Abstract
Smallpox was eradicated in the 1970s, but new outbreaks could be seeded by bioterrorism or accidental release. Substantial vaccine-induced morbidity and mortality make pre-emptive mass vaccination controversial, and if vaccination is voluntary, then there is a conflict between self- and group interests. This conflict can be framed as a tragedy of the commons, in which herd immunity plays the role of the commons, and free-riding (i.e. not vaccinating pre-emptively) is analogous to exploiting the commons. This game has been analysed previously for a particular post-outbreak vaccination scenario. We consider several post-outbreak vaccination scenarios and compare the expected increase in mortality that results from voluntary versus imposed vaccination. Below a threshold level of post-outbreak vaccination effort, expected mortality is independent of the level of response effort. A lag between an outbreak starting and a response being initiated increases the post-outbreak vaccination effort necessary to reduce mortality. For some post-outbreak vaccination scenarios, even modest response lags make it impractical to reduce mortality by increasing post-outbreak vaccination effort. In such situations, if decreasing the response lag is impossible, the only practical way to reduce mortality is to make the vaccine safer (greater post-outbreak vaccination effort leads only to fewer people vaccinating pre-emptively).
Keywords: vaccination, smallpox, game theory, bioterrorism, epidemiology, cost-effectiveness
1. Introduction
The number of annual cases of smallpox in the early 1950s, just prior to the World Health Organization's global eradication programme, is estimated at 50 million [1]. The eradication campaign was successful [1], but samples of the variola virus are still kept in at least two known laboratories in Russia and the USA [2]. In a worrying incident in July 2014, previously forgotten vials containing samples of smallpox, some of which were viable, were found in a laboratory at the National Institutes of Health campus in Bethesda, MD, USA [3]. Thus, the threat of the reintroduction of smallpox, whether inadvertently or in a bioterrorist attack, is still present.
Consequently, some countries—notably the USA—are interested in measures to protect their populations from potential smallpox infection. Prophylactic vaccination for smallpox carries a high cost (relative to other vaccines in use today), as the probability of death following vaccination—or ‘risk from being vaccinated’—is 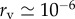 and serious side-effects occur with probability ∼10−3 [1]. Of course, infection with smallpox carries a much greater risk, because the case fatality proportion—the ‘risk from infection’—is ri ≃ 0.3 [1]. (See table 1 for a summary of parameter estimates.)
and serious side-effects occur with probability ∼10−3 [1]. Of course, infection with smallpox carries a much greater risk, because the case fatality proportion—the ‘risk from infection’—is ri ≃ 0.3 [1]. (See table 1 for a summary of parameter estimates.)
Table 1.
Summary of the fundamental (i.e. not derived) numerical parameters in our analysis, together with estimated values. Note that in [4] the probability of an outbreak was denoted r rather than a. Here, we use r for the relative risk, as in [5]. The proportion of the population infected initially by a bioterrorist attack or accidental release, α, corresponds to infection of 5000 individuals in a population of 290 million (after [4]).
| quantity | interpretation | value | source |
|---|---|---|---|
| rv | mortality risk from vaccination (probability) | 10−6 | [4] |
| ri | mortality risk from infection (probability) | 0.3 | [4] |
| ℛ0 | basic reproductive ratio | 5 | [6–8] |
| tser | mean serial interval | 22 days | [9, p. 141] |
| 1/σ | mean latent period (SEIRV) | 15 days | [1, p. 188] and [4] |
 |
vaccination effort parameter (exact interpretation depends on model) | see table 4 | |
| tlag | response lag before initiation of post-outbreak vaccination | 0 days, except in §7.5 | |
| a | probability of attack or accidental release per lifetime | 0.01 | [4] |
| α | proportion of susceptibles initially infected in an outbreak | 5000/290 × 106 ≃ 1.72 × 10−5 | [4] |
The substantial vaccine-induced morbidity and mortality associated with smallpox vaccination make pre-emptive mass vaccination controversial. If vaccination is voluntary, then there is a conflict between self- and group interests. This conflict can be framed as a tragedy of the commons, in which herd immunity plays the role of the commons, and free-riding (i.e. not vaccinating pre-emptively) is analogous to exploiting the commons. A previous game-theoretical study by Bauch et al. [4] examined this conflict of interest, and focused on the trade-off between prophylactic vaccination and post-outbreak mass vaccination (which has been shown to outperform contact-traced vaccination in a bioterrorism setting [10]). In particular, they showed that if the decision regarding pre-emptive vaccination is left to the individual, then the vaccine coverage achieved will be suboptimal from the group perspective. Bauch et al. [4] assumed that, once a post-outbreak vaccination campaign begins, individuals will be vaccinated at a constant rate determined by existing infrastructure.
Various mechanisms might drive the rate of vaccination. Vaccination at a constant rate might be achieved if vaccination centres are flooded by individuals seeking the vaccine, and are operating at peak capacity. However, public responsiveness to such a campaign is hard to predict. If demand for the vaccine does not exceed the maximal rate of distribution by public health services, the post-outbreak dynamics might play out differently, depending on the public's reaction patterns. For example, media reports on the number of new cases might influence individuals to obtain the vaccine; in that case, it is reasonable to model the vaccination rate as proportional to smallpox incidence.
In this paper, we return to the problem posed by Bauch et al. [4], but compare a variety of possible post-outbreak vaccination scenarios (described intuitively in §2 and in precise mathematical terms in §5). Whereas the scenario considered in [4] could only be analysed numerically, several of the vaccination scenarios that we consider here can be addressed analytically to obtain exact results. To this end, in §3, we make some adjustments to the game-theoretical framework of Bauch & Earn [5], so that it can be applied to the scenarios we investigate here.
Throughout this paper, we use smallpox as an illustrative example. However, our analyses can be applied to any vaccine-preventable infectious disease that could be used for bioterrorism or released accidentally, and for which the susceptible–infectious–removed (SIR) or susceptible–exposed–infectious–removed (SEIR) models are applicable (see §5). Our qualitative results appear to be robust to which post-outbreak vaccination scenario is considered and the specific parameter values (we prove this in some cases), but the precise numerical values will vary.
We calculate the vaccination coverage obtained by voluntary pre-emptive vaccination and assess the costs of this policy when compared with mandatory vaccination. The group-optimal pre-emptive vaccine coverage is discussed in §4. We discuss parameter estimates and the procedure used to compare the various models fairly in §6. We compare the predictions of the various models, and emphasize important considerations for public health in §7. Notation and definitions are summarized in tables 1–3.
Table 3.
Summary of other notation.
| quantity | interpretation |
|---|---|
| P | probability that an individual chooses to vaccinate pre-emptively (this defines the individual's strategy) |
| p | pre-outbreak vaccine coverage (proportion of the population vaccinated pre-emptively) |
| pg | the group optimum, i.e. the proportion of the population vaccinated pre-emptively which minimizes mortality |
| pi | the individual equilibrium, i.e. the level of pre-outbreak vaccine coverage which is the unique Nash equilibrium, as described in §3 |
| C(p) | the mortality cost, i.e. the proportion of the population that is expected to die, given pre-emptive vaccine coverage p |
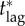 |
the critical lag, i.e. the response lag beyond which mortality is independent of vaccination effort (see §7.5.1) |
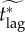 |
the effective critical lag, i.e. the response lag beyond which mortality is identical for all feasible values of vaccination effort (see §7.5.2) |
2. Vaccination scenarios
In this section, we give a brief description of the various post-outbreak vaccination scenarios considered in this paper.
Media coverage of a smallpox outbreak is likely to influence individual decisions concerning vaccination. Measures of severity of the outbreak that are likely to appear in the media include:
— death rates, as in ‘300 people died of smallpox today’,
— total number of people currently infected (prevalence), as in ‘there are now 30 000 people sick with smallpox’, and
— new cases (incidence), as in ‘200 new cases of smallpox were confirmed today’.
We consider separately how each of these types of information could affect smallpox vaccine uptake; in each case, we assume that the vaccination rate is proportional to the relevant quantity (e.g. prevalence). Note that, in standard epidemiological models [6], death rate is proportional to prevalence, so the first and second cases above are mathematically identical.
As a type of ‘null model’ for media-induced vaccination, we also consider the situation in which
— Vaccination rate is simply proportional to the size of the remaining susceptible population; this corresponds to a constant per capita vaccination rate for susceptible individuals (see the electronic supplementary material, appendix B.1). This can be regarded as a ‘null model’ to compare with models for the scenarios above in the following sense: individuals’ proclivity to vaccinate is constant over time, and does not depend on the state of the epidemic (i.e. on prevalence or incidence, which are likely to be reported by the media), whereas the vaccination rate falls as the number of susceptibles decreases over time, meaning that fewer individuals per unit time are inclined to vaccinate.
We also consider two scenarios in which vaccine uptake is not influenced by the media, but is constrained by the capabilities of public health authorities:
— If an outbreak occurs, immediately vaccinate a proportion of the susceptible population. The proportion might describe the efficacy of a post-outbreak campaign in convincing those who have thus far avoided vaccination. Individuals who remain un-vaccinated after this post-outbreak campaign would be persons holding particularly radical anti-vaccine opinions.
— Susceptible individuals are vaccinated at a constant rate until there are no more susceptibles remaining.
Finally, for each of the above scenarios, we investigate the effect of a lag between the start of an outbreak and the initiation of the post-outbreak vaccination response (allowing for public health authorities to organize a response to the outbreak). Bauch et al. [4] assumed such a response lag in their model, which is otherwise identical to the final scenario described above.
The epidemic models associated with each of the above five scenarios are described in detail in §5.
3. Game-theoretical formulation
In this section, we adapt the game-theoretical framework of Bauch & Earn [5] to our current problem. We assume that all individuals have full knowledge and are rational (in the game-theoretical sense; see [11]).
We denote the proportion of the population vaccinated pre-emptively as p. Because a proportion rv of those vaccinated will die, the pre-outbreak vaccine coverage (the proportion of the population that is immune prior to the outbreak) is peff = p(1 − rv)/(1 − prv) [4], which is slightly smaller than p. But, because none of the mathematical analysis and conclusions which follow are affected by this, and because the difference between p and peff is negligible, we refer to p as the pre-outbreak vaccine coverage level for simplicity (as in [4]).
Let a ∈ [0, 1] be the probability of an outbreak (‘a’ for ‘bioterrorist attack probability’ or ‘accidental release probability’) per lifetime (or whatever time period is under consideration). Consider two pure strategies: vaccinate and delay. The former vaccinates pre-emptively, before the beginning of an outbreak, and so receives (expected) pay-off − rv; the latter delays vaccination until after an outbreak (at which point she/he may still be vaccinated during the public health post-outbreak vaccination campaign), and receives pay-off
| 3.1 |
where πp and ψp are the probabilities of a delayer being infected, or vaccinated, respectively, after an outbreak (the delayer infection and vaccination probabilities are discussed in more detail in §5). A mixed strategy is specified by the probability P that an individual will choose to vaccinate pre-emptively. We also assume rv < ari because if it were not so, even if all delayers were infected in an outbreak, the risk of dying in an outbreak would be smaller than the risk of dying from the side-effects of the vaccine, hence there would be no reason to vaccinate.
