Abstract
Approach, avoidance and the supervisory control system are fundamental to human behavior. Much past research has examined the neurophysiological models relating trait approach and avoidance. Using measures of electroencephalographic (EEG) frontal asymmetry, trait approach has been associated with greater left-frontal activity and trait avoidance has been associated with greater right-frontal activity. However, traits related to the supervisory control system have not been previously associated with frontal asymmetry. The current study sought to test whether trait positive urgency, measuring the tendency towards rash action in response to extreme positive emotional states, would relate to frontal alpha asymmetry. One hundred twenty-six individuals completed a measure of positive urgency and resting EEG recordings. Greater positive urgency was associated with greater relative left-frontal EEG activity. Source localization revealed that this relationship appeared to originate from reduced right-frontal activity in the inferior frontal gyrus. These results clarify that the link between frontal asymmetry and positive urgency is related to reduced right-frontal activity. Reduced right-frontal activity may be a potential neurobiological trait related to the supervisory control system.
Keywords: frontal asymmetry, impulsivity, positive urgency, approach motivation
At the core of human functioning are three personality systems of approach, avoidance and supervisory control. Approach motivational responses have been theorized to be part of a behavioral approach system (BAS; Gray, 1970, 1987; Gray and McNaughton, 2000), behavioral activation system (also BAS; Fowles, 1987), behavioral facilitation system (Depue and Collins, 1999) and goal-approach system (Carver and Scheier, 2008; Elliott, 2008). In contrast, avoidance motivational responses have been theorized to be part of a withdrawal or freezing system and have been referred to as a behavioral inhibition system (BIS; Gray, 1970, 1987), fight-flight-freeze system (Gray and McNaughton, 2000) and threat avoidance system (Carver and Scheier, 2008; Elliott, 2008). Essential to the approach and avoidance system is a third supervisory control system theorized to generate effortful control, constraint, self-control (Kochanska and Knaack, 2003; Rothbart and Rueda, 2005; Nigg, 2006; Carver and Connor-Smith, 2010) and is linked to cognitive constructs of executive control and inhibitory function (Aron et al., 2004, 2014; Hester and Garavan, 2009). Generally, the supervisory system is in place to regulate both the approach and avoidance systems using effortful control to override motivational impulses (Carver and Connor-Smith, 2010). This system is thought to be inversely related to trait impulsivity, because trait impulsivity is strongly related to deficits in inhibitory control, effortful control and executive functions (Logan et al., 1997; Enticott et al., 2006).
In the past two decades, many biological models have been based on dimensions of approach and avoidance (see Fowles, 2002; Gray, 1994; Depue and Collins, 1999; Elliott and Thrash, 2002; Caspi et al., 2005; Rothbart and Hwang, 2005; Caspi and Shiner, 2006; Gable and Harmon-Jones, 2010). These models propose that approach and avoidance systems are related to distinct brain areas, and that individual differences in trait neural processes may reflect the sensitivity of each system. For much of the past century, research has demonstrated that the left and right-frontal cortical regions are asymmetrically related to approach and avoidance motivational and emotional (emotive) tendencies. Specifically, the left-frontal cortex is associated with emotive processes related to approach, whereas the right-frontal cortex is associated with emotive processes related to withdrawal (Goldstein, 1939; Rossi and Rosadini, 1967). In humans, approach and avoidant asymmetrical activations measured by suppression of the alpha frequency band activity during resting or baseline electroencephalographic (EEG) recordings appear as stable traits (for reviews see Coan and Allen, 2004; Harmon-Jones et al., 2010). Because of the strong association between motivational direction and frontal asymmetry, frontal asymmetry has been linked to trait measures of motivational direction using the BIS/BAS derived by Carver and White (1994). Greater BAS is associated with greater left-frontal activation (Harmon-Jones and Allen, 1997; Harmon-Jones and Sigelman, 2001; Coan and Allen, 2003; Harmon-Jones, 2006; Amodio et al., 2008; Gable and Harmon-Jones, 2008; Harmon-Jones et al., 2009, 2010; De Pascalis et al., 2013), and greater BIS is associated with greater right-frontal activation (Sutton and Davidson, 1997; Balconi and Mazza, 2009; Shackman et al., 2009; Balconi, 2011).
