Abstract
Montane species distributions interrupted by valleys can lead to range fragmentation, differentiation and ultimately speciation. Paleoclimatic fluctuations may accentuate or reduce such diversification by temporally altering the extent of montane habitat and may affect species differentially. We examined how an entire montane bird community of the Western Ghats—a linear, coastal tropical mountain range—responds to topographic valleys that host different habitats. Using genetic data from 23 species (356 individuals) collected across nine locations, we examined if different species in the community reveal spatial concordance in population differentiation, and whether the timing of these divergences correlate with climatic events. Our results reveal a nested effect of valleys, with several species (10 of 23) demonstrating the oldest divergences associated with the widest and deepest valley in the mountain range, the Palghat Gap. Further, a subset of these 10 species revealed younger divergences across shallower, narrower valleys. We recovered discordant divergence times for all valley-affected montane birds, mostly in the Pleistocene, supporting the Pliestocene-pump hypotheses and highlighting the role of climatic fluctuations during this period in driving species evolution. A majority of species remain unaffected by valleys, perhaps owing to geneflow or extinction–recolonization dynamics. Studying almost the entire community allowed us to uncover a range of species’ responses, including some generalizable and other unpredicted patterns.
Keywords: Shola, sky island, phylogenetic, Palghat Gap, Shenkottah Gap, India
1. Introduction
Montane habitats harbour high biodiversity and serve as excellent laboratories to study evolution and diversification [1]. Montane or sky island systems are characterized by specific environmental conditions, limiting species that occur there. Topographic dips in mountain ranges, which lead to valleys of unsuitable habitat and micro-climate, cause discontinuities in species' distributions, thereby affecting their genetic structure and evolutionary trajectories [2]. Understanding evolution and diversification in montane habitats is particularly important as these habitats are disproportionately threatened by climate change [3] and anthropogenic deforestation [4].
Biogeographic barriers, like the Makassar Strait of Wallace's line, or the Isthmuses of Tehuantepec and Panama (which separate South and North America), correlate with genetic divergences across several species inhabiting these regions (reviewed in [5]). Identifying biogeographic barriers that are consistent across taxa elucidates the geographic drivers of species' distribution and biodiversity, and helps to delineate areas of endemism [6].
Topographic dips in mountain ranges function as biogeographic barriers for montane taxa, with some affecting species concordantly (e.g. Africa [7] and Americas [8]), while others create discordant divergences (e.g. Africa [9]). But mountain valleys can act as bridges or barriers, depending on the climatic conditions. Climatic fluctuations can change montane habitat distributions [10,11], enhancing the effects of biogeographic barriers [12]. Empirical evidence for the effects of climatic fluctuations comes from several montane regions where phylogenetic divergence correlates with the Pliocene in African forest robins [13] and in a Western Ghat sky island insectivore [14], Pleistocene in Australasian sky island species [15] and older Miocene events for South American Tanagers [16]. However, species may respond differentially to topography and climate. Comparative phylogeographic analyses have thus far been conducted on subsets of bird communities; studies from the Americas [17–19], Africa [12,20,21], Australia [22] and southeast Asia [2] exhibit varied patterns among the species sampled. Such studies include species pairs that are often picked to make specific, often extreme, comparisons like wet/humid species versus dry [17,23]. Joseph et al. [24] found higher genetic divergences among rainforest species than in co-distributed generalist species. While comparing extremely ecologically divergent species pairs is interesting, it is possible that examining phylogeographic patterns in an entire montane bird community might reveal more generalizable insights and reveal a gradient of genetic effects.
The Indian subcontinent hosts the highest global oscine (songbird) diversity [25] and includes the biodiverse Western Ghats. The sky islands of the Western Ghats host Tropical Montane Cloud Forests [26], or Sholas as they are known locally, spread across a 700 km range (figure 1) with 17 endemic bird species. Unlike the Andes, where recent topographic changes led to diversification of montane species (for example flycatchers [27] and hummingbirds [28]), the Western Ghats' topography is relatively older (ca 50 Myr, arising owing to a series of uplifts during the Indian plate movement between 160 and 50 Ma [29]), and perhaps existed when songbirds (or oscines) arrived in India (ca 34 Ma) [25].
Figure 1.