The pay-off to an individual playing a mixed strategy (vaccinating with probability P) in a population in which the coverage level is p is given by
| 3.2 |
Equivalently, defining the relative risk of vaccination compared with infection as
| 3.3 |
we have E(P, p) = −ri[rP + (1 − P)a(πp + ψpr)]. Because the parameter ri simply scales the game pay-off by a constant, it does not change the dynamics. We therefore use the rescaled pay-off function
| 3.4 |
Suppose that a proportion ε of the population vaccinate with probability P and 1 − ε vaccinate with probability Q. Following Bauch & Earn [5], we assume 100% vaccine efficacy, which implies coverage level p = εP + (1 − ε)Q. (Note that in a homogeneous population where all individuals play the same strategy P, i.e. ε = 1, the coverage is p = P.) The pay-offs to individuals playing P and Q in such a population are then
| 3.5a |
or
| 3.5b |
respectively, and the pay-off gain to an individual playing P rather than that to Q in this population is
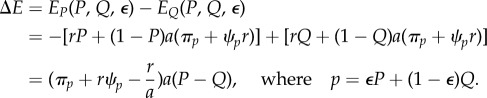 |
3.6 |
A strategy P* is a Nash equilibrium (NE) if and only if (iff), in a population in which all individuals are playing P*, no player employing a different strategy can achieve a higher pay-off. Mathematically, this means that for any other strategy Q ∈ [0, 1] if the proportion playing Q is small enough (i.e. 1 − ε is sufficiently small), then the pay-off gain to strategy P* is non-negative, i.e. ΔE(P*, Q, ε) ≥ 0. When such an NE exists, we refer to this strategy as the individual equilibrium and denote it by pi. This equilibrium is ‘individual’ in the sense that it is determined by individuals attempting to maximize their pay-offs (unlike the group optimum discussed in §4). Note, however, that this is a population game [5,12], so the pay-off to individuals depends on the frequencies of strategies in the entire population.
Additionally, consider a scenario whereby strategy P invades a population playing strategy Q. If, in this scenario, strategies P that are closer to the NE P* than the prevalent strategy Q obtain a higher pay-off than the prevalent strategy, then P* is called a convergently stable Nash equilibrium (CSNE). Mathematically, this is equivalent to demanding that if ε ≪ 1, then
and
In order to proceed with the analysis, it is necessary to derive the probabilities πp and ψp from an epidemiological model, either numerically or analytically (see the electronic supplementary material, appendix E). Proofs of existence and uniqueness of a CSNE are given for several cases in the electronic supplementary material, appendix G. These proofs depend on πp being a decreasing function of p. We have shown this to be true when post-outbreak vaccination is instantaneous or proportional to incidence, and also when vaccination is proportional to prevalence and αϕprev > γ(1−α). Based on biological intuition corroborated with simulations, we have assumed that πp decreases with p for all the models considered here. This has also recently been proved for other post-outbreak vaccination models not considered here (F Bai, F Brauer 2015, personal communication).
4. Group optimum
From the perspective of a public health official (i.e. group interest), it is desirable to attain the vaccine coverage that minimizes mortality. From this group perspective, a strategy is specified by the proportion p of the population that is pre-emptively vaccinated. The currency with which we compare strategies is the mortality cost C(p), i.e. the proportion of the population that is expected to die (either from smallpox infection or from vaccination),
| 4.1 |
where we have ignored a factor of ri as in equation (3.4). The minimum mortality cost yields the group optimum coverage level, which we denote pg. The minimum of C(p) on [0, 1] may be attained either at a local minimum in (0, 1) or at one of the endpoints
| 4.2a |
and
| 4.2b |
To completely specify the cost C(p), we need the probabilities πp and ψp, derived from the epidemiological model (see §5 and the electronic supplementary material, appendix E), just as for the individual equilibrium. We have found an exact analytical expression for pg in one subcase (see the electronic supplementary material, appendix H) and calculated it numerically in the other cases.
5. Epidemiological models
In order to find the group optimum (pg) and individual equilibrium (pi), two key quantities are calculated from the epidemic models: the delayer infection probability πp (the probability of a delayer being infected after an outbreak) and the delayer vaccination probability ψp (the probability that a delayer is eventually vaccinated, given an outbreak).
Both πp and ψp depend on the disease dynamics and the post-outbreak vaccination scenario. In the following, we assume that, in the absence of post-outbreak vaccination, the SIR model is adequate to represent the disease dynamics [13, §4]. The models do not include vital dynamics (births and deaths from all natural causes other than the disease), because the mean serial interval (also called the disease generation time, tser = 22 days [9, p. 141]) is much smaller than the mean lifetime (approx. 80 years in the USA [14]). Note that, for diseases for which the outbreak time scale is similar to the mean lifetime, vital dynamics can easily be included in the analysis (e.g. as in [5], where much longer term dynamics were considered).
Let S(t), I(t), R(t) and V(t) be the proportions of susceptible, infected, removed (recovered or dead from smallpox infection) and vaccinated individuals (immune or dead from vaccination), respectively, at time t. Our basic framework is the SIRV model, described by the differential equations
| 5.1a |
| 5.1b |
| 5.1c |
| 5.1d |
where 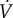 must be non-negative as indicated and is defined differently for each of the distinct scenarios of post-outbreak vaccination described in §2.
must be non-negative as indicated and is defined differently for each of the distinct scenarios of post-outbreak vaccination described in §2.
We assume that no one has natural immunity or retains immunity from vaccination decades earlier. This is an approximation, because many living individuals were vaccinated before smallpox was declared eradicated in 1979 [1], and many of those vaccinated individuals are probably still immune (vaccine-derived immunity seems to wane quite slowly and lifelong immunity is common [15]). However, smallpox is considered to have been eliminated in the USA as early as 1950, and while routine vaccination continued in some states well after that [1], the proportion of US residents younger than 60 who have been vaccinated is likely to be very small.
Thus, we assume that the coverage level prior to an outbreak is p, the proportion pre-emptively vaccinated. Consequently, prior to the outbreak, a proportion 1 − p of the population is susceptible. When a bioterrorist attack or accidental release takes place (at time t = 0), an initial attack proportion α of the susceptible population is infected. Thus,
| 5.2a |
| 5.2b |
| 5.2c |
| 5.2d |
After an outbreak, the epidemic is over when no one remains infective (I = 0). In the electronic supplementary material, appendix C, we show rigorously that this is guaranteed to occur, either in finite time or in the limit as t → ∞. In either case, we use the subscript ∞ to refer to the time at which the epidemic ends. Thus, S∞, I∞, R∞ and V∞ refer to the proportions of the population in the susceptible, infective, removed and vaccinated compartments at the end of the epidemic. With this notation, the probabilities of infection and vaccination for delayers are, respectively,
| 5.3a |
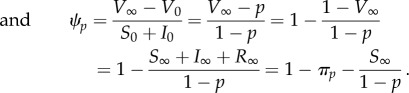 |
5.3b |
We emphasize that R is the proportion of the population that has been infected (and consequently is either immune or has died); hence, R(0) = 0, because anyone who is immune at time t = 0 is immune from vaccination. Intuitively, there is no endemic equilibrium in these models, because the combination of vaccination and natural spread of disease must eventually cause susceptibles to be so rare that the disease cannot spread (recall that these models neglect vital dynamics).
Lastly, note that πp is undefined at p = 1 (i.e. if everyone pre-emptively vaccinates), as there are no delayers for whom to calculate the probability of being infected. We define π1 as the limit of the delayer infection probability,
| 5.4 |
i.e. π1 is the limit of πp as pre-emptive vaccination approaches full coverage. In the electronic supplementary material, appendix D, we show that this limit is equal to the proportion of susceptibles initially infected in an outbreak, i.e. π1 = α for all models considered.
In the following, we describe (and interpret mechanistically) various models that we compare, and present some analytical results. In all models, the vaccination rate depends on a vaccination effort parameter,  the exact interpretation of which is model dependent.
the exact interpretation of which is model dependent.
5.1. Vaccination rate ∝ disease prevalence
In this model, vaccination occurs at a rate proportional to disease prevalence (I). A plausible scenario to which such a model would apply is if people respond to media reports on disease prevalence. As a result of increasing disease prevalence, the public might perceive the risk of being infected as higher, and be moved to vaccinate. Consequently,
| 5.5 |
where
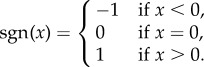 |
This model could also represent the case where vaccination rate is proportional to death rate, i.e. people vaccinate in response to media reports on new disease-induced deaths. Because the death rate is proportional to the rate at which the removed compartment, R, grows, which is proportional to I, the vaccination rate would also be proportional to I.
In the electronic supplementary material, appendix E.1.1, we find the final size relations [16–18] for the model defined by equation (5.5). These are given by
| 5.6 |
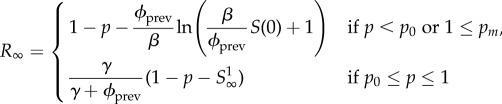 |
5.7 |
and
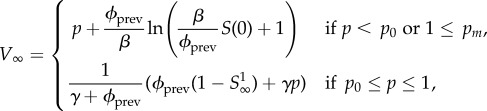 |
5.8 |
with
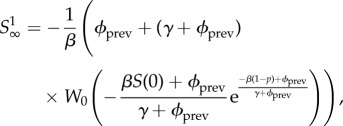 |
5.9 |
| 5.10 |
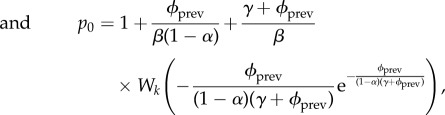 |
5.11 |
where k = 0 if pm < 1 and k = −1 if pm ≥ 1. W0 is the principal branch of Lambert's W function [19,20], and W−1 is its other real branch (see the electronic supplementary material, appendix A). pm is the unique maximum of the function 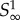 .
. 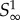 has two roots, one at p = 1, and the other at p0, which need not lie in the interval [0, 1] (p0 is a formal root and need not correspond to a meaningful probability). Note that if pm > 1 then p0 > pm, and if pm < 1 then p0 < pm.
has two roots, one at p = 1, and the other at p0, which need not lie in the interval [0, 1] (p0 is a formal root and need not correspond to a meaningful probability). Note that if pm > 1 then p0 > pm, and if pm < 1 then p0 < pm.
If pm > 1, then no delayers will remain susceptible at the end of the epidemic (i.e. all delayers will be either vaccinated or infected), regardless of the initial vaccine coverage level p. Moreover, if pm < 1, but p0 < 0, there are always some delayers who remain susceptible at the end of the epidemic, regardless of the initial coverage level p. If 0 < p0 < pm < 1, then for p ∈ [0, p0] there will be no susceptibles left at the end of the epidemic, and for p ∈ (p0, 1) there will be some remaining susceptibles. Thus, there is a wide range of parameter values for which some susceptibles remain at the end of the epidemic; in such cases, πp + ψp < 1. Numerical evidence and biological intuition suggest that πp is a decreasing function of p, and we assume that this is the case from here on (this is proven for pm ≥ 1 in the electronic supplementary material, appendix E.1.3).