In contrast to the strong link between frontal asymmetry and approach/avoidance systems, past research has almost entirely neglected the relationship between frontal asymmetry and the supervisory control system. Some recent work has hypothesized that frontal asymmetry may be associated with traits and behaviors related to the supervisory control system (Grimshaw and Carmel, 2014). For example, greater baseline left-frontal activation is associated with trait sensation seeking (Santesso et al., 2008), and right-frontal theta and delta activity relate to greater behavioral risk taking (Gianotti et al., 2009). Some work has suggested that this asymmetric activity may relate to the right inferior frontal gyrus (for review see, Aron et al., 2014). For example, the right inferior frontal gyrus has been linked with response inhibition on a go/no-go task (Schiller et al., 2013) and inability to ignore drug-related cues cues in active cocaine users (Hester and Garavan, 2009). In sum, this past work suggests that the supervisory control system may be asymmetrically related to frontal-cortical activity (Aron et al., 2004; Knoch et al., 2006; Peterson et al., 2008; Cyders et al., 2014). However, to date research has not forged a connection between trait asymmetrical alpha activity and traits related to the supervisory system, such as impulsivity.
Research investigating the importance of trait impulsivity has begun to focus on trait urgency, or the tendency to act impulsively during intense emotional states. Along these lines, Cyders et al. (2007) developed the construct of positive urgency, or the tendency to act impulsively when experiencing positive emotions. Positive emotion-based urgency appears to play a role in a number of important domains such as drinking behavior (Cyders et al., 2009; 2010; Wray et al., 2012), drug use (Zapolski et al., 2009), risky driving behaviors (Pearson et al., 2013) and sexual aggression (Mouilso et al., 2013). Although much past work investigating positive urgency as a risk factor demonstrates that positive urgency reflects failure of the supervisory control system, the neurophysiological mechanisms associated with positive urgency are unclear. Because positive urgency appears to be a stable facet of impulsivity, it is likely related to trait neurophysiological processes such as frontal asymmetry. In addition, past work demonstrating asymmetrical inhibitory function suggests that the pre-potent reward-based responding as measured by positive urgency would require supervisory control to maintain long term goals. Frontal asymmetry may serve as a biomarker of an individual’s tendency towards rash action. Revealing such relationships is part of a growing recognition of the importance of identifying neurophysiological markers associated with personality traits.
In this study we examined whether resting frontal asymmetry is related to trait positive urgency, BAS and BIS. We hypothesize that trait positive urgency will be associated with an increase in relative left (vs right) frontal activity. Consistent with past research linking reduced right-frontal activity and impulsive behaviors, we hypothesize that relatively greater left-frontal activity may result from a decrease in right-frontal activity.
Materials and Methods
Participants
One hundred twenty-six (68 female, 58 male) right-handed introductory psychology students participated in exchange for course credit.
Procedure
Participants completed the study individually. First, participants were asked to complete individual difference measures of handedness, BIS/BAS and positive urgency. Following the completion of the questionnaires, EEG electrodes were applied, and resting EEG activity was assessed for 8 min. Handedness was assessed by asking participants to report which hand they use to perform 13 simple behaviors (i.e. write, use a hammer, hold a match when striking it). All participants were right-handed.
Trait positive urgency
The positive urgency measure (PUM) was developed to identify the tendency to engage in impulsive behaviors when in a positive mood (Cyders et al., 2007). Positive urgency is measured across 14 items, such as, ‘I am surprised at the things I do while in a great mood’; ‘When I get really happy about something, I tend to do things that can have bad consequences’ (Cyders et al., 2007, p. 110). Higher PUM scores indicate greater levels of impulsive tendencies during positive moods. Positive urgency has been identified as a component of impulsivity independent from BAS (Cyders and Smith, 2007). Data from two participants were not included because they failed to complete the PUM.