Western Ghat sky islands: (a) sampling locations, (b) elevation profile (adapted from [14]). (Online version in colour.)
We investigate comparative phylogeographic patterns in an almost entire community of songbirds in the montane Shola habitat across the sky islands of Western Ghats, India. We attempted to capture as many species as possible (with mist-nets) from the community in order to recover a differential impact of generalists and specialists in the community. Although all species examined here are found in these montane habitats, some species occur all the way to the lowest elevations (coastal, sea level), with no (or less) allopatry, and we expect such species to exhibit less genetic divergence than purely montane species. This would result in varied species responses in the community. Specifically, we use genetic data from 22 of 25 oscine birds and one non-passerine in the Shola community from across the Western Ghats to ask: (i) if there is spatial concordance of genetic differentiation across valleys in the Western Ghats between species, and (ii) when genetic divergence across these valleys took place and if there is temporal concordance across the bird community. Together, answering these questions will allow us to investigate the dominant biogeographic paradigm for this region, specifically exploring vicariant events resulting in temporal concordance across species.
2. Material and methods
Field sampling was conducted at nine locations across the Western Ghats sky islands, hereafter Shola Sky Islands. We planned our sampling such that we had samples from at least four major island groups (A, B, C and D; figure 1) divided by the three deepest valleys in this region: Chaliyar River valley between A (Wayanad and northern hills) and B (Nilgiri mountains), Palghat Gap between B and C (Anamalai–Palani–Highwavies Hills) and Shenkottah Gap between C and D (Agasthyamalai).
We defined the montane bird community of the Western Ghats as species occurring above 1400 m.a.s.l. assessed from our 5-year ringing data and further supported by independent auditory and visual point-count data from other studies (electronic supplementary material, S1). We sampled 22 oscine species (including two species complexes with two species each) and one columbiform (Wood Pigeon) comprising 30 races in all, with 11 endemic species from both forests and grasslands. We were unable to sample two canopy species, Nectarina minima and Dicaeum concolor. Eight species were exclusively found in the high elevations (more than 1000 m) where the highest mountains range between 1700 and 2600 m (figure 1). Our data consist of sequences from two nuclear (fifth intron of nuclear b-fibrinogen (FIB) and the third intron of the muscle-specific kinase gene (MUSK)) and two mitochondrial (Cytochrome-b (Cyt-b) and NADH dehydrogenase 3 (ND3)) genes from blood samples of 356 individuals belonging to 23 species. Field and laboratory methods are detailed in electronic supplementary material, S2.
(a). Phylogenetic methods
We built maximum-likelihood and Bayesian phylogenetic trees (details in electronic supplementary material, S2). We considered that a phylogeographic break was recovered for a species if we observed reciprocal monophyly (within the species across the four sampling localities). Final Bayesian trees with posterior support and maximum-likelihood bootstrap values at the nodes of divergence are presented in electronic supplementary material, S3. We also assessed divergence dates using Bayesian methods (electronic supplementary material, S2). The time to most recent common ancestor (TMRCA) of the internal node in the phylogenetic tree was considered as the divergence time corresponding to the biogeographic barrier (figure 1).
(b). Temporal concordance in divergences
We explicitly tested for simultaneous divergence in the 10 taxa using MTML-msBayes, a hierarchical approximate Bayesian computation method that permits across-species demographic variation, inter-gene variability in coalescent times and DNA mutation rate heterogeneity [30]. This implementation allows us to estimate the hyper-parameter PSI: the number of different divergent times across the 10 species pairs in our study. PSI was allowed to vary from 1 (concordant divergence) to 10 (incongruent divergence). Several iterations of divergence time (τ) prior were examined to explore the parameter space adequately (details in electronic supplementary material, S2). As MsBayes is considered to show a bias towards inferring a concordant divergence largely owing to broad uninformative prior distributions used [31], we also analysed the data with DPP-msBayes [32], which allows for more flexible priors and uses a non-parametric approach—Drichlet-process priors on divergence models [32].
3. Results
(a). Where do spatially concordant divergences occur in montane birds of the Western Ghats?