Finally, because the mean infectious period (7 days; see table 2 and §6.1) is longer than the time required to complete the vaccination programme (possibly as short as 3 days [21]), it is interesting to take the limit γ → 0 (corresponding to an infinite infectious period) while keeping ℛ0 = β/γ fixed. In this limit, pm → ∞ so S∞ = 0 (equation (5.6)), which is in accordance with the assumption—made in [4]—that individuals are ultimately either removed or vaccinated.
Table 2.
Summary of derived parameters.
| quantity | interpretation | value |
|---|---|---|
| r = rv/ri | relative risk (from being vaccinated compared with natural infection) | 10−6/0.3 ≃ 3.33 × 10−6 |
| 1/γ | mean time infectious (SIRV) | tser = 22 days |
| 1/γ | mean time infectious (SEIRV) | tser − (1/σ) = 7 days |
| β | transmission rate | γℛ0 |
| πp | probability that an un-vaccinated individual will eventually be infected if the vaccine coverage level in the population is p | derived from epidemic model in §5 |
| ψp | probability of an individual un-vaccinated at the beginning of the epidemic becoming vaccinated, given vaccine coverage level p | derived from epidemic model in §5 |
We show in the electronic supplementary material, appendix G.1, that there is always a unique CSNE, that is, a ‘best strategy’ from the individual perspective. Moreover, an analytical expression for this individual equilibrium can be found if
| 5.12a |
| 5.12b |
In addition, we find an analytical formula for the group optimum when ϕprev ≥ γ (1 − α)/α (see the electronic supplementary material, appendix H, for details).
5.2. Vaccination rate ∝ incidence
A vaccination rate proportional to incidence again reflects media-induced vaccination. However, in this model, the public reacts to reports of new cases, rather than reports of the total number of sick individuals. Thus,
| 5.13 |
In the electronic supplementary material, appendix E.2, we show that
| 5.14 |
| 5.15 |
| 5.16 |
Again, because there are susceptible individuals left at the end of the epidemic, πp ≠ 1 − ψp. We show that ∂pπp < 0 (in the electronic supplementary material, appendix E.2) and find that there is a unique CSNE, pi, for which an exact formula is derived in the electronic supplementary material, appendix G.2.
5.3. Vaccination rate ∝ proportion still susceptible
In this scenario, susceptible individuals vaccinate at a rate
| 5.17 |
This is a null model, in the sense that susceptible individuals have a constant probability per unit time of being vaccinated, ϕsusc, independent of the state of the outbreak, as shown in the electronic supplementary material, appendix B.1.
We were able to obtain analytical final size relations for this model (see the electronic supplementary material, appendix E.3), but we found the formulae too cumbersome to be useful. Thus, the remainder of our analysis of this model was performed by integrating the differential equations numerically. In our numerical simulations, we always find that πp decreases with p (in the electronic supplementary material, appendix G.3, our proof of the existence of a CSNE depends on this being true).
5.4. Instantaneous vaccination of a proportion ϕinst of the population
Some experts believe that the entire USA could be vaccinated in 3 days [21], which is less than the latent period of smallpox. Consequently, instantaneous vaccination of a proportion ϕinst of the population remaining susceptible after the outbreak is also a realistic scenario to model. In this case, once vaccination has occurred, the disease simply spreads according to the standard SIR model,
| 5.18a |
| 5.18b |
| 5.18c |
with initial conditions given by
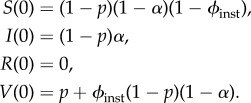 |
Note that in this scenario we deviate from the convention we use for all the other models, in which S(0) is the initial density of susceptibles prior to the beginning of the post-outbreak vaccination response. Here, S(0) is the density of susceptibles after the post-outbreak vaccination response has taken place.
For this scenario, we find (in the electronic supplementary material, appendix E.4)
| 5.19 |
and
| 5.20 |
We also show that πp is a decreasing function of p, ψp is constant and πp + ψp ≠ 1 (see the electronic supplementary material, appendix E.4). In addition, we have proved that, for this model, there is always a unique CSNE, for which we derive an exact formula in the electronic supplementary material, appendix G.4.
5.5. Constant rate vaccination
This is the model of Bauch et al. [4], in which vaccination occurs at a constant rate  Note that in [4] vaccination begins after a response lag tlag, which is the public health services' response time. This lag is taken to be tlag = 0 except in §7.5.
Note that in [4] vaccination begins after a response lag tlag, which is the public health services' response time. This lag is taken to be tlag = 0 except in §7.5.
For consistency with [4], we included an exposed (but not infective) stage (E), in this model, making it an SEIRV model. This contrasts all the other scenarios, which we have modelled using a simpler SIRV formulation. Our choice of the SIRV framework for the new scenarios is motivated by mathematical tractability and by work subsequent to [4], indicating that SEIR dynamics are captured by an appropriately parametrized SIR model (see §6.1, but see §7.5.1 for an exception).
The model equations for the constant rate vaccination scenario are
| 5.21a |
| 5.21b |
| 5.21c |
| 5.21d |
| 5.21e |
We have not found a final size relation for this model.
Under the biologically plausible assumption that πp decreases with p (verified by simulation), Bauch et al. [4] have shown the existence of a unique CSNE for this model.
6. Parameter estimates, fair comparisons of models and numerical procedures
Because one of the models we investigate includes an exposed class, and the vaccination effort parameter  has a different meaning in each scenario we examine, fair comparisons of model results are not completely straightforward. In this section, we consider how the various models can be compared.
has a different meaning in each scenario we examine, fair comparisons of model results are not completely straightforward. In this section, we consider how the various models can be compared.
6.1. SIR versus SEIR
It is well known that similar dynamics are obtained with the standard SIR and SEIR models with identical basic reproductive number, ℛ0, if the mean infectious period in the SIR model is set equal to the sum of the mean latent and infectious periods in the SEIR model [6, p. 668]. More generally, models can be fairly compared if they have the same mean serial interval [13, §4].
Estimates of the basic reproductive ratio ℛ0 of smallpox vary in the range 3 ≤ ℛ0 ≤ 10 [6–8]. Following [4], we take ℛ0 = 5. We take the mean serial interval to be tser = 22 days, as in [9, p. 141] (but note that [4] used tser = 14 days, and [22] estimated tser = 17.7 days).
In the constant rate vaccination model, we take the mean latent period to be 1/σ = 15 days [9, p. 141] (based on summing the incubation and prodrom periods, which typically last 12 and 3 days, respectively; see [1, p. 188]). In an SEIR model, the mean serial interval is the sum of the mean latent and infectious periods [13,23]; hence, 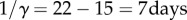 and β = γℛ0 = 5/7 per day. In the SIRV models, we take 1/γ = tser, whereas β is modified so that ℛ0 = 5 (i.e. β = γℛ0 = 5/22 per day).
and β = γℛ0 = 5/7 per day. In the SIRV models, we take 1/γ = tser, whereas β is modified so that ℛ0 = 5 (i.e. β = γℛ0 = 5/22 per day).
6.2. Vaccination effort parameter 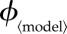
Public health policy changes will affect the vaccination effort parameter  where 〈model〉 refers to any of ‘prev’, ‘inc’, ‘susc’, ‘inst’ or ‘const’. In order to compare the outcomes of the various vaccination scenarios, for each vaccination model, we find the fair comparison value of
where 〈model〉 refers to any of ‘prev’, ‘inc’, ‘susc’, ‘inst’ or ‘const’. In order to compare the outcomes of the various vaccination scenarios, for each vaccination model, we find the fair comparison value of  that is, the value of
that is, the value of  that yields a maximal vaccination rate that is equal to the fixed rate in the constant rate vaccination model of Bauch et al. [4],
that yields a maximal vaccination rate that is equal to the fixed rate in the constant rate vaccination model of Bauch et al. [4], 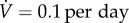 (see description under
(see description under  below). This allows us to identify, for each scenario, ranges of
below). This allows us to identify, for each scenario, ranges of  that can feasibly be attained in reality (i.e.
that can feasibly be attained in reality (i.e.  between 0 and the fair comparison value). Our aim is then to compare the different vaccination strategies in terms of vaccine doses used and total expected mortality (we will be interested in the values of these observables at both the individual equilibrium and the group optimum). The fair comparison values are summarized in table 4.
between 0 and the fair comparison value). Our aim is then to compare the different vaccination strategies in terms of vaccine doses used and total expected mortality (we will be interested in the values of these observables at both the individual equilibrium and the group optimum). The fair comparison values are summarized in table 4.
-
ϕprev.
In the prevalence model,
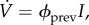 the vaccination rate is proportional to the prevalence, I, and the vaccination effort parameter ϕprev is the rate of vaccination per infected individual. In the electronic supplementary material, appendix F.1, we calculate the maximal vaccination rate as a function of the model parameters, α, β, γ and ϕprev and p. We find that the maximal vaccination rate for a given initial coverage, p, decreases with the vaccination effort, ϕprev. We also find that, when α, β and γ are as in tables 1 and 2, a maximal vaccination rate of 0.1 per day is obtained when ϕprev ≈ 1582 per day.
the vaccination rate is proportional to the prevalence, I, and the vaccination effort parameter ϕprev is the rate of vaccination per infected individual. In the electronic supplementary material, appendix F.1, we calculate the maximal vaccination rate as a function of the model parameters, α, β, γ and ϕprev and p. We find that the maximal vaccination rate for a given initial coverage, p, decreases with the vaccination effort, ϕprev. We also find that, when α, β and γ are as in tables 1 and 2, a maximal vaccination rate of 0.1 per day is obtained when ϕprev ≈ 1582 per day. -
ϕinc.
In the incidence model,
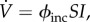 the vaccination effort parameter ϕinc is the vaccination rate per infective per susceptible. In the electronic supplementary material, appendix F.2, we calculate the maximal vaccination rate, as a function of the model parameters, α, β, γ and ϕinc. We show that the maximal vaccination rate,
the vaccination effort parameter ϕinc is the vaccination rate per infective per susceptible. In the electronic supplementary material, appendix F.2, we calculate the maximal vaccination rate, as a function of the model parameters, α, β, γ and ϕinc. We show that the maximal vaccination rate, 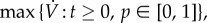 is an increasing function of ϕinc, and that in order to obtain a maximal vaccination rate of 0.1 per day or lower, with α, β and γ as in tables 1 and 2, one needs ϕinc ≈ 5190 per day.
is an increasing function of ϕinc, and that in order to obtain a maximal vaccination rate of 0.1 per day or lower, with α, β and γ as in tables 1 and 2, one needs ϕinc ≈ 5190 per day. -
ϕsusc.
