Supplemental Digital Content is available in the text.
Keywords: arrhythmias, cardiac; atrial fibrillation; catheter ablation
Background—
There is a lack of data on the comparative efficacy and procedural safety of open irrigated radiofrequency (RF) and cryoballoon catheter (CB) ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation.
Methods and Results—
In a prospective, noninferiority study, 315 patients were randomly assigned to RF (n=159) or CB (n=156) ablation. The primary end point was freedom from atrial arrhythmia with absence of persistent complications. Patients were largely comparable between groups with more vascular disease in the RF group (8.2% versus 2.6% for CB; P=0.028). The primary end point at 12 months was achieved by 70.7% with RF and 73.6% with CB (multiple procedure success), including 31 redo procedures in each group (19.5% of RF versus 19.9% of CB; P=0.933). For the intention-to-treat population, noninferiority of CB was revealed for the predefined inferiority margin (risk difference, 0.029; 95% confidence interval, −0.074 to 0.132; P<0.001). Rates at 6 months were 63.1% and 64.1% for the RF and CB groups (single procedure success), and noninferiority was confirmed (risk difference, 0.010; 95% confidence interval, −0.097 to 0.116; P=0.002). Periprocedural complications for the index procedure were more frequent in the CB group (5.0% RF, 12.2% CB; P=0.022) with a significant difference in phrenic nerve palsies (0% RF, 5.8% CB; P=0.002).
Conclusion—
This large, prospective, randomized, controlled study demonstrates noninferiority of CB ablation versus RF ablation for treating patients with paroxysmal atrial fibrillation.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00774566.
Atrial fibrillation (AF), the most common form of cardiac arrhythmia, is associated with a risk of associated complications such as stroke and heart failure, in addition to a higher rate of mortality.1,2 Sinus rhythm can often be restored with electric cardioversion; however, the rate of AF recurrence is high, even with administration of antiarrhythmic drugs (AADs).3 In addition to their relatively low efficacy, AADs have the disadvantage of causing adverse events, often leading to discontinuation.4,5 Catheter ablation is a well-established technique for treating paroxysmal AF via pulmonary vein (PV) isolation, with a variety of energy sources used, most commonly radiofrequency (RF) or cryoenergy.6 RF ablation has been shown to be a highly effective first- or second-line treatment for AF; however, it is associated with more immediate and severe complications compared with drug therapy.7–10 Incidences of PV stenosis, thromboembolic complications, cardiac perforations with pericardial tamponade, esophageal fistulas, and phrenic nerve palsies (PNPs) have been reported.7,11
Clinical Perspective on p 1319
The more recently introduced strategy of using a cryoballoon catheter (CB) for PV isolation has produced encouraging results in a number of trials. Neumann et al12 documented maintained sinus rhythm over a median follow-up period of 12 months in 74% of patients with mainly paroxysmal or persistent AF who underwent the procedure. In a separate long-term study in patients with paroxysmal AF, freedom from AF recurrence was reported for 53% of patients after 5 years.13 A number of studies have compared ablation with CB with ablation with RF for PV isolation. In general, the obtained data demonstrate equivalent efficacy and similar safety profiles for the 2 techniques, although CB has been associated with a trend for reduced incidences of cardiac perforations with pericardial tamponade but with higher rates of PNP compared with RF ablation.14–17
Although these studies have provided a wealth of information on the 2 ablation energy sources, the absence of randomization in all but 1 of these trials is a significant drawback. The FreezeAF trial was designed to overcome this shortcoming by using a randomized, controlled, noninferiority design to directly compare RF with CB for treating patients with paroxysmal AF (http://www.clinicaltrials.gov; NCT00774566).
Methods
Study Design
FreezeAF is a randomized, controlled, prospective, noninferiority clinical trial designed to assess the efficacy and safety of PV isolation performed with either a CB or an irrigated RF ablation catheter.18 The main objective of the study was to determine whether cryoablation was not inferior to RF open irrigated tip ablation for the treatment of paroxysmal AF. The total follow-up period was 12 months, with additional evaluation of the end points carried out at 6 months after the index procedure.
All patients enrolled in the study provided written, informed consent. Furthermore, the trial protocol was approved by the ethics committee of the University of Freiburg on September 15, 2008, and the study was carried out in accordance with the Declaration of Helsinki.