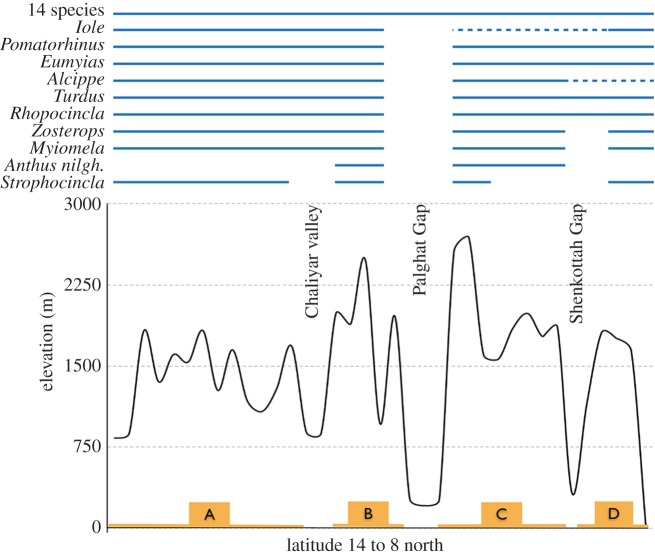
Ten of the 23 species examined showed genetic divergences (see the phylogenetic trees in electronic supplementary material, S3, and summary table in electronic supplementary material, S2) that were spatially concordant with valleys, while a majority [13] showed no such effects across the distribution examined. While the distributions of four of these species extend to lower elevations, potentially enabling geneflow, the exceptions included the small, high-elevation restricted endemic flycatcher Ficedula nigrorufa and other high-elevation birds like the Hypsipetes, Columba and Zoothera dauma, which showed no genetic divergence (figure 2). We recovered phylogenetic divergence that correlated with the Palghat Gap in 10 species, of which half were high-elevation (more than 1000 m) restricted while others are thought to be elevationally widespread in the Western Ghats (figure 2). The laughing thrush Strophocincla spp. complex was affected by all three valleys examined, while Myiomela spp. and Zosterops palpebrosus were affected only by Palghat Gap and Shenkottah Gap. Although the Zosterops distribution extends well below the valleys, we recovered unexpected phylogenetic divergence in the montane populations. We found the grassland endemic, Nilgiri Pipit Anthus nilghiriensis, to be genetically distinct across the only two highest mountain-tops that it occurs in, the Nilgiris and the Anamalai–Palni Hills (regions B and C in figure 1).
Figure 2.
Phylogenetic effect of valleys on montane bird community in the Western Ghats. Bottom half indicates the elevational profile of the sampled region and the upper half represents the phylogenetic data for each species. The break in the horizontal lines (blue online) in the upper half indicates phylogenetic division (inferred from phylogenetic trees; electronic supplementary material, S3); dashed lines (blue online): no data. Note that Anthus sp. distribution extends only to mountains B and C. (Online version in colour.)
(b). Is there temporal concordance among phylogenetic divergences in the Shola Sky Islands?
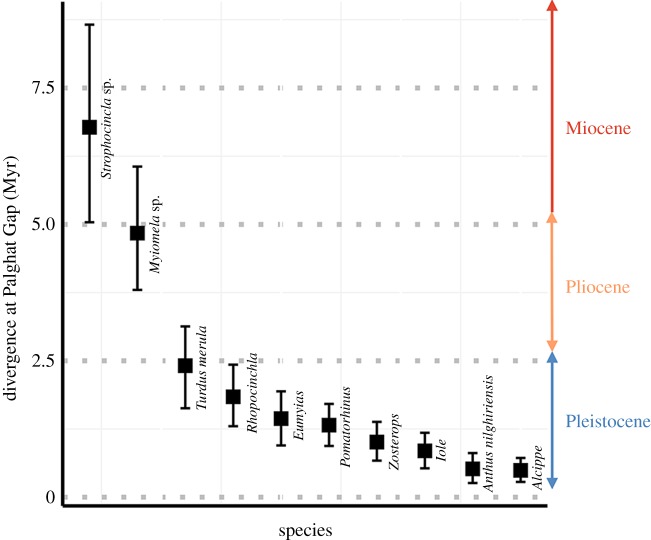
For almost all species, the oldest divergence was across the Palghat Gap. The oldest divergences were for two species complexes, Strophocincla and Myiomela, with a mean TMRCA of 6.78 (95% highest posterior density (hpd): 5.04–8.66) and 4.84 (95% hpd: 3.8–6.06) Ma, respectively. Of the other eight species that showed genetic differentiation, the mean divergence time for five species were between 2.5 and 1 Ma, while for all other species, the mean TMRCA was less than a million years (figure 3). The populations of the other 13 species that did not show any genetic divergence were also very young, with mean TMRCA less than a million years (electronic supplementary material, S4). Our examination of temporal concordance revealed that the two old divergences of Strophocincla and Myiomela were concordant when analysed with msBayes (ψ = 1, narrow and low Ω) but were discordant with DPP-msBayes (approximate posterior probability of two divergences = 0.997, GLM-adjusted posterior probability = 1). The eight younger divergences in the Pleistocene were also recovered to be discordant by both MTML-msBayes (ψ = 8, broad and high Ω) and DPP-msBayes (approximate posterior probability of eight divergences = 0.999, GLM-adjusted posterior probability = 1). As DPP-msBayes permits specification of more flexible priors while msBayes tends towards concordance [31,32], here we interpret all our study taxa to have discordant divergences.
