerebral mass, and MB lobes and peduncles—as an index of overall brain size (henceforth referred to as brain remainder).
(f). Variables and statistical analyses
All analyses were performed using SPSS v. 20 software (IBM corp. 2011). We used general linear models to test relationships of continuous and categorical covariates with species-mean brain investment (the ratio of MB calyx to brain remainder volume, and the antennal lobe to optic lobe volume ratio). We present the results of analyses run on all subjects for each species. Queens and workers could differ in brain architecture [45], and we did not sample equal numbers of each caste for all species. To check for caste differences, we repeated all analyses on data for each caste separately; in every case, patterns of statistical significance for each caste were identical to the results for the full dataset, including post hoc analyses, and we do not report the results for these tests on separate castes.
We performed a phlylogenetic independent-contrasts analysis to account for possible effects of species relatedness on the patterns we measured. We used Compare v. 4.6b software [46] and published phylogenies of Polistinae and Eumeninae [30–32]. All branch lengths were set to one, except in the case of unresolved nodes or nodes for which no information was available; these were set to a small branch length values (0.001) as recommended by Martins [46].
3. Results
(a). Mushroom body calyx: central processing investment
Colony size was negatively related to species mean MB calyx investment (figure 1a; R2 = 0.54, d.f. = 27, p < 0.001), contrary to the predictions of social complexity models. This effect was driven by inclusion of solitary species in the analysis; contrary to social recognition hypothesis predictions, colony size was not related to mean MB calyx investment among social species (figure 1a; R2 = 0.01, d.f. = 21, p = 0.60). No significant differences were found when the effects of phylogeny were taken into account (independent contrasts, r = 0.15, d.f. = 26, p = 0.45). Mode of colony founding (solitary, independent and swarm) was significantly related to species mean MB calyx investment (figure 1a; F2,26 = 38.6, p < 0.001) but solitary species had significantly higher MB calyx investment and independent versus swarm-founding social species did not differ (Tukey HSD post hoc tests). Caste determination categories differed in MB calyx investment (figure 2; F3,25 = 23.0, p < 0.001). Solitary species had significantly higher MB calyx investment; social species with no castes, size or physiological castes, and morphologically distinct castes did not differ (figure 2; Tukey HSD post hoc tests).
Figure 1.
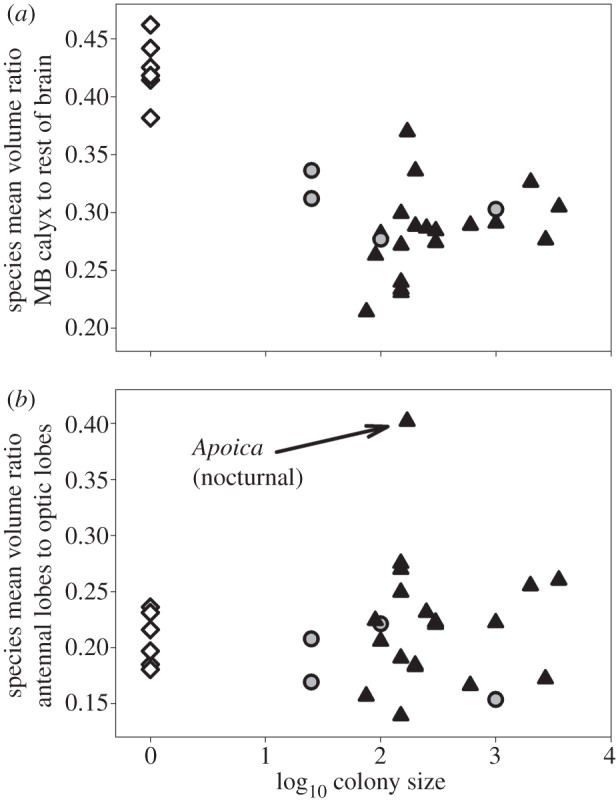
Scatter plots of the relationships between mature colony size (number of adult wasps in collected nests, log10 scale) and brain structure for 29 species of vespid wasps. Diamonds, solitary species; circles, independent-founding species; triangles, swarm-founding species. (a) Relationship of colony size with investment in central processing brain tissue: MB calyx relative to brain size. (b) Relationship of colony size with investment in peripheral processing brain tissue: antennal lobe to optic lobe volume ratio. The data point for the single nocturnal species (Apoica pallens) is indicated.