With
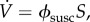 the vaccination effort parameter ϕsusc is the vaccination rate per susceptible individual (alternatively, ϕsusc can be interpreted as the probability per unit time of a delayer being vaccinated; see the electronic supplementary material, appendix B.1). In this model, the vaccination rate
the vaccination effort parameter ϕsusc is the vaccination rate per susceptible individual (alternatively, ϕsusc can be interpreted as the probability per unit time of a delayer being vaccinated; see the electronic supplementary material, appendix B.1). In this model, the vaccination rate 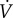 is always decreasing, because S can only decrease, so
is always decreasing, because S can only decrease, so 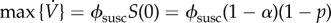 (cf. equation (5.2a)). Because the maximal vaccination rate decreases with increasing initial coverage, p, the maximal vaccination rate is attained with no pre-emptive vaccination (p = 0). Because S(0) = 1 − α, the maximal vaccination rate is
(cf. equation (5.2a)). Because the maximal vaccination rate decreases with increasing initial coverage, p, the maximal vaccination rate is attained with no pre-emptive vaccination (p = 0). Because S(0) = 1 − α, the maximal vaccination rate is 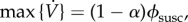 and a vaccination rate of 0.1 per day is attained for ϕsusc = 0.1/(1 − α) ≈ 0.1 per day (because α ≪ 1).
and a vaccination rate of 0.1 per day is attained for ϕsusc = 0.1/(1 − α) ≈ 0.1 per day (because α ≪ 1). -
ϕinst.
For instantaneous vaccination, the vaccination effort parameter ϕinst is the proportion of susceptibles instantaneously vaccinated when an outbreak occurs. Thus, ϕinst ∈ [0, 1]. The vaccination rate is either 0 (if ϕinst = 0) or effectively infinite (if 0 < ϕinst ≤ 1, because vaccination occurs all at once). We thus consider the entire range 0 ≤ ϕinst ≤ 1, because there is no value of ϕinst that results in a vaccination rate of 0.1 per day.
-
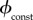 .With
.With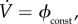 the vaccination rate is constant, so
the vaccination rate is constant, so 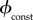 is simply the proportion of the total population that can be vaccinated per unit time. Bauch et al. [4] estimated
is simply the proportion of the total population that can be vaccinated per unit time. Bauch et al. [4] estimated 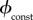 for New York City to be
for New York City to be
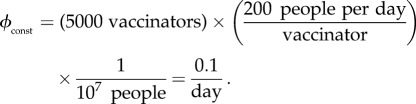
6.1 A rate of
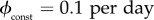 means the entire population can be vaccinated in 10 days.
means the entire population can be vaccinated in 10 days.
Table 4.
Summary of notable levels of the vaccination effort parameter,  for the different models. The first column contains ‘fair comparison’ values for the vaccination effort parameters of the various models, as calculated in §6.2. In our simulations, we allowed
for the different models. The first column contains ‘fair comparison’ values for the vaccination effort parameters of the various models, as calculated in §6.2. In our simulations, we allowed 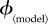 to range between 0 and values generally above the ‘fair comparison’ values (except for ϕinst, for which we used the entire possible range of [0, 1]). The second column contains the minimal values of the vaccination effort parameter (
to range between 0 and values generally above the ‘fair comparison’ values (except for ϕinst, for which we used the entire possible range of [0, 1]). The second column contains the minimal values of the vaccination effort parameter (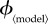 ) for which the individual equilibrium is to delay (that is,
) for which the individual equilibrium is to delay (that is, 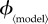 at the end of the mortality plateau; see §7.2.1).
at the end of the mortality plateau; see §7.2.1).
| model | ‘fair comparison’ value | value at end of mortality plateau |
|---|---|---|
| ϕprev | 1582 per day | 571 per day |
| ϕsusc | 0.1 per day | 0.08 per day |
| ϕinc | 5190 per day | 1137 per day |
| ϕinst | — | 0.82 per day |
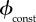 |
0.1 per day | 0.015 per day |
6.3. Numerical procedures
When generating figures 1–4, calculations of the following quantities were necessary: the mortality cost, C(p) (equation (4.1)), the group optimum, pg (§4), and the individual equilibrium, pi (§3).
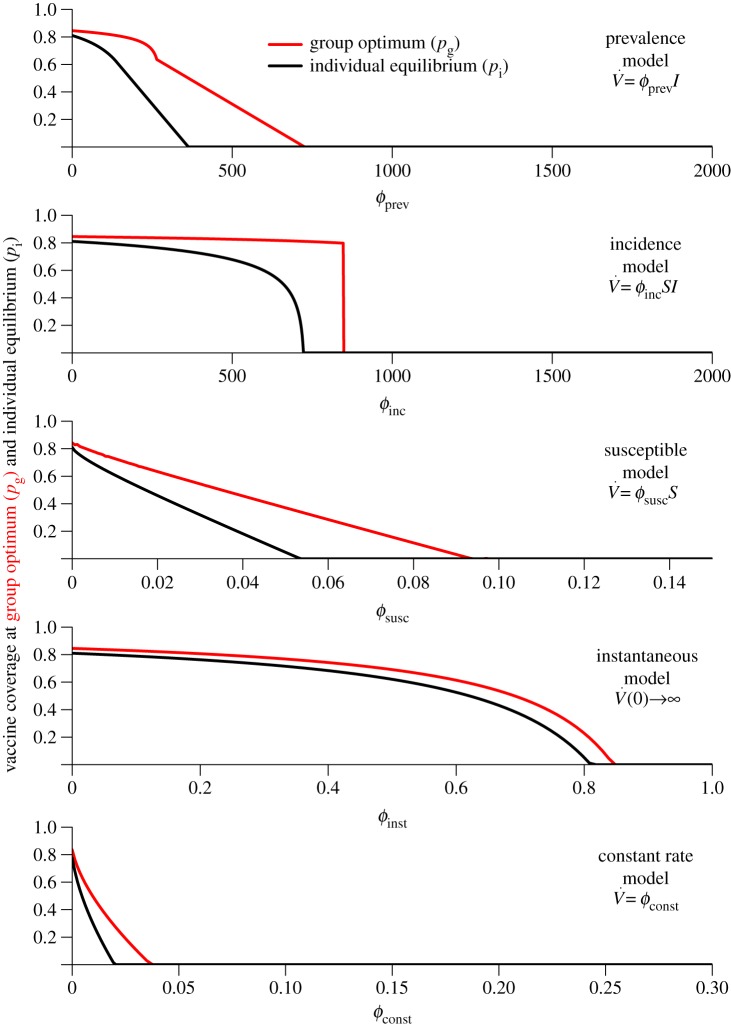
Figure 1.
Variation of the group optimum pg (red) and the individual equilibrium pi (black) with 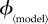 (each panel presents results for a different vaccination model). Note the different ranges of
(each panel presents results for a different vaccination model). Note the different ranges of 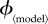 (on the abscissa) for different models. (Online version in colour.)
(on the abscissa) for different models. (Online version in colour.)
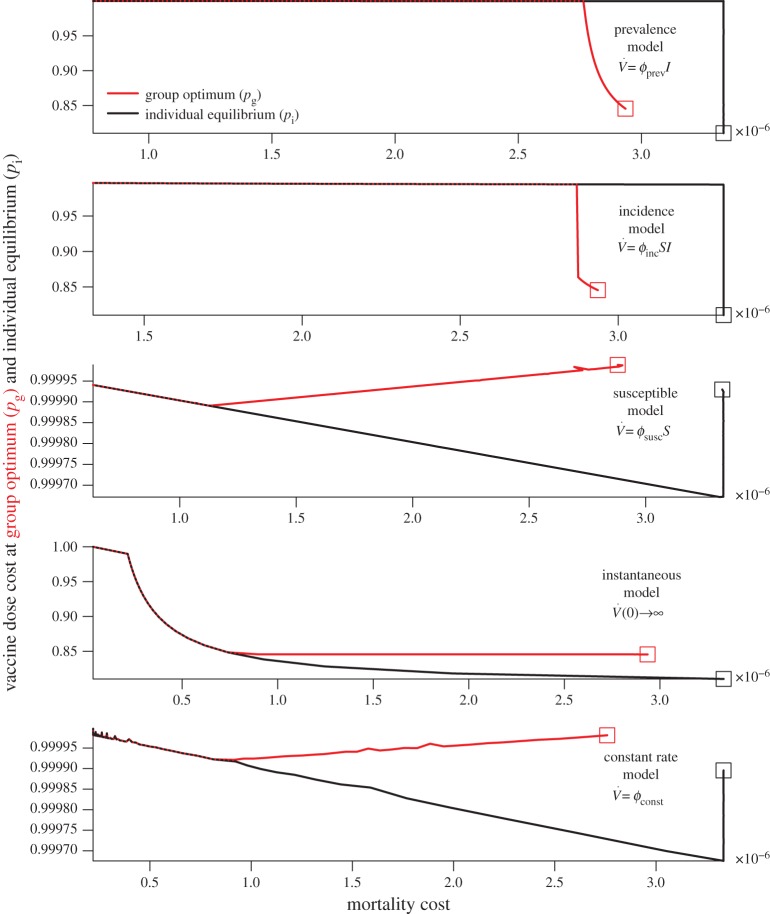
Figure 4.
Vaccine dose cost as a function of mortality cost at the group optimum, pg (red), and the individual equilibrium, pi (black), for the different models. Squares represent values at lowest 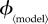 simulated. (Online version in colour.)
simulated. (Online version in colour.)
To find pg, C(p) was numerically minimized using the optimize function in R [24]. pi was found by implementing the procedures described in the electronic supplementary material, appendix G, for the various models, using R's uniroot function.
The calculations of both pg and pi depend on πp and ψp, the probabilities of a delayer being infected or vaccinated, respectively (equation (5.3)). For the models in which the vaccination rate is proportional to prevalence or incidence, we used the final size relations reported in §§5.1 and 5.2, respectively, to calculate πp and ψp. For the remaining models, πp and ψp were obtained by numerically integrating the differential equations using the deSolve package [25] in R [24].
When generating figure 5, for all the models πp and ψp were calculated by numerical integration of the differential equations.
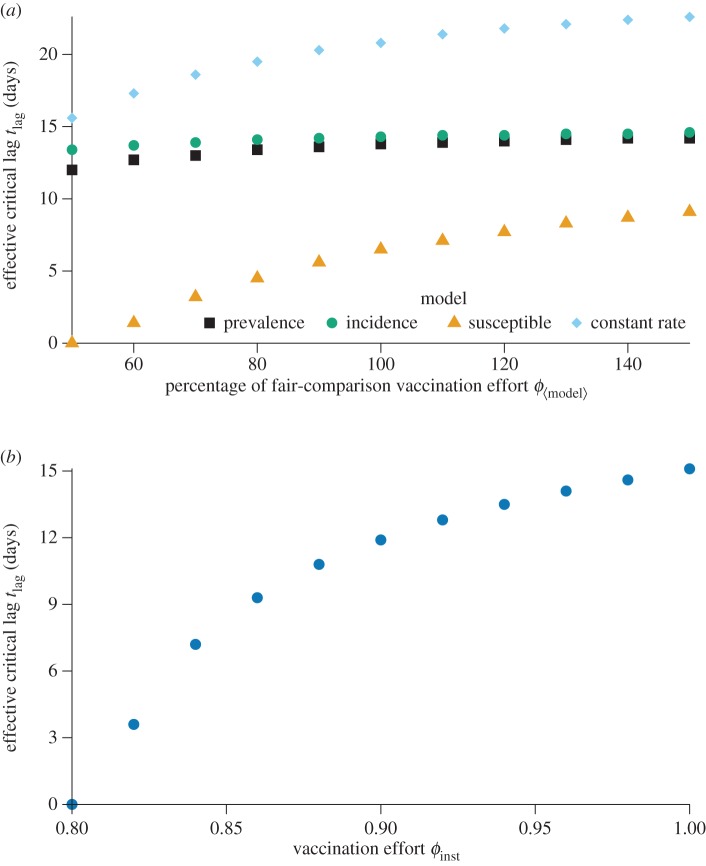
Figure 5.