Patients
Patients 18 to 75 years of age who had experienced at least 2 episodes of AF within the last 3 months were consecutively enrolled in the study. Further eligibility criteria were at least 1 episode of AF confirmed by ECG and documentation of at least 1 ineffective AAD treatment, including β-blockers. Patients were excluded if they had previously undergone left atrial (LA) ablation or surgery, if their LA was >55 mm in diameter, or if there was evidence of LA thrombus. Further exclusion criteria included unstable angina, myocardial infarction within the previous 3 months, cardiac surgery or percutaneous transluminal coronary angioplasty within the previous 3 months, an ejection fraction <40%, heart failure grade III to IV (New York Heart Association criteria), stroke or transient ischemic attack within the previous 6 months, pregnancy, or a life expectancy of <1 year. A full list of inclusion and exclusion criteria has previously been published.18 Preprocedurally, LA thrombi were excluded by transesophageal echocardiography. Additionally, the patients underwent either computed tomography or magnetic resonance imaging of the LA to determine physiological abnormalities and to exclude PV stenosis. Patients were assigned a CHA2DS2-VASc score to indicate the risk of thromboembolism and a HAS-Bled score to indicate the risk of bleeding.19,20 After providing consent, patients were randomized with an allocation ratio of 1:1 to either the CB or RF procedure. Random numbers were generated with SAS software (Cary, NC) in which block randomization with randomly selected block sizes was applied.
Ablation Procedures
For both groups of patients, a single or double transseptal puncture was performed after arterial and venous access had been achieved. PV angiography and measurement of PV potentials were carried out both before and after PV isolation with the use of a circular mapping catheter. RF-mediated antral ablation was performed with a 3.5-mm irrigated tip catheter in conjunction with a 3-dimensional navigation system (Ensite NavX/Velocity, St. Jude Medical, St. Paul, MN; Carto-3, Biosense Webster, Inc, Diamond Bar, CA). Cryoablation of the PV ostia was carried out with the Arctic Front Cryo Ablation Catheter System and FlexCath Steerable Sheath (Medtronic, Inc, Minneapolis, MN). The 28-mm CB was preferentially used; however, a switch to the 23-mm device was allowed if necessary, as were touchups with a conventional cryocatheter (Freezor Max, Medtronic, Inc). For each PV, at least 2 applications of cryoenergy 2 times for 300 seconds with first-generation CBs and 2 times for 240 seconds with second-generation CBs were used according to the manufacturer’s recommendation. Additional applications could be used if deemed necessary.
All patients received anticoagulation therapy during the 4 weeks before and for at least 6 months after the index procedure. Periprocedurally, administration of the anticoagulation therapy was uninterrupted, regardless of the selected drug. A bridging regimen with subcutaneous heparin was administered only if the patients received phenprocoumon and the international normalized ratio was <2 at the time of the procedure. Heparin was administered during the procedure to maintain an activated clotting time of 250 to 350 seconds. All AADs were discontinued 4 to 5 half-lives before the procedure. β-Blockers were the only AAD administered afterward. Patients were monitored during the hospital stay and at clinic visits at 3, 6, 9, and 12 months after the ablation procedure. A blanking period of 3 months was used. A further computed tomography or magnetic resonance imaging was carried out at the 3-month clinic visit to evaluate the PVs. Furthermore, at least one 24-hour Holter ECG was performed at the 3- and 9-month clinical visits to check for atrial arrhythmias. At 6 and 12 months, a 7- to 14-day Holter ECG was carried out to test for long-term recurrences. In the case of observed AF, a second ablation procedure was allowed starting 6 months after the index procedure, exclusively using the same energy source to which the patient had initially been randomized.
Outcomes
The primary end point was defined as the absence of atrial arrhythmias in combination with absence of persistent complications during the 6- and 12-month follow-up periods (coprimary end points). Persistent complications were defined as any new PV stenosis, PNP, cerebrovascular accident, bleedings, or vascular complications that occurred during or within after 48 hours after the procedure. Data of patients who either died or declined a clinical follow-up with rhythm assessment were considered missing. Adjudicators were not blinded to the group assignment on the determination of AF recurrence. Each documented episode of an atrial arrhythmia >30 seconds after the 3-month blanking period was considered a failure. The 6-month follow-up was used to evaluate the outcome of the index procedure; the 12-month visit additionally took into account any redo procedures. Secondary end points included procedural data, total radiation exposure, total procedure duration, and occurrence of adverse events, including PNP (assessed with breathing or pacing maneuvers at the discretion of the physician), pericardial effusion, and vascular complications. The assessment of quality of life was planned in the initial protocol version but was not further pursued. Major bleeding was defined as any bleeding or vascular complication requiring additional therapy. Minor bleeding was defined as any bleeding or vascular complication (hematoma, pseudoaneurysm, atriovenous fistula) that required prolonged hospitalization but could be managed conservatively. Detailed definitions of the components of each of the primary and secondary outcomes have been previously published.18
Statistical Analysis
Analysis of the primary end points was carried out for both the intention-to-treat (ITT) population and the per-protocol (PP) population. ITT analysis included all randomized patients who received study treatment who were evaluated in the group to which they were assigned, regardless of whether or not they completely adhered to the study protocol. Patients with major protocol violations were excluded from the PP analysis. Major protocol deviations were defined as AAD treatment after the ablation procedure, redo procedures before the 6-month follow-up, crossover, and LA ablation strategies other than PV isolation. Before the analysis, each patient was assigned to the appropriate population according to the occurrence of protocol deviations. For the purpose of statistical analysis, patients with protocol deviations during the 6-month follow-up were additionally excluded from the 12-month PP analysis set.