Figure 2.
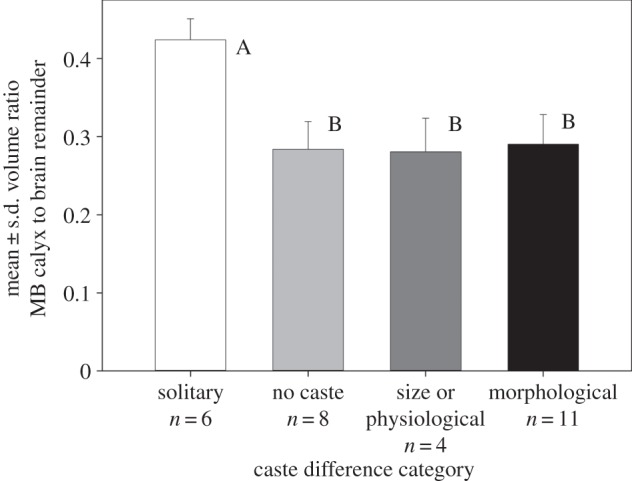
Bar graph showing mean ± s.d. across species of MB calyx investment versus degree of queen/worker caste differentiation for 29 species of vespid wasps. Letters represent results of post hoc comparisons among means; bars with the same letter were not significantly different. Sample sizes of species for each caste category are given below.
(b). Antennal/optic lobe ratio: peripheral sensory investment
The antennal to optic lobe volume ratio did not covary with colony size (figure 1b; R2 = 0.008, d.f. = 27, p = 0.64), and did not differ among caste determination categories (F3,25 = 0.60, p = 0.62).
4. Discussion
(a). Distributed cognition and social brain evolution in insects
We found no support for the social complexity hypothesis nor the individual recognition hypothesis. Instead, our data on MB calyx investment supported the main prediction of the distributed cognition hypothesis: solitary species had significantly greater MB calyx investment than social species. Among social species none of the three measures of social complexity (colony size, mode of colony founding and caste determination category) covaried with MB calyx investment. These patterns suggest the transition from solitary nesting to obligate sociality in Vespidae was accompanied by an average decrease in investment in brain tissue for central cognitive processing at the individual level. Further changes in colony structure or caste differentiation were not associated with brain architecture.
All the solitary species we sampled were in a single clade that was the sister taxon to the social species (electronic supplementary material, figure S1), and the phylogenetically corrected analysis did not support a significant relationship of MB investment with social behaviour. The generality of decreased MB investment with the evolution of insect sociality should be tested in other insect taxa, including solitary and social species such as bees and termites/roaches [47,48], to act as important checks on the generality of our findings. Elaborations of social structure that occurred after sociality evolved were not associated with directional changes in MB calyx investment. These patterns stand in marked contrast to the general association of increased sociality with greater investment in brain central processing regions among several taxa of vertebrates [6,7,10,11]. Our conclusion that increases in social complexity were not relevant to brain evolution relies on the assumption that the proxies for social complexity we analysed—colony size, mode of colony founding and degree of caste differentiation—reflect cognitively relevant variation in social structure. Some of the proxies we used are comparable with indicators of social complexity that are related to brain investment among vertebrates. Although our understanding of how insects encode and process social information is minimal [49], we believe our taxon sampling effectively captured the wide range of social structures presented by the Vespidae.
We did not find evidence for differences among grades of sociality with antennal/optic lobe investment. The nocturnal paper wasp Apoica appeared to have an exceptionally high antennal/optic volume ratio, possible reflecting adaptation to activity in low-light conditions [42].
(b). Revisiting the predictions of the social brain hypothesis: linking societies and individuals
For most vertebrate taxa (possibly excepting naked mole rats), the patterns expected under the social brain hypothesis are relatively straightforward: group members will face an increase in socially imposed cognitive challenges as social complexity increases; this should be matched by a similar pattern of increased brain investment. We suggest this is true because of the fundamental way vertebrate societies are structured: vertebrate social groups are ecological contexts where individual reproductive agendas are pursued [8]. As social complexity increases, social interactions play a greater role (relative to other ecological factors, such as the needs to forage and avoid predators) in fitness variation. Social prowess may even become a predominant selective force on cognition [8,9]. Social conflict probably plays a major role in these patterns.