Variation of the effective critical lag 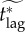 (§7.5.2) with vaccination effort
(§7.5.2) with vaccination effort 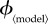 . (a) Effective critical lag versus percentage of the fair comparison vaccination effort when the fair comparison vaccination effort is defined. (b) Effective critical lag versus vaccination effort ϕinst when vaccination is instantaneous. (Online version in colour.)
. (a) Effective critical lag versus percentage of the fair comparison vaccination effort when the fair comparison vaccination effort is defined. (b) Effective critical lag versus vaccination effort ϕinst when vaccination is instantaneous. (Online version in colour.)
7. Results and discussion
7.1. Group optimum versus individual equilibrium
Figure 1 shows the group optimum pg (red) and individual equilibrium pi (black), as the vaccination effort parameter  is varied, for the different models. As expected, the group-optimal coverage is never smaller than the individual equilibrium, and both decrease as
is varied, for the different models. As expected, the group-optimal coverage is never smaller than the individual equilibrium, and both decrease as  is increased. The difference, pi − pg, tends to grow initially with
is increased. The difference, pi − pg, tends to grow initially with  but eventually decreases to 0, because the coverage at both the group optimum and individual equilibrium always drops to 0 if the vaccination rate parameter
but eventually decreases to 0, because the coverage at both the group optimum and individual equilibrium always drops to 0 if the vaccination rate parameter  is increased sufficiently. It is also evident that the difference between the group-optimal coverage and the individual equilibrium depends strongly on the vaccination model used. In general, this difference is much smaller for the instantaneous and constant rate vaccination models than it is for the other models in which vaccination is affected by the state of the outbreak.
is increased sufficiently. It is also evident that the difference between the group-optimal coverage and the individual equilibrium depends strongly on the vaccination model used. In general, this difference is much smaller for the instantaneous and constant rate vaccination models than it is for the other models in which vaccination is affected by the state of the outbreak.
7.2. Mortality cost versus vaccination cost
Figure 2 shows the mortality cost (proportion of the population that dies; figure 2a) and the vaccination cost (proportion of the population that is vaccinated by the end of the outbreak; figure 2b) as functions of the vaccination effort parameter,  , for the various post-outbreak response scenarios.
, for the various post-outbreak response scenarios.
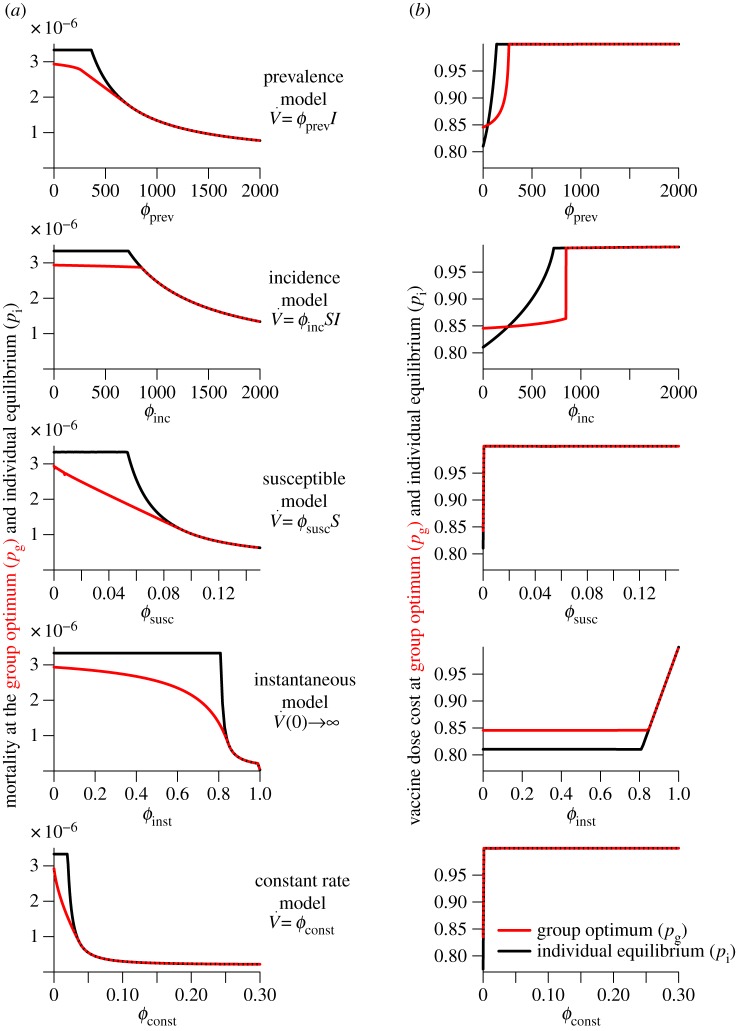
Figure 2.
Variation of the mortality cost (proportion of the population that dies) and vaccine dose cost (proportion of the population that is vaccinated by the end of the outbreak) as functions of the vaccination effort parameter 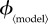 , for different vaccination models. Each row depicts the mortality costs (panel a) and vaccine dose costs (panel b), at the group optimum (red) and individual equilibrium (black) for one model. Note the different ranges of
, for different vaccination models. Each row depicts the mortality costs (panel a) and vaccine dose costs (panel b), at the group optimum (red) and individual equilibrium (black) for one model. Note the different ranges of 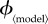 for different models. (Online version in colour.)
for different models. (Online version in colour.)
7.2.1. Mortality plateau
The most striking feature of figure 2 is the plateau in mortality cost at the individual equilibrium for low values of  This plateau can be explained using the Bishop–Cannings theorem [12,26], which implies that if the individual equilibrium is a mixed strategy then the pay-off for vaccinating and delaying must be the same. For low values of
This plateau can be explained using the Bishop–Cannings theorem [12,26], which implies that if the individual equilibrium is a mixed strategy then the pay-off for vaccinating and delaying must be the same. For low values of  the individual equilibrium is mixed (0 < pi < 1), so the mortality cost associated with vaccinating is the same as for delaying, which is therefore the same as the overall mortality cost. Because the mortality cost for vaccinating is equal to the risk from vaccination (rv, or r in normalized units; cf. equation (3.3) and tables 1 and 2), the overall mortality cost is constant at rv (or r in normalized units) as long as the individual equilibrium is mixed. As
the individual equilibrium is mixed (0 < pi < 1), so the mortality cost associated with vaccinating is the same as for delaying, which is therefore the same as the overall mortality cost. Because the mortality cost for vaccinating is equal to the risk from vaccination (rv, or r in normalized units; cf. equation (3.3) and tables 1 and 2), the overall mortality cost is constant at rv (or r in normalized units) as long as the individual equilibrium is mixed. As  is increased, the individual equilibrium pi is decreased (see §7.1). When pi reaches 0, there is a pure strategy equilibrium (i.e. always delay), so the Bishop–Cannings theorem no longer applies; then, the overall mortality is the mortality of delayers, which is −a[riπp + ψprv] (see equation (3.1)) and this decreases as
is increased, the individual equilibrium pi is decreased (see §7.1). When pi reaches 0, there is a pure strategy equilibrium (i.e. always delay), so the Bishop–Cannings theorem no longer applies; then, the overall mortality is the mortality of delayers, which is −a[riπp + ψprv] (see equation (3.1)) and this decreases as  is increased (because the epidemic is extinguished faster).
is increased (because the epidemic is extinguished faster).
7.2.2. Public health strategy implications of the mortality plateau
There is an important implication of the plateau in mortality that occurs for small  if vaccination is voluntary: in order to achieve any reduction in overall mortality, the post-outbreak vaccination response must be so strong that no individual would choose to vaccinate pre-emptively (pi = 0, i.e. the equilibrium is for everyone to delay). Only if the post-outbreak vaccination response is already sufficiently efficient (
if vaccination is voluntary: in order to achieve any reduction in overall mortality, the post-outbreak vaccination response must be so strong that no individual would choose to vaccinate pre-emptively (pi = 0, i.e. the equilibrium is for everyone to delay). Only if the post-outbreak vaccination response is already sufficiently efficient ( is already sufficiently large; figure 1) can outbreak size (and hence overall mortality) be reduced by further enhancing the post-outbreak vaccination response (i.e. by increasing
is already sufficiently large; figure 1) can outbreak size (and hence overall mortality) be reduced by further enhancing the post-outbreak vaccination response (i.e. by increasing  ).
).
Note that, for every model examined here, the right-hand (high effort) edge of the mortality plateau in figure 1 occurs for a value of vaccination effort  lower than the fair comparison value (table 4). Thus, at the fair comparison values of
lower than the fair comparison value (table 4). Thus, at the fair comparison values of  the individual equilibrium is always to delay vaccination, and mortality can be reduced by increasing vaccination effort,
the individual equilibrium is always to delay vaccination, and mortality can be reduced by increasing vaccination effort, 
However, in §7.5, we show that any lag between the start of an outbreak and the beginning of post-outbreak vaccination extends the mortality plateau to higher vaccination efforts,  and a long enough lag makes reducing mortality by increasing vaccination effort impossible. We discuss the implications of this for public health strategies further in §7.5.
and a long enough lag makes reducing mortality by increasing vaccination effort impossible. We discuss the implications of this for public health strategies further in §7.5.
7.2.3. Generality of the mortality plateau
It is important to note that the mortality plateau described earlier is a general phenomenon that applies not only to the post-outbreak vaccination scenarios examined here, but also to any reasonable post-outbreak vaccination scenario. More precisely, suppose public health agencies have some measure of control over a vaccination effort parameter, ϕ. Suppose also that ϕ = 0 corresponds to no possibility of obtaining vaccine post-outbreak, and that the probabilities of a delayer being infected or vaccinated after an outbreak (πp and ψp, respectively) are continuous functions of p and ϕ (for 0 ≤ p < 1 and ϕ ≥ 0). As in §3, the costs for delaying and vaccinating individuals are then a(riπp + rvψp) and rv, respectively. Now suppose the following additionally:
(1) If there is no possibility of being vaccinated post-outbreak (ϕ = 0), and no one is vaccinated pre-emptively (p = 0), then individuals are at greater risk than if they had been vaccinated pre-emptively (i.e. a(riπp + rvψp)|p =0,ϕ =0 > rv).
(2) As the initial coverage approaches 100% (p → 1), the disease does not spread any further than the initial infected cohort (πp → α). Note that, as shown in the electronic supplementary material, appendix D, this assumption holds for all of the models considered in this paper, and the mathematical argument used to show this is quite general.
(3) The risk that a delayer is infected in the initial infection event and then dies is smaller than the risk of mortality from the vaccine alone (αri < rv).
The vaccination game with this post-outbreak vaccination scenario is a population game, and thus must have at least one Nash equilibrium [27, theorem 2.1.1, p. 24].
So for, low enough vaccination effort ϕ, if coverage p is low, it is more costly to delay than to vaccinate (from the first assumption above). Conversely, if coverage p is high enough, the third assumption above implies that delaying is preferable to vaccinating pre-emptively. It follows that any individual equilibrium that results from the vaccination game is a mixed NE (0 < pi < 1). The preceding argument presented in §7.2.1 (using the Bishop–Cannings theorem) now implies the existence of a plateau in mortality.