Trait BIS/BAS
The BIS/BAS scales contain three subscales of BAS and one scale of BIS assessed across 20 items. BIS is assessed through seven items and relates to responses in anticipation of punishment. The following item is an example of the BIS component: ‘I worry about making mistakes’. Higher BIS scores indicate greater levels of behavior inhibition. The three subscales of BAS include: BAS Reward Responsiveness, BAS Drive and BAS Fun-Seeking. BAS Reward Responsiveness is assessed through five items that measure response to the anticipation of reward. BAS Drive looks at persistent goal pursuit through four items. BAS Fun-Seeking is comprised of four items reflecting a desire for new rewards and a willingness to approach potential rewards. All BAS items from each subscale were averaged to obtain an overall index score of BAS; higher BAS scores indicate greater levels of approach motivation. We report means, standard deviations and Cronbach’s alphas for PUM, BIS, BAS and BAS subscales in Table 1.
Table 1.
Means and SDs of PUM, BIS and BAS
| Scale | Mean (SD) | Cronbachs (α) |
|---|---|---|
| PUM | 2.01 (0.71) | 0.92 |
| BIS | 2.87 (0.52) | 0.73 |
| BAS | 3.11 (0.35) | 0.81 |
| BAS RR | 3.44 (0.42) | 0.74 |
| BAS DRIVE | 2.79 (0.55) | 0.73 |
| BAS FUN | 2.98 (0.54) | 0.62 |
Note. Possible ranges for each scale are the following: for PUM, 1–5; for BIS/BAS, 1–7. Means and standard deviations (in parentheses) for PUM, BIS and BAS scales are from the current sample. PUM, positive urgency measure; BIS, behavioral inhibition system; BAS, behavioral activation system; BAS RR, behavioral activation system reward responsiveness; BAS DRIVE, behavioral activation system drive; BAS FUN, behavioral activation fun seeking.
EEG assessment and processing
EEG was recorded using a stretch lycra cap with 64 mounted tin electrodes (Electro-Caps, Eaton, OH). EEG activity was referenced to an electrode placed on the left earlobe and a ground electrode was mounted midway between FPZ and FZ. Electrode impedances were under 5000 Ω and homologous sites were within 1000 Ω of each other. Signals were amplified using Neuroscan SynAmps RT amplifier unit (El Paso, TX). Signals were low-pass filtered at 100 Hz, high-pass filtered at 0.05 Hz, notched filtered at 60 Hz and digitized at 2000 Hz.
Eight minutes of resting data were acquired while participants focused their gaze in front of them; 4 min with eyes open (O) and 4 min with eyes closed (C). Two sequences were used and were alternated between participants: C-O-O-C-O-C-C-O and O-C-C-O-C-O-O-C. Artifacts (e.g. aberrant signals due to muscle movement or large non-blink eye movements) were removed manually. Following the removal of artifacts, a regression-based eye movement correction was utilized to remove blinks from the data files (Semlitsch et al., 1986). Lastly, the data were visually inspected ensuring proper correction.
Consistent with past studies measuring trait frontal-cortical activation using alpha band power (see Coan and Allen, 2004; Harmon-Jones et al., 2010 for reviews), power spectra epochs 1.024 s in duration were extracted through a Hamming window (50% taper of distal ends). Alpha power is inversely related to regional brain activity as evidenced by hemodynamic measures (Cook et al., 1998; Goldman et al., 2002; Feige et al., 2005) verbal tasks, (Davidson et al., 1990; Jauk et al., 2012), and motor tasks (Harmon-Jones, 2006; Gable et al., 2013). Data were re-referenced using a common average reference. Consecutive epochs were overlapped by 50% to minimize data loss due to windowing. We investigated the classical alpha broadband within 8–13 Hz (Shackman et al., 2010). Power values were obtained using a fast Fourier transformation and aggregated across all resting minutes. Consistent with much past work investigating frontal asymmetry (Harmon-Jones et al., 2011; Stewart et al., 2011), asymmetry indexes (log right minus log left) were computed for homologous sites F6/5 and F8/7. Index scores were created by averaging the asymmetry indices. Because alpha power is inversely related to cortical activity (Lindsley and Wicke, 1974), higher scores indicate greater left hemisphere activity. These sites were aggregated to create an index of relative left-frontal activity. Data from five participants were not recorded due to equipment malfunction. One participant was excluded because their baseline activity was >3 SDs from the mean. In order to examine whether heterogeneity in trait positive urgency, BIS and BAS is associated with individual differences in resting frontal activity, we conducted individual regression analyses testing whether each self-report measure relates to the index of relative left-frontal activity.