There is an important difference between vertebrate and insect societies in how social complexity arises. Simple vertebrate societies are often family groups, but larger vertebrate societies comprise increasingly distant relatives or even collections of non-relatives. This is not true of social insects: excepting unicolonial invasive ants [50], most insect societies are family groups. Changes in insect colony size and social complexity involve modifications of family structure, with a general trend towards increasing individual specialization and division of labour among group members [18,19]. In some senses, insect colonies can be viewed as extended phenotypes of the reproductive(s). We found evidence for a marked decrease, rather than an increase, in MB calyx investment that accompanied the origins of sociality in one social insect clade. Our data further suggest the most important transition was from solitary life to simple societies, with further transitions in social structure having no measurable effect on brain architecture. This pattern indicates super-organismal attributes, such as distributed cognition, arose early in social evolution among Vespidae.
Supplementary Material
Acknowledgements
Marie Clifford, Robin Harris, Emily Johnson, Nola MacAloon, Yamile Molina, Abigail Mudd, Christopher Papa, Eve Swearingen, James Warren and Nazaneen Zahedi assisted with histology and neuroanatomy. Collections were made under research permits from the governments of Costa Rica, Ecuador and Taiwan.
Authors' contributions
S.O'D. designed the study. S.O'D. collected specimens. S.J.B., SD., P.K., S.M. and E.S. collected histological data. S.O'D. analysed the data. S.O'D., S.J.B. and S.D. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
S.O'D. was supported by National Science Foundation grant no. 1209072 and Drexel University startup funds; S.D. was supported by Drexel University Arts and Sciences Dean's Office funds.
References
- 1.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 2.Barton RA, Purvis A, Harvey PH. 1995. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Phil. Trans. R. Soc. Lond. B 348, 381–392. ( 10.1098/rstb.1995.0076) [DOI] [PubMed] [Google Scholar]
- 3.Ratcliffe JM, Brock Fenton MB, Shettleworth SJ. 2006. Behavioral flexibility positively correlated with relative brain volume in predatory bats. Brain Behav. Evol. 67, 165–176. ( 10.1159/000090980) [DOI] [PubMed] [Google Scholar]
- 4.Safi K, Dechmann DK. 2005. Adaptation of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera). Proc. R. Soc. B. 272, 179–186. ( 10.1098/rspb.2004.2924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catania KC, Henry EC. 2006. Touching on somatosensory specializations in mammals. Curr. Opin. Neurobiol. 16, 467–473. ( 10.1016/j.conb.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 6.Dunbar R. 2003. Evolution of the social brain. Science 302, 1160–1161. ( 10.1126/science.1092116) [DOI] [PubMed] [Google Scholar]
- 7.Perez-Barberıa FJ, Shultz S, Dunbar RIM. 2007. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution 61, 2811–2821. ( 10.1111/j.1558-5646.2007.00229.x) [DOI] [PubMed] [Google Scholar]
- 8.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shultz S, Dunbar RIM. 2006. Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B 273, 207–215. ( 10.1098/rspb.2005.3283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bshary R, Gingins S, Vail AL. 2014. Social cognition in fishes. Trends Cogn. Sci. 18, 465–471. ( 10.1016/j.tics.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 11.West RJD. 2014. The evolution of large brain size in birds is related to social, not genetic, monogamy. Biol. J. Linn. Soc. 111, 668–678. ( 10.1111/bij.12193) [DOI] [Google Scholar]
- 12.Mooney SJ, Peragine DE, Hathaway GA, Holmes MM. 2014. A game of thrones: neural plasticity in mammalian social hierarchies. Soc. Neurosci. 9, 108–117. ( 10.1080/17470919.2014.882862) [DOI] [PubMed] [Google Scholar]