7.2.4. Vaccination cost plateau
Figure 2b also show a plateau for sufficiently large vaccination efforts (except for the constant rate vaccination model). Unlike the mortality plateau, this vaccination cost plateau is not rigorously a constant (it changes very slightly as a function of  ), but it is certainly a plateau for all intents and purposes. This plateau occurs because overall vaccination rises with the vaccination effort,
), but it is certainly a plateau for all intents and purposes. This plateau occurs because overall vaccination rises with the vaccination effort,  and cannot exceed V∞ = 1, so vaccination costs must eventually taper off.
and cannot exceed V∞ = 1, so vaccination costs must eventually taper off.
7.2.5. Perceived versus real risks
The general public is likely to overestimate vaccine-induced mortality [28–30], which would tend to decrease the pre-outbreak vaccine coverage under voluntary vaccination. The game-theoretical framework we employ assumes individuals behave rationally and possess perfect information on which to base their decisions, but it is possible to relax the assumption of perfect information while maintaining that of rationality. Thus, to account for misinformation regarding the dangers of vaccination (possibly as a result of vaccine scares), we can interpret ri and rv as the perceived risks of infection and vaccination (rather than the actual risks) to predict the effective level of vaccine coverage prior to an outbreak (note that perceived risks are to be used to predict the individual equilibrium, pi, but not when predicting the group optimum pg, nor when predicting the mortality and vaccination costs at either of these coverages). Consequently, public health agencies can potentially reduce mortality by attempting to influence the public's estimate of r (the risk of vaccination relative to infection). For example, risk perception might be influenced by a media campaign aiming to increase the accuracy of the public's perception of vaccine safety and promote pre-emptive vaccination.
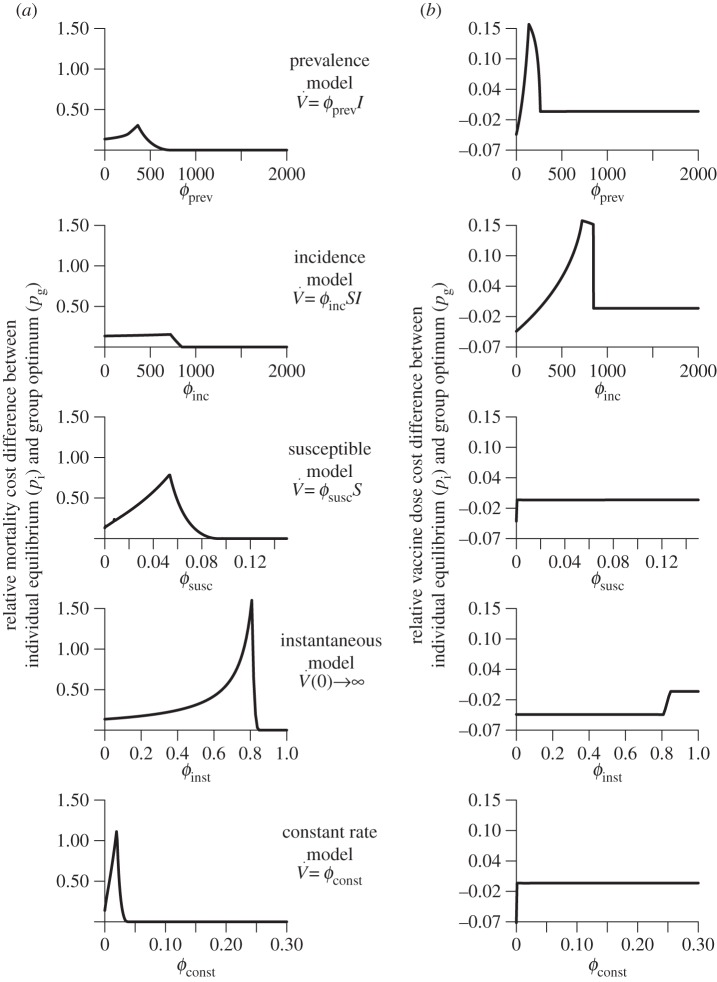
7.3. Comparison of relative costs
In figure 3a, we look at the relative mortality cost difference, that is, in units of the cost of optimal mandatory vaccination. Explicitly, we examine how (C(pi) − C(pg))/C(pg) varies with  for each model. Similarly, we plot the relative difference in vaccination (V∞(pi) − V∞(pg))/V∞(pg) (figure 3b), which is the relative vaccine dose cost difference between voluntary and mandatory vaccination.
for each model. Similarly, we plot the relative difference in vaccination (V∞(pi) − V∞(pg))/V∞(pg) (figure 3b), which is the relative vaccine dose cost difference between voluntary and mandatory vaccination.
Figure 3.
Variation of relative difference in mortality and vaccine dose costs with 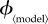 , for different vaccination models. Each row depicts the relative mortality cost difference (panel a) and relative vaccine dose cost difference (panel b) for one model. Note the different ranges of
, for different vaccination models. Each row depicts the relative mortality cost difference (panel a) and relative vaccine dose cost difference (panel b) for one model. Note the different ranges of 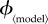 for different models.
for different models.
7.3.1. Large variation in relative mortality cost
Observe that, in figure 3a, the relative mortality cost difference is always non-negative (as expected from the definition of the group optimum as the pre-outbreak coverage for which expected mortality cost is minimal). There is substantial variability among the models in the dependence of the relative mortality cost differences on the vaccination parameter  In particular, if vaccination rate is proportional to incidence or prevalence, variation in relative mortality cost is an order of magnitude smaller than if vaccination is instantaneous or at a constant rate. The vaccination scenario that exhibits the largest variation in relative mortality costs is instantaneous vaccination. In this scenario, a voluntary vaccination policy could result in over 150% more deaths than if vaccination were mandatory.
In particular, if vaccination rate is proportional to incidence or prevalence, variation in relative mortality cost is an order of magnitude smaller than if vaccination is instantaneous or at a constant rate. The vaccination scenario that exhibits the largest variation in relative mortality costs is instantaneous vaccination. In this scenario, a voluntary vaccination policy could result in over 150% more deaths than if vaccination were mandatory.
7.3.2. Modest variation in relative vaccine dose cost
There is also substantial variability in the pattern of variation of relative vaccine dose cost as a function of  among the different models (figure 3b). However, for all the models, variation in relative vaccine dose cost as a function of
among the different models (figure 3b). However, for all the models, variation in relative vaccine dose cost as a function of  is much less than the corresponding variation in relative mortality cost. The maximum variation in relative vaccine dose cost reaches approximately 16% for the models in which vaccination is proportional to prevalence or incidence. This relatively large variation can be attributed to low pre-outbreak vaccination coverage (at the individual equilibrium) causing high disease prevalence and incidence; consequently, because vaccination rate is proportional to prevalence or incidence, there is correspondingly high post-outbreak vaccination, which overshoots that which would be required to minimize group mortality. In these two situations, the vaccine dose cost at the individual equilibrium can be greater than at the group optimum. In any case, the relatively small difference in overall vaccine dose costs, both as a function of vaccination effort (
is much less than the corresponding variation in relative mortality cost. The maximum variation in relative vaccine dose cost reaches approximately 16% for the models in which vaccination is proportional to prevalence or incidence. This relatively large variation can be attributed to low pre-outbreak vaccination coverage (at the individual equilibrium) causing high disease prevalence and incidence; consequently, because vaccination rate is proportional to prevalence or incidence, there is correspondingly high post-outbreak vaccination, which overshoots that which would be required to minimize group mortality. In these two situations, the vaccine dose cost at the individual equilibrium can be greater than at the group optimum. In any case, the relatively small difference in overall vaccine dose costs, both as a function of vaccination effort ( ) and among vaccination scenarios (see figure 2b), suggests that vaccine dose cost should probably not be a factor in public health policy decisions.
) and among vaccination scenarios (see figure 2b), suggests that vaccine dose cost should probably not be a factor in public health policy decisions.
7.4. Vaccine dose cost as a function of mortality cost
Figures 2 and 3 present mortality costs and vaccine dose costs as functions of vaccination effort for the various models. Because the meaning of the vaccination effort parameter  differs among models, it is not straightforward to make meaningful comparisons among the various models (which is why we calculated ‘fair comparison’ values in §6). In this section, we display results for the various models, factoring out the vaccination effort parameter. For each model, figure 4 shows the vaccine dose cost as a function of mortality cost. In health economics terms, this can be considered a cost-effectiveness analysis [31].
differs among models, it is not straightforward to make meaningful comparisons among the various models (which is why we calculated ‘fair comparison’ values in §6). In this section, we display results for the various models, factoring out the vaccination effort parameter. For each model, figure 4 shows the vaccine dose cost as a function of mortality cost. In health economics terms, this can be considered a cost-effectiveness analysis [31].
In figure 4, the squares indicate the point in the mortality-cost–vaccination-cost plane where the vaccination effort ( ) is the lowest that we considered. Increasing vaccination effort (while remaining at the individual equilibrium or the group optimum) corresponds to moving away from the square along the plotted curves.
) is the lowest that we considered. Increasing vaccination effort (while remaining at the individual equilibrium or the group optimum) corresponds to moving away from the square along the plotted curves.
The graphs in figure 4 allow us to answer practical questions such as ‘If we want to ensure that no more than one in every 10 million citizens dies, how many vaccine doses are required in each scenario?’ or ‘If we have a stockpile of vaccine doses sufficient for 30% of the population, what percentage of the population can be expected to die if there is an outbreak in each scenario?’ Of course, by construction the graphs do not indicate how much effort ( ) is required to achieve the desired results. We emphasize that—as shown in the previous section—vaccine dose cost at the individual equilibrium or group optimum hardly varies as a function of vaccination effort (
) is required to achieve the desired results. We emphasize that—as shown in the previous section—vaccine dose cost at the individual equilibrium or group optimum hardly varies as a function of vaccination effort ( ), so the ‘practical’ questions are not necessarily well posed (e.g. if we have sufficient vaccine doses for only 30% of the population, then neither the individual equilibrium nor the group optimum can ever be achieved). This is true for all the models with parameters appropriate for smallpox; for another disease graphs like figure 4 could have genuine practical value for public health policy analysis (e.g. setting ℛ0 = 1.25 and keeping all other model parameters as in tables 1 and 2 causes the vaccination cost to vary between 25% and 99.999%).
), so the ‘practical’ questions are not necessarily well posed (e.g. if we have sufficient vaccine doses for only 30% of the population, then neither the individual equilibrium nor the group optimum can ever be achieved). This is true for all the models with parameters appropriate for smallpox; for another disease graphs like figure 4 could have genuine practical value for public health policy analysis (e.g. setting ℛ0 = 1.25 and keeping all other model parameters as in tables 1 and 2 causes the vaccination cost to vary between 25% and 99.999%).