Source Localization
We utilized standardized low-resolution brain electromagnetic tomography (sLORETA) to estimate the intracerebral electrical sources that generated the scalp-recorded alpha band frequency activity (Pascual-Marqui, 2002). sLORETA computes electric neuronal activity as current density and has been validated in comparison with fMRI, MRI and PET (Dierks et al., 2000; Worrell et al., 2000; Vitacco et al., 2002; Mulert et al., 2004; Pizzagalli et al., 2005; Zumsteg et al., 2006). Using the electrode positions determined by the MNI 152 scalp, the subcortical areas are partitioned in 6239 voxels at 5 × 5 × 5 mm spatial resolution. We report areas of neural activity in accordance to standard anatomical labels using MNI space corrected to Talairach space.
Results
Relationship between frontal activity and positive urgency
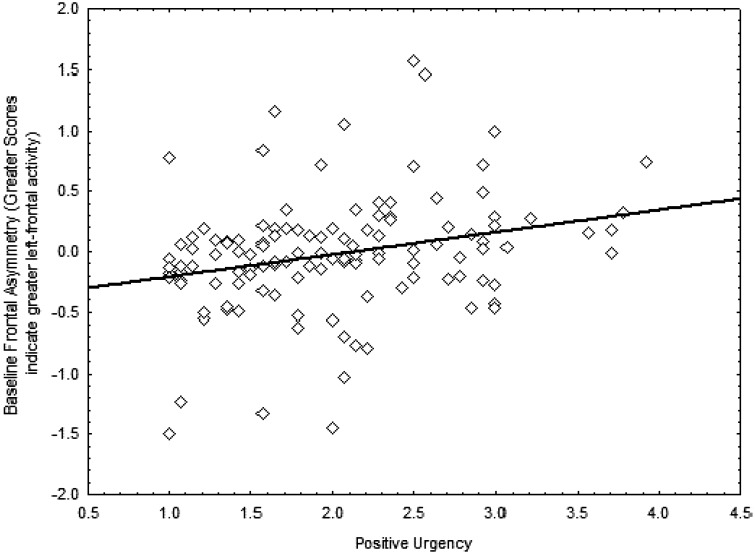
We first examined whether heterogeneity in trait positive urgency can be associated with individual differences in baseline frontal activity. Frontal asymmetry was positively related to positive urgency, β = 0.27 [0.09, 0.44], t(119) = 3.05, P = 0.003 (see Figure 1). Individuals with greater left-frontal activity at baseline reported greater trait positive urgency.
Fig. 1.
Relationship between greater resting left-frontal activity and positive urgency.
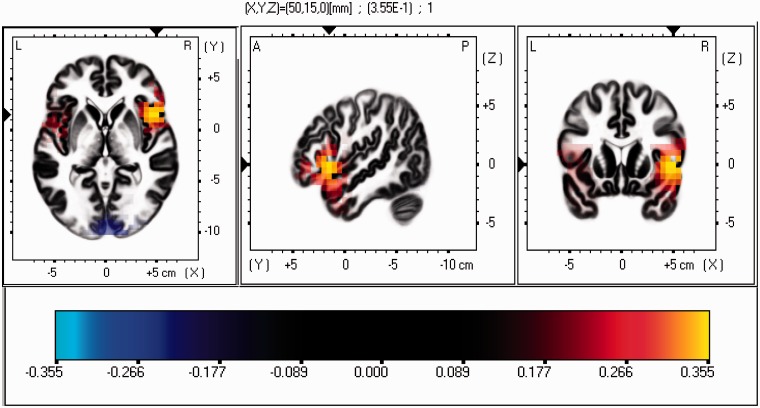
Based on sLORETA statistics sub-program and visual inspection, current source density analyses of the relationship between PUM scores and alpha power identified the right inferior frontal gyrus (MNI coordinates: X = 50, Y = 15, Z = 0) as the origin of this relationship (See Figure 2). These results suggest that PUM scores relate to reduced right-frontal activity at the inferior frontal gyrus.