The test problem for the assessment of noninferiority was formulated in terms of the rate of achievement of the primary end point 12 months after the CB procedure (pCB) and after the RF procedure (pRF), whereas the null hypothesis was defined as follows: H0: pCB−pRF≤−δ, where δ=0.15.18
An analogous test problem was formulated for the related rates after the 6-month follow-up, with this null hypothesis tested only if that for the 12-month follow-up was rejected; otherwise, both null hypotheses were accepted. As a result of the application of this multiple testing procedure, the experiment-wise type I error rate was controlled in the strong sense. The tests were carried out by applying the noninferiority test for rates according to Farrington and Manning21 at a 1-sided significance level of α=2.5%. Multiple imputation was applied to deal with missing values for the primary end points. The multiple imputation by chained equations algorithm22,23 was used with a logistic regression model for the primary end points and the group assignment as the covariable. To adequately reflect the variability caused by imputed data, 100 imputed data sets were used. The results of the related analyses were pooled by the Rubin rule.24 An additional sensitivity analysis was performed, imputing all missing data sets as failures.
Descriptive statistics were used to describe the secondary variables, with means and standard deviations, medians and first and third quartiles, or absolute and relative frequencies given as appropriate. P values for these variables are not adjusted for multiplicity. For ordinal and continuous variables, the P values for treatment group comparisons were determined with the 2-sided Wilcoxon rank-sum test, whereas the 2-sided χ2 test was used to compare categorical variables. Statistical analysis was performed with R 3.13, with the multiple imputation by chained equations package version 2.22 used for multiple imputation.25
Sample Size Calculation
The originally planned sample size was calculated assuming equal rates of 0.78 for achieving the primary outcome, which was based on the findings of previous studies and experience with the procedures.3,12 To reach a power of 1−β = 80% for the 1-sided Farrington-Manning test at a level of α=2.5%, the required sample size was 2×122=244 patients.21 Owing to the uncertainty about the assumption of an overall rate of 0.78, a blinded sample size recalculation was prespecified in the protocol.18 The overall rate observed in March 2011 was 0.65, smaller than the anticipated rate. Using this rate for both groups but leaving all other quantities of the initial sample size calculation (especially the noninferiority margin) unchanged led to an increase in sample size to a total of 2×157=314 patients.
Results
Patients
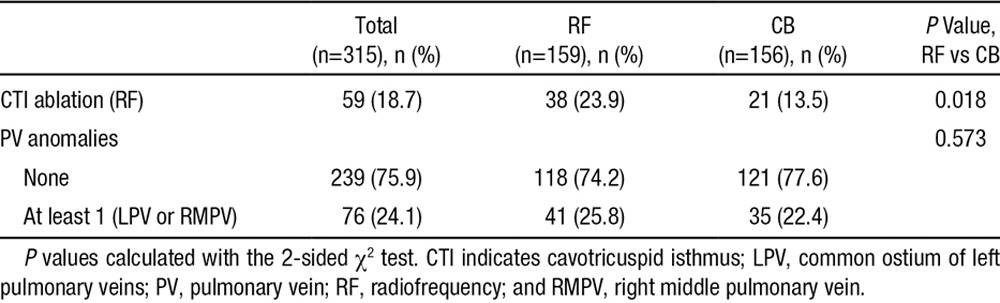
A total of 322 patients were included, with 7 not receiving treatment as part of the trial because of a withdrawal of consent but without their group assignment known, leaving 315 patients randomly assigned. This resulted in a final ITT population of 315 patients, 159 allocated to receive RF ablation and 156 allocated to receive CB ablation. Eleven patients had protocol deviations at 6 months (5 in RF, 6 in CB) and 33 at 12 months (18 in RF, 15 in CB), leaving 304 and 282 patients, respectively, for the PP analysis (Figure). More male than female patients underwent the procedure (60.6% male overall), with no significant difference in the proportions of each in the 2 groups (Table 1). In addition, the age of the patients did not vary between groups (P=0.871). In terms of comorbidities, there were no significant differences in the proportions of patients who presented with coronary artery disease, hypertension, diabetes mellitus, stroke, aortic insufficiency, mitral regurgitation, tricuspid valve insufficiency, or multiple valvular defects. There was a slightly lower proportion of patients with vascular disease in the CB group (P=0.028).