Figure 3.
Molecular divergence time estimates (TMRCA—in Myr ± hpd) at the Palghat Gap (largest valley) for species that showed any genetic divergence. (Online version in colour.)
(c). Nested effect of valleys
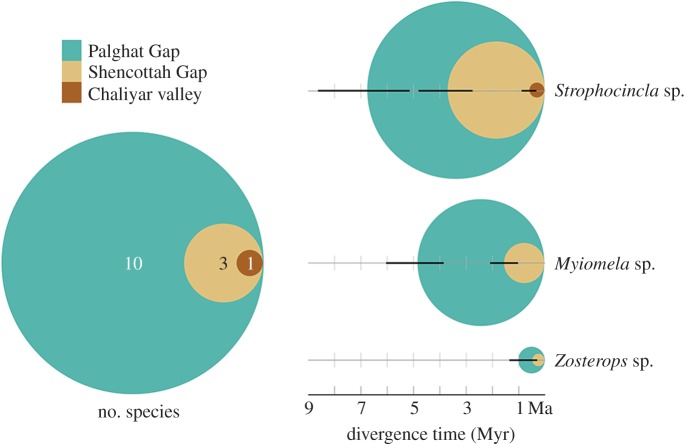
The montane birds of the Western Ghats are impacted by valleys in a nested pattern, with the Palghat Gap (between B and C) being the most important biogeographic divide, impacting most species (all 10 species that showed any genetic differentiation) followed by the Shenkottah Gap (between C and D) and then by the Chaliyar river valley (between A and B; figure 2). Species that were affected by two barriers were additionally affected by the Shenkottah Gap and then finally by the Chaliyar river, in a nested pattern. A similar nested pattern in age of divergences was observed, with those at Palghat Gap being older than at the Shenkottah Gap, followed by the Chaliyar River (figure 4).
Figure 4.
Nested effect of valleys on bird community. The panel on the left indicates the number of species showing genetic impacts—smaller, nested circles indicate a subset of species impacted by the respective valleys. The panel on the right indicates the age of divergence of three study taxa, with the older divergences always at Palghat Gap and younger divergences at smaller valleys. (Online version in colour.)
4. Discussion
We assessed the impact of habitat valleys and topographical dips on patterns of genetic differentiation in an entire montane bird community. Our results reveal that (i) not all species are affected to a similar extent and (ii) species are affected by topographic valleys in a nested pattern: the deepest gaps impact more species than do shallower gaps. Correspondingly, divergences across deeper gaps are older than those across shallower gaps. We suggest that climate-driven habitat shifts may accentuate genetic differentiation between locations concordant with deep and wide valleys, resulting in this nested pattern.
(a). Nested patterns of divergences in time and space
The relative impact of different topographic features revealed a consistent hierarchy or nestedness in the importance of different gaps, with the Palghat Gap affecting more species and creating more ancient divergences, followed by the impacts of the Shenkottah Gap and the Chaliyar river valley. We suggest that the relatively greater impact of the Palghat Gap may be owing to it being a wider (40 km wide compared with 10 km Shenkottah and 2–3 km wide Chaliyar valley) and deeper gap than the other two. Although no systematic studies have been conducted comparing species of differing ecologies and dispersal abilities, an impact of this Gap has also been found in small, restricted species like frogs [33] and some plants [34] and even in large-bodied, wide-ranging species like elephants [35]. However, certain older taxa like centipedes [36] and some long-lived trees [37] do not appear to be affected by this Gap.