Fig. 2.
Current source density analyses of the correlation between positive urgency and alpha power (less cortical activity) in the frontal cortex revealed the origin at the right inferior frontal gyrus (MNI coordinates: X = 50, Y = 15, Z = 0). The strength of the correlation coefficient is identified by the color scale. Red/yellow source localizations are associated with less cortical activity (greater alpha power).
Relationship between frontal activity and BIS/BAS
Next, we examined whether BIS/BAS scores were associated with individual differences in resting frontal activity. The frontal asymmetry index was not correlated with BIS, β = 0.10 [−0.08, 0.28], t(119) = 1.10, P = 0.27. Also, frontal asymmetry was not correlated with BAS, β = −0.08 [−0.26, 0.10], t(118) = −0.88, P = 0.38. Positive urgency remained a moderate predictor of left-frontal activity when controlling for BIS, β = 0.27 [0.10, 0.45], t(118) = 3.11, P = 0.002, or BAS, β = 0.26 [0.09, 0.44], t(117) = 2.96, P = 0.004. See Table 2 for the relationships between PUM, BIS and BAS. Results suggest that BIS/BAS did not relate to frontal asymmetry in the current sample.
Table 2.
Correlations between PUM and BIS, BAS and BAS subscales
| Scale | Pearsons r | P-value |
|---|---|---|
| BIS | −0.14 | 0.15 |
| BAS | −0.12 | 0.21 |
| BAS RR | −0.31 | 0.00* |
| BAS DRIVE | −0.08 | 0.42 |
| BAS FUN | 0.11 | 0.24 |
PUM, positive urgency measure; BIS, behavioral inhibition System; BAS, behavioral activation system; BAS RR, behavioral activation system reward responsiveness; BAS DRIVE, behavioral activation system drive; BAS FUN, behavioral activation fun seeking.
Our confidence intervals (CIs) are a range of plausible values for the relationship between positive urgency and left-frontal activity. Values outside the CI are relatively implausible. The lower bound estimate (lower limit) suggests that positive urgency remains a small predictor of greater left-frontal-cortical activity.
Discussion
This study revealed that baseline frontal-cortical activity measured through frontal asymmetry is associated with greater trait positive urgency. Consistent with predictions, greater relative left-frontal activity related to greater trait impulsivity. Source localization of this relationship revealed its origin as reduced activity in the right inferior frontal gyrus. These results suggest that the relationship between greater relative left-frontal activity and positive urgency stem from relatively greater left-frontal activity because of less right-frontal activity in the inferior frontal gyrus. Reduced right-frontal cortical activity seems to be associated with reduced functioning of the supervisory control system. Greater relative left-frontal asymmetry has predominantly been associated with approach temperament and behaviors. These new findings suggest that greater relative left-frontal asymmetry associated with the supervisory control system is driven by reduced right-frontal activation.
Past research suggests that reduced right-frontal activity through temporary or permanent lesions results in greater approach-related behaviors such as mania or aggression (Sackeim et al., 1982; d’Alfonso et al., 2000). Other work suggests that right-frontal activity relates to the supervisory control system, as evidenced by enhanced impulsivity (Knoch et al., 2006; Aron et al., 2014). The current findings provide new insight into the link between personality traits and neurophysiological markers. Previous research has neglected to research the connection between asymmetric cortical activity specific to the alpha band (inverse of cortical activity) and trait individual differences in impulsivity. This is the first instance of research exploring the relationship between positive urgency and baseline cortical activity.
Results from this study suggest that reduced right-frontal cortical activity may be a neurophysiological marker of an individual’s propensity towards rash action under intense positive emotional states. Positive urgency is a unique personality construct that predicts a wide range of risky behaviors. The current results suggest that positive urgency is related to reduce right-frontal activity. Baseline cortical asymmetry may play a role in promoting risky behaviors. Better understanding the neurophysiological correlates of positive urgency may contribute to our understanding of how urgency contributes to substance use and pathologies associated with impulsivity. For example, the neural correlates associated with positive urgency may shed light on pathological and addictive behaviors. Future research should investigate the potentially mediational role of frontal asymmetry in disorders and behaviors associated with positive urgency.