- 13.Gowlett J, Gamble C, Dunbar R. 2012. Human evolution and the archaeology of the social brain. Curr. Anthropol. 53, 693–722. ( 10.1086/667994) [DOI] [Google Scholar]
- 14.Faulkes CG, Bennett NC. 2013. Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Phil. Trans. R. Soc. B 368, 20120347 ( 10.1098/rstb.2012.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNally L, Jackson AL. 2013. Cooperation creates selection for tactical deception. Proc. R. Soc. B 280, 20130699 ( 10.1098/rspb.2013.0699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronenberg W, Riveros AJ. 2009. Social brains and behavior- past and present. In Organization of insect societies (eds Gadau J, Fewell J.), pp. 377–401. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Riveros AJ, Seid MA, Wcislo WT. 2012. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 83, 1043–1049. ( 10.1016/j.anbehav.2012.01.032) [DOI] [Google Scholar]
- 18.Jeanne RL. 2003. Social complexity in the Hymenoptera, with special attention to wasps. In Genes, behaviors and evolution of social insects (eds Kitkuchi T, Azuma N, Higashi S.), pp. 81–121. Sapporo, Japan: Hokkaido University Press. [Google Scholar]
- 19.Hölldobler B, Wilson EO. 2009. The superorganism. New York, NY: WW Norton. [Google Scholar]
- 20.Jeanne RL. 1991. The swarm-founding Polistinae. In The social biology of wasps (eds Ross KG, Matthews RW.), pp. 191–231. Ithaca, NY: Comstock Publishing Associates. [Google Scholar]
- 21.O'Donnell S. 1998. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu. Rev. Entomol. 43, 323–346. ( 10.1146/annurev.ento.43.1.323) [DOI] [PubMed] [Google Scholar]
- 22.Davis RL. 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28, 275–302. ( 10.1146/annurev.neuro.28.061604.135651) [DOI] [PubMed] [Google Scholar]
- 23.Farris SM, Robinson GE, Fahrbach SE. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones TA, Donlan NA, O'Donnell S. 2009. Growth and pruning of mushroom body Kenyon cell dendrites during worker behavioral development in the paper wasp, Polybia aequatorialis (Hymenoptera: Vespidae). Neurobiol. Learn. Mem. 92, 485–495. ( 10.1016/j.nlm.2009.06.007) [DOI] [PubMed] [Google Scholar]
- 25.Seid MA, Harris KM, Traniello JFA. 2005. Age-related changes in the number and organization of synapses in the lip region of the mushroom bodies in the ant Pheidole dentata. J. Comp. Neurol. 488, 269–277. ( 10.1002/cne.20545) [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell S, Donlan NA, Jones TA. 2004. Mushroom body structural plasticity is associated with temporal polyethism in eusocial wasp workers. Neurosci. Lett. 356, 159–162. ( 10.1016/j.neulet.2003.11.053) [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell S, Donlan NA, Jones TA. 2007. Developmental and dominance-associated differences in mushroom body organization in the paper wasp, Mischocyttarus mastigophorus. J. Neurobiol. 67, 39–46. ( 10.1002/neu.20324) [DOI] [PubMed] [Google Scholar]
- 28.Farris SM. 2008. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav. Evol. 72, 1–15. ( 10.1159/000139457) [DOI] [PubMed] [Google Scholar]
- 29.Farris SM, Schulmeister S. 2011. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc. R. Soc. B 278, 940–951. ( 10.1098/rspb.2010.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel JW, Carpenter JM. 1994. Comparing methods: adaptive traits and tests of adaptation. In Phylogenetics in ecology (eds Eggleton P, Vane-Wright R.), pp. 79–101. London, UK: Harcourt Brace. [Google Scholar]
- 31.Carpenter JM, Kojima J-I, Wenzel JW. 2000. Polybia, paraphyly, and polistine phylogeny. Am. Mus. Novit. 3298, 1–24. () [DOI] [Google Scholar]
- 32.Hermes MG, Melo GAR, Carpenter JM. 2014. The higher-level phylogenetic relationships of the Eumeninae (Insecta, Hymenoptera, Vespidae), with emphasis on Eumenes sensu lato. Cladistics 30, 453–484. ( 10.1111/cla.12059) [DOI] [PubMed] [Google Scholar]