Although the Shenkottah and the Palghat Gaps are geologically very old (pre-Cambrian), the impact on recent and relatively vagile colonizers like birds, especially those with small ranges, is likely to be through more recent climatic effects [38]. However, we indicate that in scenarios where climate-mediated habitat shifts lead to genetic structure in species, topography provides the background for climate to act upon. Species are thus more affected by the deeper, wider valleys than narrower, shallow ones—a probable paradigm for montane species. Such patterns, including nestedness, may not be apparent in other mountain ranges with many parallel ranges and complex topography. It is possible that this clear effect was recovered here possibly owing to the very linear Shola Sky Island system of the Western Ghats with relatively simple topography. Specifically, in the Western Ghats, our results suggest the Palghat Gap to be the most significant biogeographic barrier, possibly affecting patterns of diversity and distribution across varied taxa.
(b). Pleistocene divergences of montane bird community
Recent examination of montane bird diversification revealed that most African montane birds diverged from their sister taxa in the Pliocene–Miocene boundary and not in the Pleistocene [12]. However, in this study, the examination of the entire bird community reveals that all species diverged discordantly, with a majority of the species in the Pleistocene, while the only two older divergences are Myiomela and Strophocincla in the Pliocene–Miocene. The Pleistocene period is known to have had dramatic fluctuations in dry and wet periods driving the extent of wet evergreen habitats in India (reviewed in [39]) and in southeast Asia (e.g. [40]) and our dating of the phylogenetic divergences are consistent with the ‘Pleistocene-pump’ ([16], review in [41]), which suggests enhanced divergence during this period.
(c). Why are some montane species not impacted by valleys?
Some montane-restricted endemic species (F. nigrorufa and Schoenicola platyura) that we expected to respond to valleys like other specialists, did not show any population divergence across their range, and had young TMRCA. Such patterns could be caused (non-exclusively) as follows. (i) Species could actively disperse across these barriers, though current information does not support this. (ii) Extinction recolonization responses to paleoclimatic cycles are well documented in montane taxa [11,40] and have been proposed as an explanation for a similar lack of genetic structure in a high-altitude specialist bird in Australian sky islands [24]. (iii) Incomplete lineage sorting owing to recent divergence has been implicated in other bird species that do not indicate apparent divergence (see review in [42]). We suggest that some of these factors together with recolonization during inter-glacials may be responsible for the apparent lack of phylogeographic pattern in some species. Future studies could attempt to differentiate between these hypotheses.
(d). Insights from examining an entire community
Montane species in the Western Ghats revealed differing responses to topographic (and habitat) valleys. The highest genetic differentiation was observed in montane-restricted species (such as in [18,22]), while widespread generalists such as Pycnonotus jocosus, grassland generalists like Anthus rufulus, open country birds such as Saxicola caprata and large-bodied species like Columba elphinstonii and Z. dauma were not genetically differentiated. Both of these patterns are expected based on existing data on other species (e.g. [43]). However, our study also revealed cryptic structure in some species, such as Z. palpebrosus, a widespread species that extends to lower elevations.
While our study supports the role of climate in species diversification as suggested in the literature [12], we propose the additional importance of the physical structure of barriers, such as depth and width of valleys. Topography provides the canvas for climate-mediated habitat shifts to occur. Deeper, wider valleys allow greater climatic effect, resulting in greater genetic differentiation.
Finally, we emphasize that studying the entire community allowed us to uncover these varied, and often species-specific responses. An examination of species traits that affect genetic divergence may provide insights into diversification patterns. That species were differentially impacted in the evolutionary past emphasizes that changing climate may elicit idiosyncratic responses from species in such hotspots of biodiversity.