The current results did not find that trait behavioral approach sensitivity related to baseline frontal asymmetry. However, much past research demonstrates that greater left-frontal activation evoked by approach-motivated emotional states is related to individual differences in approach motivation (Harmon-Jones et al., 2010; Gable and Poole, 2012, 2014). Perhaps the link between approach/avoidance systems and frontal asymmetry may be largely driven by situational context, such as emotional/motivation states. The relationship between individual differences in frontal asymmetry and approach/avoidance systems may be more pronounced in the context of emotional responses (Coan et al., 2006). However, because the current research found a robust association between positive urgency and baseline frontal activity, the link between baseline frontal asymmetry and trait impulsivity may be less influenced by situational context.
Of note in the current findings is that positive urgency and greater relative left-frontal cortical activity have been associated with positive affect. However, it is unlikely that the current results are dependent on positive mood. Past work examining greater relative left-frontal activity has linked greater left-frontal activity with negative affects such as anger (Poole and Gable, 2014), suggesting that it is approach motivation, rather that positive affect that evokes relatively greater left-frontal activity. However, it is unlikely that the association between greater left-frontal asymmetry and positive urgency is due to enhanced trait approach motivation. In the current findings, the relationship between relative left-frontal cortical activity and positive urgency was not diminished when controlling for the variance in behavioral approach. Examination of the psychometric properties of PUM and BAS reveals that positive urgency represents a distinct factor from those represented by the subscales of BAS (Cyders et al., 2007). Indeed, the current findings support this distinction; overall, positive urgency was unrelated to the BAS scales. Moreover, positive urgency, but not BAS scales, related to resting frontal asymmetry. Future research is needed to examine the neural correlates of the supervisory control system governing approach and avoidance motivational states.
Investigating neurophysiological measures associated with traits related to impulsivity are key to better understanding the supervisory control system mediating the approach and avoidance systems. The current results help to clarify that the link between trait positive urgency and greater left-frontal activity is driven by reduced right-frontal activity. Because much past work has associated frontal asymmetry with approach and avoidant systems, the current results suggest that deficits in the supervisory control system may be related to neural substrates associated with these motivational systems.
This is the first study to link the trait neurophysiological marker of resting alpha asymmetry with trait impulsivity, as measured by positive urgency. These results suggest a potential underlying neurobiological mechanism for the development and maintenance of trait positive urgency. Emotion-based rash action associated with relatively greater left-frontal activity may be a means through which individuals have increased reactive approach-related tendencies and affect. These results are in line with a growing recognition of the importance of identifying neural or neurophysiological markers of personality traits related to core systems of human behavior (Nusslock et al., 2012; Cyders et al., 2014). Such markers can increase understanding of the physiology of traits and the underlying mechanisms of these systems.
Conflict of Interest
None declared.
References
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45(1):11–9. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences. 2014;18(4):177–85. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Balconi M. Frontal brain oscillation modulation in facial emotion comprehension: the role of reward and inhibitory systems in subliminal and supraliminal processing. Journal of Cognitive Psychology. 2011;23(6):723–35. [Google Scholar]
- Balconi M, Mazza G. Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues: ERS/ERD and coherence measures of alpha band. International Journal of Psychophysiology. 2009;74(2):158–65. doi: 10.1016/j.ijpsycho.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Carver CS, Connor-Smith J. Personality and coping. Annual Review of Psychology. 2010;61:679–704. doi: 10.1146/annurev.psych.093008.100352. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Feedback processes in the simultaneous regulation of action and affect. In: Shah JY, Gardner WL, editors. Handbook of Motivation Science. New York: Guilford Press; 2008. pp. 308–24. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319–33. [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annual Review of Psychology. 2005;56:453–84. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Caspi A, Shiner RL. Personality development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of Child Psychology: Vol. 3. Social, Emotional, and Personality Development. New York: Wiley; 2006. pp. 300–65. [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–14. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67(1):7–50. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology. 1998;107(6):408–14. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi KA, Kareken DA. Negative urgency mediates the relationship between amygdala and orbitofrontal cortex activation to negative emotional stimuli and general risk-taking. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu123. doi: 10.1093/cercor/bhu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Mood-based rash action and its components: Positive and negative urgency. Personality and Individual Differences. 2007;43(4):839–50. [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development andvalidation of a measure of positive urgency. Psychological Assessment. 2007;19(1):107–18. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104(2):193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Zapolski TC, Combs JL, Settles RF, Fillmore MT, Smith GT. Experimental effect of positive urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychology of Addictive Behaviors. 2010;24(3):367–75. doi: 10.1037/a0019494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Alfonso AA, van Honk J, Hermans E, Postma A, de Haan EH. Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subjects. Neuroscience Letters. 2000;280(3):195–8. doi: 10.1016/s0304-3940(00)00781-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27(5):528–43. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Cozzuto G, Caprara GV, Alessandri G. Relations among EEG-alpha asymmetry, BIS/BAS, and dispositional optimism. Biological Psychology. 2013;94(1):198–209. doi: 10.1016/j.biopsycho.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Dierks T, Jelic V, Pascual-Marqui RD, et al. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer’s disease. Clinical Neurophysiology. 2000;111(10):1817–24. doi: 10.1016/s1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, editor. Handbook of Approach and Avoidance Motivation. Mawah, NJ: Erlbaum; 2008. [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82(5):804–18. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Ogloff JR, Bradshaw JL. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Personality and Individual Differences. 2006;41(2):285–94. [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. Journal of Neurophysiology. 2005;93(5):2864–72. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. Journal of Research in Personality. 1987;21(4):417–35. [Google Scholar]
- Fowles DC. Comprehensive Handbook of Psychopathology. New York: Springer US; 2002. Biological variables in psychopathology: a psychobiological perspective; pp. 85–104. [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychological Science. 2008;19(5):476–82. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. The blues broaden, but the nasty narrows attentional consequences of negative affects low and high in motivational intensity. Psychological Science. 2010;21(2):211–5. doi: 10.1177/0956797609359622. [DOI] [PubMed] [Google Scholar]
- Gable PA, Poole BD. Time flies when you’re having approach-motivated fun effects of motivational intensity on time perception. Psychological Science. 2012;23(8):879–86. doi: 10.1177/0956797611435817. [DOI] [PubMed] [Google Scholar]
- Gable PA, Poole BD. Influence of trait behavioral inhibition and behavioral approach motivation systems on the LPP and frontal asymmetry to anger pictures. Social Cognitive and Affective Neuroscience. 2014;9(2):182–90. doi: 10.1093/scan/nss130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable PA, Poole BD, Cook MS. Asymmetrical hemisphere activation enhances global–local processing. Brain and Cognition. 2013;83(3):337–41. doi: 10.1016/j.bandc.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Gianotti LR, Knoch D, Faber PL, et al. Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychological Science. 2009;20(1):33–8. doi: 10.1111/j.1467-9280.2008.02260.x. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13(18):2487–92. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein K. The Organism: A Holistic Approach to Biology Derived from Pathological Data in Man. New York, NY: American Book; 1939. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8(3):249–66. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Gray JA. Personality dimensions and emotion systems. In: Ekman P, Davidson RJ, editors. The Nature of Emotion: Fundamental Questions. New York: Oxford University Press; 1994. pp. 329–31. [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 2000. [Google Scholar]