Table 1.
Patient Characteristics

Figure.
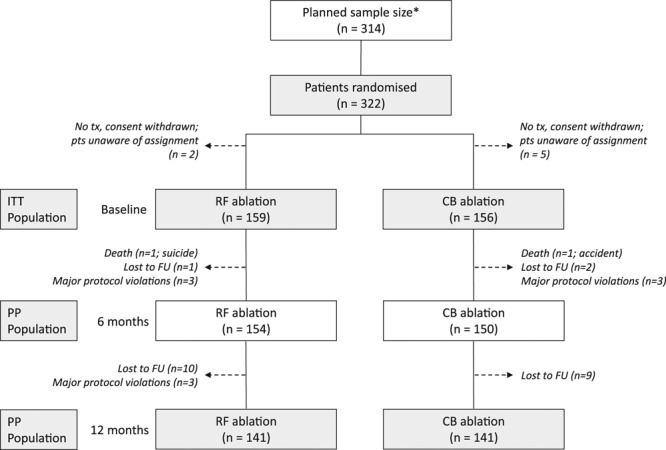
Patient flow, including planned sample size recalculation. *The initial sample size calculation resulted in 244 patients, which was readjusted in March 2011 after a prespecified blinded sample size recalculation (see Methods). Patients (pts) withdrawing consent before treatment (tx) were unaware of the assignment and were excluded from the population. Patients who refused a 12-month follow-up were contacted by phone to verify that they were alive although no heart rhythm was obtained. CB indicates cryoballoon ablation; FU, follow-up; ITT, intention to treat; PP, per protocol; and RF, radiofrequency catheter ablation.
Patients were treated with a variety of anticoagulants, with the majority in both groups receiving phenprocoumon (74.0%; Table 2). A high proportion of patients in each group were being treated with β-blockers (89.5%), and a relevant number were receiving an angiotensin-converting enzyme inhibitor (39.0%). Similar proportions of patients in each group were being treated with an angiotensin II type 1 antagonist, a diuretic, or a statin. Except for apixaban (4 patients in CB group, 0 in RF), there were no significant differences between the 2 treatment groups with regard to any of the prescribed medications.
Table 2.
Prior Medication

In terms of risk for thromboembolism and bleeding, the CHA2DS2-VASc and HAS-Bled indexes, respectively, were comparable for both treatment groups (Table 3).
Table 3.
Risk Indexes

Physiological anomalies of the PVs, for example, a common ostium of the left PVs or an additional right middle PV, were distributed equally in both groups. An additional ablation of the cavotricuspid isthmus as a result of documented typical atrial flutter before or during the procedure was less frequent in the CB group (13.5% versus 23.9% in RF; P=0.018; Table 4). Ablation of the cavotricuspid isthmus was always performed with either irrigated RF or nonirrigated RF energy.
Table 4.
Electrophysiological Study of the Heart
Primary End Point
The coprimary end points were defined as absence of atrial arrhythmias in combination with absence of persistent complications during the 6- and 12-month follow-up periods. In the ITT population, the coprimary end points were reached in 63.1% of patients in the RF group and 64.1% of patients in the CB group at 6 months and in 70.7% and 73.6% of patients, respectively, at 12 months. The increase in the rate of the primary end point was attributable to redo procedures, which were allowed only after 6 months. Sixty-two patients underwent redo procedures, 31 in each group (19.5% RF versus 19.9% CB; P=0.933).
With the use of the defined margin of δ=0.15 and a 1-sided significance level of α=2.5% for the Farrington-Manning test for noninferiority,21 the null hypothesis at 12 months could be rejected (H0: pCB−pRF≤−0.15; risk difference 0.029; 95% confidence interval [CI], −0.074 to 0.132; P<0.001; Table 5). We therefore tested the analogous null hypothesis at 6 months and found that it could also be rejected (risk difference, 0.010; 95% CI, −0.097 to 0.116; P=0.002). A sensitivity analysis in which missing values were imputed as failures confirmed these results (Table I in the online-only Data Supplement).