Supplementary Material
Supplementary Material
Acknowledgements
We thank: Permits—Forest Departments of Kerala (R. Rajaraja Varma, V. Gopinathan, T. M. Manoharan and B. S. Corrie) and Tamilnadu (R. Sunderaraju); extensive discussions—Trevor Price, Sushma Reddy and R. Nandini; field—Abhilash Babu, Anusha Shankar, Chetana Purushotam, Ravi Kiran, Sahas Barve and Sriranjini Swaminathan; laboratory—Joli Borah, Nelum Wickramasinghe, Jyothi Nair, Abhinav Sur, Aleeson E. and Himanshu Chattani; analyses—Goutham Atla, Jamie Oaks, Anna Papadopoulou, Michael Hickerson and H. N. Chakrapani; logistics—Suhel Quader, Robert Stewart and Tanya Balcar; illustrations—R. Nandini and Prasenjeet Yadav; map—Arundhati Das; sequencing—Shilpa M; comments on manuscript—Robert Ricklefs, Trevor Price, Fiona Savory, Balaji Chattopadhyay, Meghana Natesh, Krishnapriya Tamma, Amruta Varudkar and other Lab3 members. Colour-blind safe colours (www.colorbrewer2.org).
Ethics
Research cleared by NCBS Animal Ethics Committee and Forest Department permits WL10–1647/2011, WL5/8844/2011
Data accessibility
All DNA sequences have been submitted to GENBANK (see the electronic supplementary material).
Authors' contributions
V.V.R. and U.R. conceived and designed the study; V.V.R. and C.K.V. coordinated and collected field data; V.V.R. and P.G.—molecular laboratory work; P.G.—sequence alignments; V.V.R., U.R. and P.G.—data analyses, drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by National Geographic Society Research and Exploration grant to V.V.R., DAE award to study Indian Biogeography, Ramanujan Fellowship and NCBS internal funding to U.R.
References
- 1.Graham CH, et al. 2014. The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography 37, 711–719. ( 10.1111/ecog.00578) [DOI] [Google Scholar]
- 2.Lim HC, Rahman MA, Lim SL, Moyle RG, Sheldon FH. 2011. Revisiting Wallace's haunt: coalescent simulations and comparative niche modeling reveal historical mechanisms that promoted avian population divergence in the Malay Archipelago. Evolution 65, 321–334. ( 10.1111/j.1558-5646.2010.01105.x) [DOI] [PubMed] [Google Scholar]
- 3.La Sorte FA, Jetz W. 2010. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. B 277, 3401–3410. ( 10.1098/rspb.2010.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soh MC, Sodhi NS, Lim SL. 2006. High sensitivity of montane bird communities to habitat disturbance in Peninsular Malaysia. Biol. Conserv. 129, 149–166. ( 10.1016/j.biocon.2005.10.030) [DOI] [Google Scholar]
- 5.Woodburne MO. 2010. The Great American biotic interchange: dispersals, tectonics, climate, sea level and holding pens. J. Mammal. Evol. 17, 245–264. ( 10.1007/s10914-010-9144-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cracraft J. 1985. Historical biogeography and patterns of differentiation within the South American avifauna: areas of endemism. Ornithol. Monogr. 6, 49–84. ( 10.2307/40168278) [DOI] [Google Scholar]
- 7.Bowie R, Fjeldså J, Hackett S, Bates J, Crowe T. 2006. Coalescent models reveal the relative roles of ancestral polymorphism, vicariance, and dispersal in shaping phylogeographical structure of an African montane forest robin. Mol. Phylogenet. Evol. 38, 171–188. ( 10.1016/j.ympev.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 8.Cadena CD, Klicka J, Ricklefs RE. 2007. Evolutionary differentiation in the Neotropical montane region: molecular phylogenetics and phylogeography of Buarremon brush-finches (Aves, Emberizidae). Mol. Phylogenet. Evol. 44, 993–1016. ( 10.1016/j.ympev.2006.12.012) [DOI] [PubMed] [Google Scholar]
- 9.Fjeldså J, Bowie R. 2008. New perspectives on the origin and diversification of Africa's forest avifauna. Afr. J. Ecol. 46, 235–247. ( 10.1111/j.1365-2028.2008.00992.x) [DOI] [Google Scholar]