- Grimshaw GM, Carmel D. An asymmetric inhibition model of hemispheric differences in emotional processing. Frontiers in Psychology. 2014;5:489. doi: 10.3389/fpsyg.2014.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E. Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology. 2006;43(6):598–603. doi: 10.1111/j.1469-8986.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–63. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84(3):451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Serra R, Gable PA. The effect of commitment on relative left frontal cortical activity: tests of the action-based model of dissonance. Personality and Social Psychology Bulletin. 2011;37(3):395–408. doi: 10.1177/0146167210397059. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK, Harris CR. Jealousy: novel methods and neural correlates. Emotion. 2009;9(1):113–7. doi: 10.1037/a0014117. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80(5):797–803. [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacology Biochemistry and Behavior. 2009;93(3):270–7. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Jauk E, Benedek M, Neubauer AC. Tackling creativity at its roots: evidence for different patterns of EEG alpha activity related to convergent and divergent modes of task processing. International Journal of Psychophysiology. 2012;84(2):219–25. doi: 10.1016/j.ijpsycho.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. The Journal of Neuroscience. 2006;26(24):6469–72. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful control as a personality characteristic of young children: Antecedents, correlates, and consequences. Journal of Personality. 2003;71(6):1087–112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Lindsley DB, Wicke JD. The electroencephalogram: autonomous electrical activity in man and animals. Bioelectric Recording Techniques. 1974;1(part B):3–83. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–4. [Google Scholar]
- Mouilso ER, Calhoun KS, Rosenbloom TG. Impulsivity and sexual assault in college men. Violence and Victims. 2013;28(3):429–42. doi: 10.1891/0886-6708.vv-d-12-00025. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Schmitt R, et al. Integration of fMRI and simultaneous EEG: Towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urosevic S, Goldstein K, Abramson LY. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology. 2012;121(3):592–601. doi: 10.1037/a0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Findings in Experimental Clinical Pharmacology. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pearson MR, Murphy EM, Doane AN. Impulsivity-like traits and risky driving behaviors among college students. Accident Analysis Prevention. 2013;53:142–8. doi: 10.1016/j.aap.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CK, Gable P, Harmon-Jones E. Asymmetrical frontal ERPs, emotion, and behavioral approach/inhibition sensitivity. Social Neuroscience. 2008;3(2):113–24. doi: 10.1080/17470910701612736. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science. 2005;16(10):805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Poole BD, Gable PA. Affective motivational direction drives asymmetric frontal hemisphere activation. Experimental Brain Research. 2014;232(7):2121–30. doi: 10.1007/s00221-014-3902-4. [DOI] [PubMed] [Google Scholar]
- Rossi GF, Rosadini G. Experimental analysis of cerebral dominance in man. In: Millkan CH, Darley FL, editors. Brain Mechanisms Underlying Speech and Language. New York: Grune and Stratton; 1967. pp. 167–84. [Google Scholar]
- Rothbart MK, Hwang J. Temperament and the development of competence and motivation. In: Elliot AJ, Dweck CS, editors. Handbook of Competence and Motivation. New York: Guilford Press; 2005. pp. 167–84. [Google Scholar]
- Rothbart MK, Rueda MR. The development of effortful control. In: Mayr U, Awh E, Keele S, editors. Developing Individuality in the Human Brain: A Tribute to Michael I. Posner. Washington: American Psychological Association; 2005. pp. 167–88. [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions: Neurologic evidence. Archives of Neurology. 1982;39(4):210–8. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Ashbaugh AR, Antony MM, McCabe RE, Schmidt LA. Frontal EEG asymmetry and sensation seeking in young adults. Biological Psychology. 2008;78(2):164–72. doi: 10.1016/j.biopsycho.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Schiller B, Gianotti LR, Nash K, Knoch D. Individual differences in inhibitory control—relationship between baseline activation in lateral PFC and an electrophysiological index of response inhibition. Cerebral Cortex. 2013;24:2430–35. doi: 10.1093/cercor/bht095. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science. 2009;20(12):1500–6. doi: 10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51(4):1319–33. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJ. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. Journal of Affective Disorders. 2011;129(1):167–74. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–10. [Google Scholar]
- Vitacco D, Brandeis D, Pascual-Marqui R, Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Human Brain Mapping. 2002;17(1):4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Lagerlund TD, Sharbrough FW, et al. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topography. 2000;12(4):273–82. doi: 10.1023/a:1023407521772. [DOI] [PubMed] [Google Scholar]
- Wray TB, Simons JS, Dvorak RD, Gaher RM. Trait-based affective processes in alcohol-involved “risk behaviors”. Addictive Behaviors. 2012;37(11):1230–9. doi: 10.1016/j.addbeh.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TC, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors. 2009;23(2):348–54. doi: 10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg D, Andrade DM, Wennberg RA. Source localization of small sharp spikes: low resolution electromagnetic tomography (LORETA) reveals two distinct cortical sources. Clinical Neurophysiology. 2006;117(6):1380–7. doi: 10.1016/j.clinph.2006.02.019. [DOI] [PubMed] [Google Scholar]