In figure 4, when the vaccination rate is proportional to either prevalence or incidence, note that, as the vaccination effort,  increases, two phases of behaviour are apparent for the costs at both pi and pg: first, vaccine dose cost rises but no change in mortality cost is observed (this is caused by the plateau in mortality described in §7.2.1). Then, for all but the instantaneous vaccination model, vaccine dose cost remains virtually constant (note the differences in the scales of the vertical axes among the various panels), but mortality costs decrease.
increases, two phases of behaviour are apparent for the costs at both pi and pg: first, vaccine dose cost rises but no change in mortality cost is observed (this is caused by the plateau in mortality described in §7.2.1). Then, for all but the instantaneous vaccination model, vaccine dose cost remains virtually constant (note the differences in the scales of the vertical axes among the various panels), but mortality costs decrease.
It is interesting to note that the dependence of vaccine costs on mortality costs at the group optimum varies among the models. For example, when vaccination is proportional to remaining susceptibles, and for the constant rate vaccination model, we see in figure 4 that at the group optimum, as mortality cost is decreased, vaccine dose cost decreases at first, but then increases. Thus, in these situations, one can lower both the mortality and the vaccine dose cost at the same time by increasing vaccination effort (in health economics terms, the decision to use higher vaccination effort has negative marginal cost in vaccine doses per life saved). This contrasts the models in which vaccination is instantaneous, or proportional to incidence or prevalence, in which we observe that, as mortality cost is decreased, the vaccine dose cost at the group optimum remains constant and then increases sharply.
Finally, for the instantaneous vaccination model, there is a range of vaccination efforts for which one can reduce mortality without increasing vaccine dose costs at the group optimum. In this parameter range, the increase in vaccine dose cost necessary to decrease mortality at the individual equilibrium is small at first, but grows larger as mortality is decreased.
7.5. Effect of vaccination response lag tlag
We have implicitly assumed that in any of the scenarios we have considered the vaccination response will begin as soon as an outbreak is seeded by a bioterrorist attack or accidental release. In contrast, Bauch et al. [4] assumed a lag of two weeks between the seeding of an outbreak and the initiation of a vaccination response. In this section, we investigate the effect of a response lag of tlag days between an outbreak being seeded and the post-outbreak vaccination campaign beginning (so far, we have assumed tlag = 0 days; in [4], tlag = 14 days was assumed).
Intuitively, adding a lag between the beginning of an outbreak and the vaccination response allows the disease to spread unhindered for some time, which increases the probability of delayers being infected, thus decreasing the pay-off for delaying. As a result, the individual equilibrium pi increases, which consequently extends the mortality plateau (§7.2.1) to higher values of 
7.5.1. The critical lag, 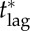
For a disease such as smallpox with 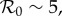 the expected final size of an uncontrolled epidemic is greater than 99.9% of the population. If no one is pre-emptively vaccinated, and the response lag after an outbreak is seeded is sufficiently long, almost everyone will have been infected before the response begins, i.e. if tlag is sufficiently long then delayers will almost certainly be infected before they can be vaccinated. Consequently, unless the probability of an outbreak (a) is negligible, delaying will be riskier than vaccinating pre-emptively, so the individual equilibrium will not be for everyone to delay: we will certainly have pi > 0. It follows that for response lags longer than some critical lag,
the expected final size of an uncontrolled epidemic is greater than 99.9% of the population. If no one is pre-emptively vaccinated, and the response lag after an outbreak is seeded is sufficiently long, almost everyone will have been infected before the response begins, i.e. if tlag is sufficiently long then delayers will almost certainly be infected before they can be vaccinated. Consequently, unless the probability of an outbreak (a) is negligible, delaying will be riskier than vaccinating pre-emptively, so the individual equilibrium will not be for everyone to delay: we will certainly have pi > 0. It follows that for response lags longer than some critical lag, 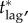 mortality cannot be reduced no matter how much effort is applied in the post-outbreak vaccination response (i.e. the mortality plateau described in §7.2.1 continues for arbitrarily large values of
mortality cannot be reduced no matter how much effort is applied in the post-outbreak vaccination response (i.e. the mortality plateau described in §7.2.1 continues for arbitrarily large values of  ).
).
A more precise argument allows us to estimate 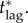 Suppose the initial coverage is p = 0 (no pre-emptive vaccination). If the risk of becoming infected and dying is larger than the risk from vaccinating, i.e. ariπ0 > rv (or, equivalently, π0 > r/a), then delaying will not be the individual equilibrium. For any vaccination scenario, the delayers' probability of being infected by the end of the outbreak (equation (5.3a)) is greater than or equal to their probability of being infected before the vaccination response begins (at time tlag),
Suppose the initial coverage is p = 0 (no pre-emptive vaccination). If the risk of becoming infected and dying is larger than the risk from vaccinating, i.e. ariπ0 > rv (or, equivalently, π0 > r/a), then delaying will not be the individual equilibrium. For any vaccination scenario, the delayers' probability of being infected by the end of the outbreak (equation (5.3a)) is greater than or equal to their probability of being infected before the vaccination response begins (at time tlag),
| 7.1 |
Therefore, if
| 7.2 |
then π0 > r/a and delaying is guaranteed not to be the individual equilibrium. But for any post-outbreak vaccination scenario that includes a response lag, when t < tlag the removed proportion of the population, R(t), follows the standard SIR model solution (with no vaccination). For the SIR model with no vaccination (p = 0; a, α and ℛ0 as in table 1), numerical simulation shows that equation (7.2) is satisfied for 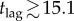 days. Hence, if the public health response lag is 16 days or longer, then it is guaranteed that (regardless of the vaccination scenario or corresponding vaccination effort,
days. Hence, if the public health response lag is 16 days or longer, then it is guaranteed that (regardless of the vaccination scenario or corresponding vaccination effort,  ) delaying will not be the individual equilibrium.
) delaying will not be the individual equilibrium.
We emphasize that our estimate of 16 days as an upper bound for 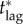 depends on a number of factors, including:
depends on a number of factors, including:
— the probability of an outbreak (a);
— the proportion of susceptibles infected in the initial outbreak (α); and
— the epidemiological model: the estimate is obtained using the SIR model, but adding an exposed class (SEIR) with parameters as in table 1 increases the critical lag. Repeating the calculation for the SEIR model yielded the upper bound
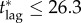 days. The reason for this difference in critical lags is that, when the outbreak is seeded, all individuals initially infected begin their latent period simultaneously, and take on average 15 days to become infectious.
days. The reason for this difference in critical lags is that, when the outbreak is seeded, all individuals initially infected begin their latent period simultaneously, and take on average 15 days to become infectious.
Thus, 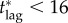 days should be regarded as a rough estimate at best. Nonetheless, the existence of a critical lag, beyond which it is impossible to reduce mortality by increasing vaccination effort, is an important consideration for public health agencies, in devising contingency plans for post-outbreak vaccination against diseases.
days should be regarded as a rough estimate at best. Nonetheless, the existence of a critical lag, beyond which it is impossible to reduce mortality by increasing vaccination effort, is an important consideration for public health agencies, in devising contingency plans for post-outbreak vaccination against diseases.
Note, however, that, in the case of a bioterrorist attack, an outbreak will probably not be discovered until individuals show symptoms, i.e. until someone's latent period has passed (12 days at a minimum). Taking this delayed detection into account, it follows that in order to avoid extending the mortality plateau to all feasible values of vaccination effort,  the response lag from discovery of the epidemic to the beginning of the post-outbreak vaccination response must, in practice, be substantially shorter than 26 days. This is in contrast to an accidental release, where public health authorities might know of the outbreak well before anyone has shown symptoms. In this latter case, because it is more likely that the critical lag has not been exceeded, it is especially important to begin the vaccination response as early as possible in order to reduce mortality.
the response lag from discovery of the epidemic to the beginning of the post-outbreak vaccination response must, in practice, be substantially shorter than 26 days. This is in contrast to an accidental release, where public health authorities might know of the outbreak well before anyone has shown symptoms. In this latter case, because it is more likely that the critical lag has not been exceeded, it is especially important to begin the vaccination response as early as possible in order to reduce mortality.
Lastly, it is important to note that the effect of a response lag on the mortality plateau presupposes that both the vaccination effort and the response lag are known to the public in advance. This limits the applicability of this effect, because, in the case of a bioterrorist attack, the response lag probably depends on when an infective first shows symptoms (which introduces a stochastic effect). Further analysis would be needed to determine the effects of a stochastic response lag on individual behaviour, and thus on mortality.
7.5.2. The effective critical lag, 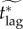
We have seen that if the response lag is longer than the critical lag (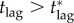 ), then no matter how large the vaccination effort (
), then no matter how large the vaccination effort ( ), it is impossible to reduce mortality. Of course, in practice, the vaccination effort cannot be arbitrarily large and will be constrained by public health resources. Given a maximum feasible vaccination effort, it would be helpful to know how long the response lag can be before the mortality plateau extends to all feasible levels of vaccination effort.
), it is impossible to reduce mortality. Of course, in practice, the vaccination effort cannot be arbitrarily large and will be constrained by public health resources. Given a maximum feasible vaccination effort, it would be helpful to know how long the response lag can be before the mortality plateau extends to all feasible levels of vaccination effort.
To address this issue, we define the effective critical lag, 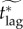 , to be the minimal response lag, such that the individual equilibrium is no longer to delay (i.e. pi > 0) given a maximum feasible vaccination effort
, to be the minimal response lag, such that the individual equilibrium is no longer to delay (i.e. pi > 0) given a maximum feasible vaccination effort
 . Thus, the critical lag
. Thus, the critical lag 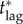 is the limit of
is the limit of 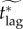 as the maximum feasible vaccination effort becomes arbitrarily large.
as the maximum feasible vaccination effort becomes arbitrarily large.
In figure 5, we plot the effective critical lag, 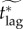 , against the vaccination effort,
, against the vaccination effort,  , for the various models examined in this paper. For the models for which fair comparison values of
, for the various models examined in this paper. For the models for which fair comparison values of  are well defined (see §6.2), we used these as estimates for feasible vaccination efforts. However, because the fair comparison level of vaccination effort is a crude estimate for the range of feasible vaccination efforts, in figure 5a, we plot the effective critical lag at values of
are well defined (see §6.2), we used these as estimates for feasible vaccination efforts. However, because the fair comparison level of vaccination effort is a crude estimate for the range of feasible vaccination efforts, in figure 5a, we plot the effective critical lag at values of  ranging from 50% to 150% of the fair comparison levels of vaccination efforts for the various models, in increments of 10% of the fair comparison level of
ranging from 50% to 150% of the fair comparison levels of vaccination efforts for the various models, in increments of 10% of the fair comparison level of 
The instantaneous vaccination model was the only model for which a fair comparison value of vaccination effort ϕinst could not be defined (see §6.2). For this model, we show the effective critical lag 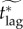 for ϕinst ranging from 0.8 to 1 (if ϕinst < 0.8, then
for ϕinst ranging from 0.8 to 1 (if ϕinst < 0.8, then 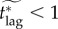 day) in figure 5b.
day) in figure 5b.
We see in figure 5 that, for some vaccination scenarios, minimizing the response lag tlag is essential: even a short lag extends the mortality plateau to all feasible vaccination effort levels, making it impossible to reduce mortality by increasing effort after the lag. We also note that, for some scenarios, a good estimate of the attainable vaccination effort is necessary, because the critical effective lag is very sensitive to the vaccination effort. These two facts further underline the importance of accurately modelling post-outbreak vaccination to inform public health decisions relating to post-outbreak contingency plans. When the response lag is longer than the effective critical lag (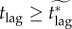 ), the only plausible way for public health officials to decrease mortality (while allowing individuals to choose whether or not to vaccinate) is to reduce the relative mortality risk (by decreasing the probability of dying from vaccination, i.e. developing a safer vaccine).
