Abstract
Despite the risks, people enjoy giving advice. One explanation is that giving beneficial advice can result in reflected glory, ego boosts or reputation enhancement. However, giving poor advice can be socially harmful (being perceived as incompetent or untrustworthy). In both circumstances, we have a vested interest in the advice follower’s success or failure, especially when it reflects specifically on us compared with when it is diffused between multiple advisors. We examined these dynamics using an Advisor-Advisee Game, where subjects acted as an Advisor to a confederate Advisee who selected one of the three options when trying to win money: accept the subject’s advice, accept the advice of a second confederate Advisor or accept both Advisors’ advice. Results showed that having one’s advice accepted, compared with being rejected, resulted in activity in the ventral striatum—a core reward area. Furthermore, the ventral striatum was only active when the subject’s advice led to the advisee winning, and not when the advisee won based on the confederate’s advice. Finally, the medial prefrontal cortex (MPFC) was more active when the Advisee won or lost money based solely on the subject’s advice compared with when the second Advisor’s advice was accepted. One explanation for these findings is that the MPFC monitors self-relevant social information, while the ventral striatum is active when others accept advice and when their success leads to reflected glory.
Keywords: advice giving, reward, reflected glory, self-relevance, medial prefrontal cortex
INTRODUCTION
Advice giving is described as an interaction between an advisor and advisee in which the advisor attempts to aid the advisee to find an answer for their problem (Lippitt, 1959). Giving advice, however, can be a risky social and vocational endeavour. Research confirms that people prefer to relay positive information to others (Rosen and Tesser, 1970) and that people weigh their advice more carefully when it reflects on them directly rather than through the medium of a third party (Jonas et al., 2005). Furthermore, advice giving may be one attempt to manipulate what others think about us (i.e. reputation seeking; Izuma, 2012). If our advice is accepted, we may feel that we have garnered another’s respect and admiration. If the advice provided leads to another’s personal success, we may feel a sense of reward through ego enhancement or reflected glory (Ortony et al., 1990; Cialdina et al., 1976). Conversely, giving the wrong advice can lead to self-conscious emotions (e.g. guilt or embarrassment) often associated with doing interpersonal harm (e.g. helping others fail) and a feeling that others may perceive us as incompetent, untrustworthy or spiteful.
Advice giving may be one way in which individuals can gain the most basic of social rewards: acceptance and respect (Baumeister and Leary, 1995). The hedonic feelings associated with giving advice are presumably modulated by brain regions involved in primary reward processes. For example, the dopamine-enriched striatum is a key area activated during the receipt of a reward (Delgado, 2007; Hare et al., 2008; Mobbs et al., 2009a,b), social cost-benefit analysis (Izuma, 2012) and complex social interactions including reciprocity and trust building (King-Casas et al., 2005; Phan et al., 2010). Furthermore, the striatum has been shown to respond when others like us (Davey et al., 2009) and when perceiving one’s own good reputation (Izuma, 2008) or social status (Ly et al., 2011), while activity in the striatum is observed when people perform acts that enhance their reputation including giving to charity (Harbaugh et al., 2007), egalitarianism (Dawes et al., 2012) and social co-operation (Rilling et al., 2002).
Although the medial prefrontal cortex (MPFC) is active during reward states (O’Doherty, 2004), it is also active during tasks that evoke self-relatedness (Mitchell et al., 2006), including when we deploy self-monitoring (Moran et al., 2009), self-judgements (Kelley et al., 2002), self-esteem (Somerville et al., 2010), and self-reflection (Johnson et al., 2006). The MPFC is also active for self-relatedness in exchanges with others, for example, when we observe similar others win money (Mobbs et al., 2009a,b), and during impression management or reputation processing (Izuma et al., 2010). It has been suggested that processing one's own reputation requires meta-cognition and the MPFC is a prime candidate for such an operation (Izuma, 2012). Indeed, the MPFC may represent future beliefs about how others will negatively perceive us (e.g. social distress and social transgressions; Eisenberger et al., 2007). If this is true, then the MPFC should be active in self-relevant situations such as when advisors experience an advisees’ positive or negative outcome based on our right or wrong advice.
We created an Advisor-Advisee Game to test the hypothesis that activation in the brain’s social reward circuitry will be increased when people accept one’s advice. Specifically, we posited that observing an Advisee win will be more rewarding if it is based on one’s own advice (i.e. Advisor A) compared with another’s advice (i.e. Advisor B). We further reasoned that the brain regions that underlie the processing of self-relevance and reputation would be more active when one gives good advice as a single agent, rather than as a member of a team with distributed responsibility for the good advice. For example, having our advice accepted, which then leads on to an Advisee winning, should result in an increase in the brain’s reward (ventral striatum) and self-monitoring (MPFC) regions when the win and loss are based solely on the subject’s (i.e. Advisor A’s) advice compared with when an Advisee accepts the same advice from two Advisors [i.e. the subject (Advisor A) plus Advisor B (a confederate)].
METHODS
Participants
Twenty-three subjects were scanned in this experiment. Seven subjects were omitted due to doubts over the veracity of the task manipulations, leaving with 16 subjects (8 men, age 24.8 ± 4.53). All were right handed, fluent speakers of English and screened for psychiatric or neurological problems. All subjects gave informed consent and were remunerated £20 for time, travel and inconvenience. This study was authorized by the Local Research Ethics Committee for Cambridge, UK.