- 33.Richards OW, Richards MJ. 1951. Observations on the social wasps of South America (Hymenoptera Vespidae). Trans. R. Entomol. Soc. 102, 1–169. ( 10.1111/j.1365-2311.1951.tb01241.x) [DOI] [Google Scholar]
- 34.Spradbery JP. 1973. Wasps. Seattle, WA: University of Washington Press. [Google Scholar]
- 35.Richards OW. 1978. The social wasps of the Americas, excluding the Vespinae. London, UK: British Museum (Natural History). [Google Scholar]
- 36.Ito Y, Itioka T. 2008. Demography of the Okinawan eusocial wasp Ropalidia fasciata (Hymenoptera: Vespidae). II. Effects of foundress group size on survival rates of colonies and foundresses, and production of progeny. Entomol. Sci. 11, 17–30. ( 10.1111/j.1479-8298.2007.00248.x) [DOI] [Google Scholar]
- 37.Gadagkar R. 1991. Belonogaster, Mischocyttarus, Parapolybia and independent-founding Ropalidia. In The social biology of wasps (eds Ross KG, Matthews RW.), pp. 149–190. Ithaca, NY: Cornell University Press. [Google Scholar]
- 38.Shima SN, Yamane S, Zucchi R. 1994. Morphological caste differences in some Neotropical swarm-founding polistine wasps. I. Apoica flavissima (Hymenoptera: Vespidae). Jpn J. Entomol. 62, 811–822. [Google Scholar]
- 39.Shima SN, Yamane S, Zucchi R. 1996. Morphological caste differences in some Neotropical swarm-founding polistine wasps. II. Polybia dimidiata (Hymenoptera, Vespidae). Jpn J. Entomol. 64, 131–144. [Google Scholar]
- 40.Hunt JH, O'Donnell S, Chernoff N, Brownie C. 2001. Observations on two Neotropical swarm-founding wasps, Agelaia yepocapa and A. panamaensis (Hymenoptera: Vespidae). Ann. Entomol. Soc. Am. 94, 555–562. ( 10.1603/0013-8746(2001)094[0555:OOTNSF]2.0.CO;2) [DOI] [Google Scholar]
- 41.Noll FB, Wenzel JW, Zucchi R. 2004. Evolution of caste in neotropical swarm-founding wasps (Hymenoptera: Vespidae; Epiponini). Am. Mus. Novit. 3467, 1–24. () [DOI] [Google Scholar]
- 42.O'Donnell S, Clifford MR, DeLeon S, Papa C, Zahedi N, Bulova SJ. 2013. Brain size and visual environment predict species differences in paperwasp sensory processing brain regions (Hymenoptera: Vespidae, Polistinae). Brain Behav. Evol. 82, 177–184. ( 10.1159/000354968) [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell S, Clifford MR, DeLeon S, Papa C, Zahedi N, Bulova SJ. 2014. A test of neuroecological predictions using paperwasp caste differences in brain structure (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 68, 529–536. ( 10.1007/s00265-013-1667-6) [DOI] [Google Scholar]
- 44.Mayhew TM. 1992. A review of recent advances in stereology for quantifying neural structure. J. Neurocytol. 21, 313–328. ( 10.1007/BF01191700) [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell S, Clifford MR, Molina Y. 2011. A comparative analysis of constraints and caste differences in brain investment among social paper wasps. Proc. Natl Acad. Sci. USA 108, 7107–7112. ( 10.1073/pnas.1017566108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins EP. 2004. COMPARE, version 4.6b. Computer programs for the statistical analysis of comparative data. Bloomington, IN: Department of Biology, Indiana University; See http://compare.bio.indiana.edu. [Google Scholar]
- 47.Danforth BN. 2002. Evolution of sociality in a primitively eusocial lineage of bees. Proc. Natl Acad. Sci. USA 99, 286–290. ( 10.1073/pnas.012387999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nalepa CA. 2011. Body size and termite evolution. Evol. Biol. 38, 243–257. ( 10.1007/s11692-011-9121-z) [DOI] [Google Scholar]
- 49.Lihoreau M, Latty T, Chittka L. 2012. An exploration of the social brain hypothesis in insects. Front. Phys. 3, 1–7. ( 10.3389/fphys.2012.00442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moffett MW. 2012. Supercolonies of billions in an invasive ant: what is a society? Behav. Ecol. 23, 925–933. ( 10.1093/beheco/ars043) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