Table 5.
Primary End-Point Analysis

At the 12-month follow-up, the PP population comprised 141 patients in both the RF and CB treatment groups. At 6 months, there were 154 in the RF group and 150 in the CB group. With the same noninferiority margin of δ=0.15 and the same 1-sided significance level of α=2.5%, the null hypothesis at 12 months could be rejected (risk difference, 0.014; 95% CI, −0.089 to 0.117; P<0.001; Table 5). Subsequent analysis of primary end-point achievement at 6 months revealed that the null hypothesis could also be rejected for this end point (risk difference 0.010; 95% CI, −0.096 to 0.117; P=0.002).
Secondary End Points
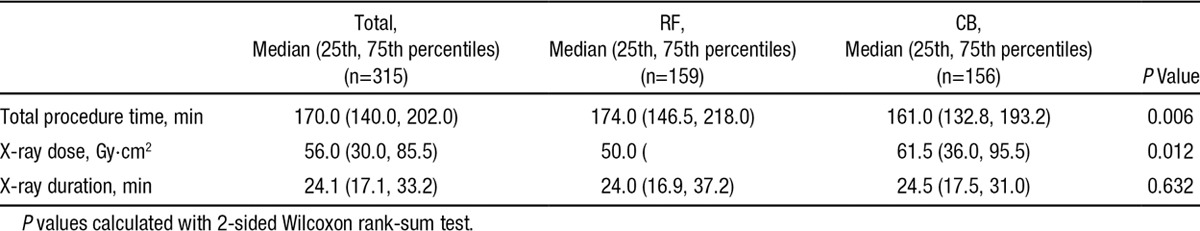
In 10 patients, a second CB of a different size (23 and 28 mm) was needed to achieve PV isolation. None needed a touchup with a conventional cryocatheter. The overall procedure time was ≈13 minutes shorter in the CB group (P=0.006). The x-ray duration was very similar in both groups (median, 24 minutes in RF and 25.5 minutes in CB; P=0.632), although the median of the total x-ray dosage was 11.5 Gy·cm2 higher for the CB procedure (P=0.012; Table 6) with an overall median x-ray dose of 56.0 Gy·cm2.
Table 6.
Secondary End Points
Complications
Few adverse events occurred during the index procedure in either treatment group (Table 7). Overall, major events occurred in 14 patients (4.4%), and minor events occurred in 13 patients (4.1%). The periprocedural complication rate was higher in the CB group (5.0% in the RF and 12.2% in the CB group; P=0.022). Specifically, 13 vascular complications (4.1%) were reported with no difference between groups, and 9 of them were classified as major. Overall, 5 pericardial effusions (1.6%) occurred. The 2 major events occurred in the CB group and needed pericardial drainage. All resolved without the need for surgery. In 9 patients, right PNP was observed during cryoablation of the right superior PV (5.8% of those with CB). Symptomatic PNPs were classified as major events (3 of 9). PNPs resolved in 4 of the 9 patients during the hospital stay, 2 before the 6-month follow-up and all before the 12-month follow-up (all were followed up throughout the 12 months). Recovery was demonstrated via fluoroscopy. No PV stenosis occurred in either group, and no transient ischemic attack/stroke was reported.
Table 7.
Number of Patients with Periprocedural Complications During the Index Procedure

Thirty-one redo procedures were performed in each group (total of 62), and 3 major complications were reported. In the RF group, 1 PV stenosis requiring PV stenting occurred, and in the CB group, 1 pericardial effusion requiring pericardial drainage and 1 vascular complication requiring additional compression therapy occurred. No PNP or transient ischemic attack/stroke was reported for the second procedure.