- 10.Sukumar R, Suresh HS, Ramesh R. 1995. Climate change and its impact on tropical montane ecosystems in southern India. J. Biogeogr. 22, 533–536. ( 10.2307/2845951) [DOI] [Google Scholar]
- 11.Knowles L. 2001. Did the Pleistocene glaciations promote divergence? Tests of explicit refugial models in montane grasshopprers. Mol. Ecol. 10, 691–701. ( 10.1046/j.1365-294x.2001.01206.x) [DOI] [PubMed] [Google Scholar]
- 12.Fjeldså J, Bowie RC, Rahbek C. 2012. The role of mountain ranges in the diversification of birds. Annu. Rev. Ecol. Evol. Syst. 43, 249–265. ( 10.1146/annurev-ecolsys-102710-145113) [DOI] [Google Scholar]
- 13.Voelker G, Outlaw RK, Bowie RCK. 2010. Pliocene forest dynamics as a primary driver of African bird speciation. Glob. Ecol. Biogeogr. 19, 111–121. ( 10.1111/j.1466-8238.2009.00500.x). [DOI] [Google Scholar]
- 14.Robin VV, Sinha A, Ramakrishnan U. 2010. Ancient geographical gaps and paleo-climate shape the phylogeography of an endemic bird in the sky islands of southern India. PLoS ONE 5, e13321 ( 10.1371/journal.pone.0013321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toussaint EF, Sagata K, Surbakti S, Hendrich L, Balke M. 2013. Australasian sky islands act as a diversity pump facilitating peripheral speciation and complex reversal from narrow endemic to widespread ecological supertramp. Ecol. Evol. 3, 1031–1049. ( 10.1002/ece3.517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjeldså J, Rahbek C. 2006. Diversification of tanagers, a species rich bird group, from lowlands to montane regions of South America. Integr. Comp. Biol. 46, 72–81. ( 10.1093/icb/icj009) [DOI] [PubMed] [Google Scholar]
- 17.Weir JT, Bermingham E, Schluter D. 2009. The great American biotic interchange in birds. Proc. Natl Acad. Sci. USA 106, 21 737–21 742. ( 10.1073/pnas.0903811106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amei A, Smith BT. 2014. Robust estimates of divergence times and selection with a Poisson random field model: a case study of comparative phylogeographic data. Genetics 196, 225–233. ( 10.153/genetics.113.157776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burney CW, Brumfield RT. 2009. Ecology predicts levels of genetic differentiation in Neotropical birds. Am. Nat. 174, 358–368. ( 10.1086/603613) [DOI] [PubMed] [Google Scholar]
- 20.Voelker G, et al. 2013. River barriers and cryptic biodiversity in an evolutionary museum. Ecol. Evol. 3, 536–545. ( 10.1002/ece3.482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowie RCK. 2011. The utility of contemporary and historical estimates of dispersal in determining response to habitat fragmentation in a tropical forest-dependent bird community. Mol. Ecol. 20, 1799–1802. ( 10.1111/j.1365-294X.2011.05032.x) [DOI] [PubMed] [Google Scholar]
- 22.Dolman G, Joseph L. 2012. A species assemblage approach to comparative phylogeography of birds in southern Australia. Ecol. Evol. 2, 354–369. ( 10.1002/ece3.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moritz C, Hoskin CJ, MacKenzie JB, Phillips BL, Tonione M, Silva N, VanDerWal J, Williams SE, Graham CH. 2009. Identification and dynamics of a cryptic suture zone in tropical rainforest. Proc. R. Soc. B 276, 1235–1244. ( 10.1098/rspb.2008.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph L, Moritz C, Hugall A. 1995. Molecular support for vicariance as a source of diversity in rainforest. Proc. R. Soc. Lond. B 260, 177–182. ( 10.1098/rspb.1995.0077) [DOI] [PubMed] [Google Scholar]
- 25.Price TD, et al. 2014. Niche filling slows the diversification of Himalayan songbirds. Nature 509, 222–225. ( 10.1038/nature13272) [DOI] [PubMed] [Google Scholar]
- 26.Bunyan M, Bardhan S, Jose S. 2012. The Shola (Tropical Montane Forest)-grassland ecosystem mosaic of Peninsular India: a review. Am. J. Plant Sci. 3, 1632–1639. ( 10.4236/ajps.2012.311198) [DOI] [Google Scholar]