), the only plausible way for public health officials to decrease mortality (while allowing individuals to choose whether or not to vaccinate) is to reduce the relative mortality risk (by decreasing the probability of dying from vaccination, i.e. developing a safer vaccine).
7.5.3. The response lag should be minimized
Based on §§7.5.1 and 7.5.2, reducing the response lag lowers expected mortality and makes it easier to decrease mortality further:
- — For vaccination efforts higher than the end of the mortality plateau, everyone will choose to delay vaccination (§7.2.1). From the discussion leading up to equation (7.1), it follows that, even in the best-case scenario where the epidemic is stopped immediately at t = tlag, mortality will be no less than
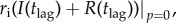
7.3 which increases with the response time tlag. Thus, increasing the response lag increases the lowest attainable mortality (even if vaccination effort can be increased without bound).
— Increasing the response lag increases the vaccination effort at the end of the mortality plateau (i.e. the minimal vaccination effort beyond which increasing vaccination effort decreases mortality). Thus, longer response lags make it harder to achieve a decrease in mortality.
However, note that if a response time lower than the effective critical lag (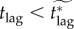 ) cannot be achieved, neither increasing the vaccination effort,
) cannot be achieved, neither increasing the vaccination effort,  , nor decreasing the response time, tlag, can decrease mortality.
, nor decreasing the response time, tlag, can decrease mortality.
8. Conclusion
We have analysed five distinct scenarios (§2) associated with a potential smallpox outbreak triggered by a bioterrorist attack or accidental release. The scenarios differ in the factors that influence individuals' perception of risk and how a post-outbreak vaccination response plays out. We examined these scenarios both with and without an assumed lag between an outbreak starting and a public health response being initiated. Our work generalizes the analysis of Bauch et al. [4], who investigated a single scenario with a response lag of 14 days.
As in [4], we considered separately group interest (optimal strategies for minimizing overall mortality) and self-interest (stable strategies for individual choices with respect to pre-emptive vaccination). From each perspective, we obtained the (imposed or expected) pre-emptive vaccination coverage (p) for each scenario (the group optimum pg in the case of group interest and the individual equilibrium pi in the case of self-interest; figure 1).
Our principal conclusions are the following.
(1) For a given level of post-outbreak vaccination effort, the group optimum pre-emptive coverage is always greater than the individual equilibrium (pg > pi; figure 1) and the expected total mortality is always less if public health authorities impose the group optimum rather than letting individuals make their own vaccination decisions (figure 2a). If no outbreak occurs, then some people will die unnecessarily from pre-emptive vaccination. Given the difficulty of estimating the probability of an attack or accidental release, it would be hard for governments to justify an imposed pre-emptive vaccination policy for a disease like smallpox for which the vaccine can cause death.
(2) The number of vaccine doses required at the group optimum and individual equilibrium does not vary substantially as a function of vaccination effort (e.g. speed of vaccine distribution post-outbreak) for any of the scenarios (figure 2b). Consequently, the economic cost of vaccine production is not likely to play a significant role in policy decisions.
(3) Total expected mortality as a function of vaccination effort depends strongly on which scenario is considered (figure 2a). Some vaccination scenarios are affected by the public reaction to media reports on the epidemic's progress, whereas some (the instantaneous and constant rate vaccination scenarios) are under the direct control of public health authorities. To assist public health authorities preparing for potential outbreaks, further research is needed to determine which factors have the greatest influence on individuals' perception of risk and which post-outbreak vaccination strategies are most feasible.
(4) For any realistic vaccination scenario, there is a range of vaccination effort levels in which increasing vaccination effort does not reduce overall mortality. In this mortality plateau, increasing vaccination effort leads only to fewer people vaccinating pre-emptively, until the individual equilibrium becomes to delay vaccination (at which point it is possible to reduce mortality by increasing the vaccination effort). Thus, under voluntary vaccination, in order for public health authorities to expect to reduce mortality by increasing vaccination effort post-outbreak, their planned post-outbreak vaccination response must be so efficient that no one would choose to vaccinate pre-emptively (pi = 0).
(5) Any lag between the beginning of an outbreak and the post-outbreak vaccination response makes it harder for higher vaccination effort levels to make a difference to overall mortality, and a large enough lag will make it impossible to reduce mortality, regardless of the level of vaccination effort. Given a maximum feasible vaccination effort level, there is an effective critical lag, beyond which it is impossible to reduce mortality by increasing vaccination effort. The dependence on the post-outbreak vaccination scenario, of both the effective critical lag at feasible levels of vaccination effort and the effect of changes in vaccination effort on the effective critical lag, further highlights the importance of researching realistic post-outbreak vaccination responses.
It is not possible to know with certainty how governments and health agencies will react, or how individuals will behave, in the event of an outbreak. However, the above-mentioned conclusions are based on our analysis of five distinct post-outbreak scenarios (and some model features that are much more generic), so it seems likely that our conclusions would remain valid if further plausible scenarios were considered.
Supplementary Material
Acknowledgements
Some preliminary work on the problem addressed in this paper was carried out by Adelicia Yu as part of her undergraduate summer research project in 2004. We are grateful to Sigal Balshine, Paul Higgs, Rufus Johnstone and two anonymous referees for valuable comments.
Funding statement
This study is supported by NSERC (D.J.D.E.) and the Trillium Foundation (C.M.).
References
- 1.Fenner F.1988. Smallpox and its eradication, Parts 1–14. In History of international public health, vol. 6. Geneva, Switzerland: World Health Organization.
- 2.Koplow DA. 2004. Smallpox: the fight to eradicate a global scourge. Oakland, CA: University of California Press. [Google Scholar]
- 3.Christensen J.2014. CDC: smallpox found in NIH storage room is alive.http://www.cnn.com/2014/07/11/health/smallpoxfound-nih-alive/ (accessed 12 August 2014).
- 4.Bauch CT, Galvani AP, Earn DJD. 2003. Group interest versus self-interest in smallpox vaccination policy. Proc. Natl Acad. Sci. USA 100, 10564–10567. ( 10.1073/pnas.1731324100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauch CT, Earn DJD. 2004. Vaccination and the theory of games. Proc. Natl Acad. Sci. USA 101, 13 391–13 394. ( 10.1073/pnas.0403823101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Eichner M, Dietz K. 2003. Transmission potential of smallpox: estimates based on detailed data from an outbreak. Am. J. Epidemiol. 158, 110–117. ( 10.1093/aje/kwg103) [DOI] [PubMed] [Google Scholar]
- 8.Gani R, Leach S. 2001. Transmission potential of smallpox in contemporary populations. Nature 414, 748–751. ( 10.1038/414748a) [DOI] [PubMed] [Google Scholar]
- 9.Krylova O. 2011. Predicting epidemiological transitions in infectious disease dynamics: smallpox in historic London (1664–1930). PhD thesis, McMaster University, Canada. [Google Scholar]
- 10.Kaplan EH, Craft DL, Wein LM. 2002. Emergency response to a smallpox attack: the case for mass vaccination. Proc. Natl Acad. Sci. USA 99, 10 935–10 940. ( 10.1073/pnas.162282799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fudenberg D, Tirole J. 1991. Game theory. Cambridge, MA: MIT Press. [Google Scholar]
- 12.Maynard Smith J. 1982. Evolution and the theory of games. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Krylova O, Earn DJD. 2013. Effects of the infectious period distribution on predicted transitions in childhood disease dynamics. J. R. Soc. Interface 10, 20130098 ( 10.1098/rsif.2013.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. 2014. World health statistics 2014. Geneva, Switzerland: WHO. [Google Scholar]
- 15.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9, 1131–1137. ( 10.1038/nm917) [DOI] [PubMed] [Google Scholar]
- 16.Kermack WO, McKendrick AG. 1927. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A 115, 700–721. ( 10.1098/rspa.1927.0118) [DOI] [Google Scholar]
- 17.Ma J, Earn DJD. 2006. Generality of the final size formula for an epidemic of a newly invading infectious disease. Bull. Math. Biol. 68, 679–702. ( 10.1007/s11538-005-9047-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earn DJD, Andrews PW, Bolker BM. 2014. Population-level effects of suppressing fever. Proc. R. Soc. B 281, 20132570 ( 10.1098/rspb.2013.2570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisstein EW. Lambert W-function. From MathWorld: a Wolfram Web Resource. http://mathworld.wolfram.com/LambertW-Function.html. (accessed 17 April 2015).
- 20.Corless RM, Gonnet GH, Hare DEG, Jeffrey DJ, Knuth DE. 1996. On the Lambert W function. Adv. Comput. Math. 5, 329–359. ( 10.1007/BF02124750) [DOI] [Google Scholar]
- 21.McNeil DG., Jr2013. Wary of attack with smallpox, U.S. buys up a costly drug. The New York Times. See http://www.nytimes.com/2013/03/13/health/us-stockpiles-smallpox-drug-in-case-of-bioterror-attack.html. (accessed 12 August 2014).
- 22.Vink MA, Bootsma MCJ, Wallinga J. 2014. Serial intervals of respiratory infectious diseases: a systematic review and analysis. Am. J. Epidemiol. 180, 865–875. ( 10.1093/aje/kwu209) [DOI] [PubMed] [Google Scholar]
- 23.Svensson A. 2007. A note on generation times in epidemic models. Math. Biosci. 208, 300–311. ( 10.1016/j.mbs.2006.10.010) [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: See http://www.R-project.org/. [Google Scholar]
- 25.Soetaert K, Petzoldt T, Setzer RW. 2010. Solving differential equations in R: package deSolve. J. Stat. Softw. 33, 1–25.20808728 [Google Scholar]
- 26.Sandholm WH. 2010. Population games and evolutionary dynamics. Economic Learning and Social Evolution. Cambridge, MA: MIT Press. [Google Scholar]
- 27.Bishop DT, Cannings C. 1978. A generalized war of attrition. J. Theor. Biol. 70, 85–124. ( 10.1016/0022-5193(78)90304-1) [DOI] [PubMed] [Google Scholar]
- 28.Leach M, Fairhead J.2007. Vaccine anxieties: global science, child health and society. Science in Society Series. London, UK: Earthscan.
- 29.Brown KF, Kroll JS, Hudson MJ, Ramsay M, Green J, Long SJ, Vincent CA, Fraser G, Sevdails N. 2010. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine 28, 4235–4248. ( 10.1016/j.vaccine.2010.04.052) [DOI] [PubMed] [Google Scholar]
- 30.Gangarosa EJ, Galazka A, Wolfe C, Phillips L, Gangarosa R, Miller E, Chen RT, Gangarosa RE. 1998. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet 351, 356–361. ( 10.1016/S0140-6736(97)04334-1) [DOI] [PubMed] [Google Scholar]
- 31.Phillips CJ. 2008. Health economics: an introduction for health professionals. London, UK: John Wiley & Sons. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.