Discussion
The randomized FreezeAF study was carried out to comprehensively evaluate noninferiority of CB compared with RF ablation in terms of absence of atrial arrhythmias and persistent complications during a follow-up period of 12 months. Patients with paroxysmal AF were enrolled and randomized to undergo PV isolation with 1 of the 2 energy sources. Patient characteristics were similar to other reports of patients with paroxysmal AF with a low mean age and a low degree of comorbidity. The 2 treatment groups were well matched in terms of sex and age and had similar rates of comorbidities. Furthermore, there were no significant differences in the proportions of patients concurrently receiving any of the anticoagulants, β-blockers, or other cardiovascular-related medications. Electrophysiology studies revealed that patients undergoing the CB procedure had a slightly lower incidence of typical atrial flutter. Therefore, the rate of an additional ablation of the tricuspid isthmus was lower. The majority of patients in both groups were assigned a CHA2DS2-VASc score of 1 or 2, indicating an intermediate risk of stroke within a year, whereas a small proportion were at major risk.19 There were also no differences in terms of the 1-year risk score for predicting a major bleed, with the majority of individuals in both groups being assigned a score of 0 to 2.20
A number of studies have demonstrated the efficacy and safety of CB ablation for the treatment of AF12,13,26–28; however, it is still unclear how this strategy compares with the well-established RF method. Here, we demonstrated noninferiority of PV isolation with CB ablation compared with using RF for a primary end point of freedom from atrial arrhythmia combined with the absence of persistent complications. At both 6 and 12 months, CB was shown to be noninferior to RF within a margin of δ=0.15. A margin of 15%, which might be considered large, was deemed acceptable at the inception of the study, given the advantages of CB with respect to dimensions other the primary end point. Although this assessment was based on multiple reports at the time of study initiation, a recent meta-analysis has summarized the potential advantages of CB over RF.29 This meta-analysis, which included a total of 14 articles and 1104 patients, found fluoroscopy time (weight mean difference, −14.23 minutes; 95% CI, −25.45 to −2.82) and overall procedure time (weight mean difference, −29.65 minutes; 95% CI, −50.77 to 8.54) to be significantly shorter, with a nonsignificant increase in ablation time (weight mean difference, 11.66 minutes; 95% CI, −10.71 to 34.34). Furthermore, CB was found to be associated with a trend for fewer major complications (odds ratio, 0.46; 95% CI, 0.11–1.83). Finally, there is also evidence that the learning curve for CB ablation was much shorter than for RF ablation.30
Noninferiority was demonstrated for both the ITT and PP populations. Furthermore, the overall rate of complications was 8.6% with no persistent complications at 12 months. Previous nonrandomized trials have indicated that CB PV isolation is equivalent to RF in terms of achieving freedom from atypical atrial flutter and tachycardia in patients with paroxysmal AF but with a higher rate of PNP.14,17,31 Recently, although not directly comparable, Malmborg et al16 reported a small, randomized study comparing CB with the circular multipolar duty-cycled RF-based PV ablation catheter in patients with persistent or paroxysmal AF. They demonstrated freedom from AF in 52% and 38% of CB and duty-cycled RF patients, respectively, after 6 months (P=0.13), with values of 46% and 34%, respectively, at 12 months (P=0.21). Wasserlauf et al17 reported a single-center, prospective, cohort study in patients undergoing catheter ablation for paroxysmal AF using CB (n=101) or RF (n=100). Freedom from AF at 1 year was 60.3% in the CB group and 61.1% in the RF group (P=0.93). Overall complication rates were equivalent; however, fewer cardiac perforations occurred with CB (0% versus 4%; P=0.042).
In addition to efficacy, a number of other factors should be taken into account in the selection of the most suitable approach to treating a patient. Safety is of paramount importance, in terms of both periprocedural factors and complications identified during follow-up. Here, we found that the mean x-ray duration was equal in both groups, whereas the x-ray dose was slightly greater for the patients who underwent the CB procedure. Previous studies have found both longer14,31 and shorter16,17 fluoroscopy times for the CB procedure. The higher amount of the x-ray dose in the CB group is explained by the need for a higher resolution of the fluoroscopy image to prove balloon occlusion. The overall procedure time was significantly shorter for the cryoenergy technique. This result has been replicated in a number of other studies.17,32,33
Rates of adverse events were low in both treatment groups, making it difficult to draw definitive conclusions. In this study, periprocedural complications were lower in the RF group, which is in potential disagreement with the nonsignificant trend for fewer complications with CB reported in the aforementioned meta-analysis.29 However, the events in our study were influenced mainly by the number of (transient) PNPs in the CB group. By the time of the 6-month follow-up period, all but 3 cases of PNPs had been resolved, and at 12 months, all patients with PNPs had recovered, which is in agreement with other studies.26,32,34 PV stenoses, on the other hand, are recognized as complications associated with RF ablation.6,35,36 In the present study, there was a single PV stenosis in a redo procedure with RF energy. Vascular complications and pericardial effusion were comparable in both groups, although the pericardial effusions in the CB group needed to be drained. No transient ischemic attack/stroke occurred in either group.