- 27.Bates JM, Zink RM. 1994. Evolution into the Andes: molecular evidence for species relationships in the genus Leptopogon. Auk 111, 507–515. [Google Scholar]
- 28.Chaves JA, Weir JT, Smith TB. 2011. Diversification in Adelomyia hummingbirds follows Andean uplift. Mol. Ecol. 20, 4564–4576. ( 10.1111/j.1365-294X.2011.05304.x) [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee S, Goswami A, Scotese CR. 2013. The longest voyage: tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Res. 23, 238–267. ( 10.1016/j.gr.2012.07.001) [DOI] [Google Scholar]
- 30.Hickerson MJ, Stahl E, Takebayashi N. 2007. msBayes: pipeline for testing comparative phylogeographic histories using hierarchical approximate Bayesian computation. BMC Bioinform. 8, 268 ( 10.1186/1471-2105-8-268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oaks JR, Sukumaran J, Esselstyn JA, Linkem CW, Siler CD, Holder MT, Brown RM. 2013. Evidence for climate-driven diversification? A caution for interpreting ABC inferences of simultaneous historical events. Evolution 67, 991–1010. ( 10.1111/j.1558-5646.2012.01840.x) [DOI] [PubMed] [Google Scholar]
- 32.Oaks JR. 2014. An improved approximate-Bayesian model-choice method for estimating shared evolutionary history. BMC Evol. Biol. 14, 150 ( 10.1186/1471-2148-14-150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair A, Gopalan SV, George S, Kumar KS, Shikano T, Merilä J. 2012. Genetic variation and differentiation in Indirana beddomii frogs endemic to the Western Ghats biodiversity hotspot. Conserv. Genet. 13, 1459–1467. ( 10.1007/s10592-012-0389-z) [DOI] [Google Scholar]
- 34.Deshpande AU, et al. 2001. Genetic diversity across natural populations of three montane plant species from the Western Ghats, India revealed by intersimple sequence repeats. Mol. Ecol. 10, 2397–2408. ( 10.1046/j.0962-1083.2001.01379.x) [DOI] [PubMed] [Google Scholar]
- 35.Vidya T, Fernando P, Melnick D, Sukumar R. 2005. Population differentiation within and among Asian elephant (Elephas maximus) populations in southern India. Heredity 94, 71–80. ( 10.1038/sj.hdy.6800568) [DOI] [PubMed] [Google Scholar]
- 36.Joshi J, Karanth P. 2013. Did southern Western Ghats of peninsular India serve as refugia for its endemic biota during the Cretaceous volcanism? Ecol. Evol. 3, 3275–3282. ( 10.1002/ece3.603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodare S. 2013. Conservation genetics and speciation in Asian forest trees. Uppsala, Sweden: Uppsala University. [Google Scholar]
- 38.Smith BT, et al. 2014. The drivers of tropical speciation. Nature 515, 406–409. ( 10.1038/nature13687) [DOI] [PubMed] [Google Scholar]
- 39.Karanth KP. 2003. Evolution of disjunct distributions among wet-zone species of the Indian subcontinent: testing various hypotheses using a phylogenetic approach. Curr. Sci. 85, 1276–1283. [Google Scholar]
- 40.Cannon CH, Morley RJ, Bush AB. 2009. The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc. Natl Acad. Sci. USA 106, 11 188–11 193. ( 10.1073/pnas.0809865106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rull V. 2011. Neotropical biodiversity: timing and potential drivers. Trends Ecol. Evol. 26, 508–513. ( 10.1016/j.tree.2011.05.011) [DOI] [PubMed] [Google Scholar]
- 42.McKay BD, Zink RM. 2010. The causes of mitochondrial DNA gene tree paraphyly in birds. Mol. Phylogenet. Evol. 54, 647–650. ( 10.1016/j.ympev.2009.08.024) [DOI] [PubMed] [Google Scholar]
- 43.Stuart-Fox DM, Schneider CJ, Moritz C, Couper PJ. 2001. Comparative phylogeography of three rainforest-restricted lizards from mid-east Queensland. Aust. J. Zool. 49, 119–127. ( 10.1071/ZO00092) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All DNA sequences have been submitted to GENBANK (see the electronic supplementary material).