Conclusions
In response to a need for more conclusive data on the efficacy and safety of CB for PV isolation, we carried out a randomized noninferiority study comparing the technique with the well-established RF ablation method. At both the 6- and 12-month follow-up, CB was demonstrated to be noninferior to RF in terms of freedom from AF and an absence of persistent complications.
Source of Funding
This is an investigator-initiated study. The study was supported with 20.000 EUR to acquire Holter monitor devices by CryoCath/Medtronic.
Disclosures
Dr Luik has received consulting fees/honoraria from Medtronic, Inc, Boston Scientific Corp, Biosense Webster, and St. Jude Medical. Dr Merkel has received consulting fees/honoraria from Medtronic, Inc, CardioFocus, Inc, and Boston Scientific Corp. Dr Schmitt has received consulting fees/honoraria from Pfizer, Inc, Bayer/Schering Pharma, Boehringer Ingelheim, and Medtronic, Inc. Dr Kieser was a member of the Data and Safety Monitoring Board of the FIRE AND ICE study (http://www.clinicaltrials.gov; identifier: NCT01490814). The other authors report no conflicts.
Supplementary Material
Footnotes
Presented as a Late-Breaking Clinical Trial at the 36th Annual Scientific Session of the Heart Rhythm Society; May 13–16, 2015; Boston, MA.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.016871/-/DC1.
CLINICAL PERSPECTIVE
This is the first large, randomized trial demonstrating that the cryoballoon technique is noninferior to the current gold standard of radiofrequency catheter ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Unlike the radiofrequency catheter ablation technique, cryoballoon is a single-shot device that does not require specially trained operators and an additional 3-dimensional mapping system. This leads to a shortening of the procedure time. However, cryoballoon was associated with the occurrence of phrenic nerve palsies, which completely resolved during the 12 months after the intervention.
References
- 1.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. doi: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. doi: 10.1016/S0002-9343(02)01236-6S. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder IC, Crijns HJ, Tieleman RG, Brügemann J, De Kam PJ, Gosselink AT, Verheugt FW, Lie KI. Chronic atrial fibrillation: success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med. 1996;156:2585–2592. doi: 10.1001/archinte.156.22.2585. doi: 10.1001/archinte.1996.00440210109011. [DOI] [PubMed] [Google Scholar]
- 4.Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012;5:CD005049. doi: 10.1002/14651858.CD005049.pub3. doi: 10.1002/14651858.CD005049.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Hollands JM, Gowan M, Riney JN, Deal EN, Kates AM. Role of new drugs for management of atrial fibrillation. Ann Pharmacother. 2012;46:1656–1670. doi: 10.1345/aph.1R155. doi: 10.1345/aph.1R155. [DOI] [PubMed] [Google Scholar]
- 6.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 7.Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace. 2015;17:370–378. doi: 10.1093/europace/euu376. doi: 10.1093/europace/euu376. [DOI] [PubMed] [Google Scholar]
- 8.Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clémenty J, Haïssaguerre M. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 9.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 10.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A RAAFT-2 Investigators. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 11.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–1803. doi: 10.1016/j.jacc.2009.02.022. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, Kurzidim K, Berkowitsch A, Koller M, Heintze J, Scholz U, Wetzel U, Schneider MA, Horstkotte D, Hamm CW, Pitschner HF. Circumferential pulmonary vein isolation with the cryoballoon technique: results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273–278. doi: 10.1016/j.jacc.2008.04.021. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Neumann T, Wójcik M, Berkowitsch A, Erkapic D, Zaltsberg S, Greiss H, Pajitnev D, Lehinant S, Schmitt J, Hamm CW, Pitschner HF, Kuniss M. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;15:1143–1149. doi: 10.1093/europace/eut021. doi: 10.1093/europace/eut021. [DOI] [PubMed] [Google Scholar]
- 14.Knecht S, Sticherling C, von Felten S, Conen D, Schaer B, Ammann P, Altmann D, Osswald S, Kühne M. Long-term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: a propensity score matched analysis. Int J Cardiol. 2014;176:645–650. doi: 10.1016/j.ijcard.2014.06.038. doi: 10.1016/j.ijcard.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Tse HF, Reek S, Timmermans C, Lee KL, Geller JC, Rodriguez LM, Ghaye B, Ayers GM, Crijns HJ, Klein HU, Lau CP. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol. 2003;42:752–758. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]
- 16.Malmborg H, Lönnerholm S, Blomström P, Blomström-Lundqvist C. Ablation of atrial fibrillation with cryoballoon or duty-cycled radiofrequency pulmonary vein ablation catheter: a randomized controlled study comparing the clinical outcome and safety; the AF-COR study. Europace. 2013;15:1567–1573. doi: 10.1093/europace/eut104. doi: 10.1093/europace/eut104. [DOI] [PubMed] [Google Scholar]
- 17.Wasserlauf J, Pelchovitz DJ, Rhyner J, Verma N, Bohn M, Li Z, Arora R, Chicos AB, Goldberger JJ, Kim SS, Lin AC, Knight BP, Passman RS. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38:483–489. doi: 10.1111/pace.12582. doi: 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
- 18.Luik A, Merkel M, Hoeren D, Riexinger T, Kieser M, Schmitt C. Rationale and design of the FreezeAF trial: a randomized controlled noninferiority trial comparing isolation of the pulmonary veins with the cryoballoon catheter versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation. Am Heart J. 2010;159:555–560.e1. doi: 10.1016/j.ahj.2010.01.008. doi: 10.1016/j.ahj.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 20.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 21.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9:1447–1454. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
- 22.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 23.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Software. 2011;45:1–67. [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 25.R Core Team. R: A LANGUAGE and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 26.Klein G, Oswald H, Gardiwal A, Lüsebrink U, Lissel C, Yu H, Drexler H. Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008;5:802–806. doi: 10.1016/j.hrthm.2008.02.014. doi: 10.1016/j.hrthm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 28.Vogt J, Heintze J, Gutleben KJ, Muntean B, Horstkotte D, Nölker G. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J Am Coll Cardiol. 2013;61:1707–1712. doi: 10.1016/j.jacc.2012.09.033. doi: 10.1016/j.jacc.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Huang Y, Cai H, Qi Y, Jia N, Shen W, Lin J, Peng F, Niu W. Is cryoballoon ablation preferable to radiofrequency ablation for treatment of atrial fibrillation by pulmonary vein isolation? A meta-analysis. PLoS One. 2014;9:e90323. doi: 10.1371/journal.pone.0090323. doi: 10.1371/journal.pone.0090323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein G, Gardiwal A, Oswald H. Catheter-based cryoablation of atrial fibrillation: state of the art. Minerva Cardioangiol. 2008;56:623–633. [PubMed] [Google Scholar]
- 31.Schmidt M, Dorwarth U, Andresen D, Brachmann J, Kuck KH, Kuniss M, Lewalter T, Spitzer S, Willems S, Senges J, Jünger C, Hoffmann E. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: results from the German Ablation Registry. J Cardiovasc Electrophysiol. 2014;25:1–7. doi: 10.1111/jce.12267. doi: 10.1111/jce.12267. [DOI] [PubMed] [Google Scholar]
- 32.Mugnai G, Chierchia GB, de Asmundis C, Sieira-Moret J, Conte G, Capulzini L, Wauters K, Rodriguez-Mañero M, Di Giovanni G, Baltogiannis G, Ciconte G, Saitoh Y, Juliá J, Brugada P. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am J Cardiol. 2014;113:1509–1513. doi: 10.1016/j.amjcard.2014.01.425. doi: 10.1016/j.amjcard.2014.01.425. [DOI] [PubMed] [Google Scholar]
- 33.Herrera Siklódy C, Arentz T, Minners J, Jesel L, Stratz C, Valina CM, Weber R, Kalusche D, Toti F, Morel O, Trenk D. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: a randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012;9:189–196. doi: 10.1016/j.hrthm.2011.09.017. doi: 10.1016/j.hrthm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Chun KR, Schmidt B, Metzner A, Tilz R, Zerm T, Köster I, Fürnkranz A, Koektuerk B, Konstantinidou M, Antz M, Ouyang F, Kuck KH. The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J. 2009;30:699–709. doi: 10.1093/eurheartj/ehn570. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu WC, Hsu TL, Tai CT, Tsai CF, Hsieh MH, Lin WS, Lin YK, Tsao HM, Ding YA, Chang MS, Chen SA. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:887–892. doi: 10.1046/j.1540-8167.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 36.Saad EB, Marrouche NF, Saad CP, Ha E, Bash D, White RD, Rhodes J, Prieto L, Martin DO, Saliba WI, Schweikert RA, Natale A. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med. 2003;138:634–638. doi: 10.7326/0003-4819-138-8-200304150-00010. doi: 10.7326/0003-4819-138-8-200304150-00010. [DOI] [PubMed] [Google Scholar]