Task
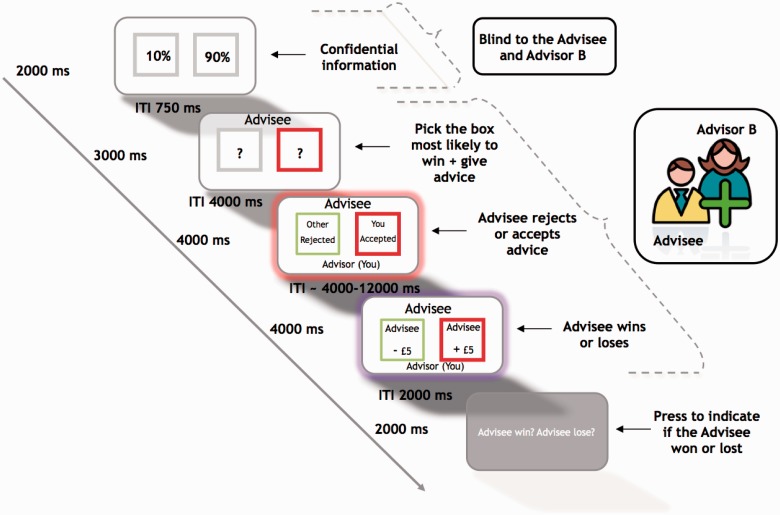
At the start of each experiment, each subject (Advisor A) was briefly introduced to two confederates, the Advisee and the second Advisor (Advisor B), and told that they were going to play a simple trust game where the Advisee had to learn which Advisor was providing the best information. Together with the two confederates, subjects were informed that for each of a series of trials they were to provide the best advice to the Advisee about which of the two boxes to choose in order to win £5 (Figure 1). In actuality, all responses by the Advisee and Advisor B were pre-programmed. Once the subject (Advisor A) was separated from the two confederates, it was explained that, while they would be given information about which box is most likely to win, Advisor B would not be given any such information and therefore would be guessing about the winning boxes. This step in the experimental rationale provided to the subject was important for two reasons. First, unless one advisor was clearly giving better advice then it would have been too difficult for the Advisee to learn to trust one advisor over another as there would have been no discriminating information. Second, if the subject thought that Advisor B had been given the same information as the subject/Advisor A, then we would have to justify why Advisor B was not suggesting the same boxes for selection by the Advisee.
Fig. 1.
Trial sequence and timing of the Advisor-Advisee Game. The subject (Advisor A), but not the Advisee or Advisor B (both confederates), was initially presented with two boxes on the screen. Each box showed the probability of that box winning if it was later selected. The subject was told that when these probability percentages were replaced with question marks, both the Advisee and Advisor B could now also see the boxes. The subject then gave advice by pressing a left or right button to signal to the Advisee which box was the most likely one to win. Following this, the subject was told that the Advisee could either accept their advice, reject their advice or accept both the subject’s and Advisor B’s advice. After a jittered ITI, the outcome of whether the Advisee won or lost was revealed to the subject. Finally, to ensure the subject was paying attention, she/he had to indicate the win or lose outcome of the Advisee.
After giving advice, subjects were told that they would be informed about all the advised information put forward, as well as the selection made by the Advisee. In other words, participants were informed as to whether the Advisee accepted their advice despite conflicting advice being provided by Advisor B, rejected their advice in favour of conflicting advice from Advisor B or accepted the advice provided by both Advisors when the Advisors were in agreement. Following the acceptance or rejection of advice, the subject passively observed which of the two boxes led to the Advisee winning or losing £5.
We indicated that if we had not given the subject this privileged information such that both advisors were essentially guessing about the advice to give, then the task for the Advisee would have been impossibly hard as there would have been no discriminating information. The task was nevertheless a challenge for the Advisee, however, as the other advisor (Advisor B) would still, even by chance, be giving the correct advice on around 50% of trials and so it would not be surprising that the Advisee did not immediately latch onto the fact that the subject was providing the most useful information. To further reduce the likelihood of suspicion, we made sure that it was clear to participants that their advice was infallible. For example, it is important to point out that the advice was not likely to have been perceived as infallible because participants were not being told that this box will definitely win when provided with the insider information. Rather, they were being given a probability (on some trials as low as 60 : 40) of that box being more likely to win and this formed the basis of the advice that they were passing on. For each trial, there was therefore a 10–40% likelihood that their advice would turn out to be wrong based on the probabilities that they had been shown beforehand. Consequently, in order to become suspicious on these grounds, they would need to track not only the percentage of times that their advice was incorrect but also to relate it to these varying probabilities. We felt that this was unlikely but, nevertheless, we took care to exclude the data from participants where suspicion was reported.
At the end of the experiment, subjects completed a questionnaire asking them to indicate on a 10-point Likert scale with 1 = not at all and 10 = very much: (Q1) ‘How rewarding did you find it, when the advisee lost after not taking your advice?’; (Q2) ‘How upsetting was it for you when the advisee rejected your advice?’’ (Q3) ‘How rewarding was it for you when you gave the advisee the right advice and they won?’; (Q4) ‘How responsible did you feel when the advisee won after giving them the right advice?’ and (Q5) ‘Do you like to give people advice?’
Image acquisition
Magnetic resonance imaging (MRI) scanning was conducted at the Medical Research Council Cognition and Brain Sciences Unit on a 3-T Tim Trio Magnetic Resonance Imaging scanner (Siemens, Germany) by using a head coil gradient set. Whole-brain data were acquired with echo planar T2*-weighted imaging (EPI), sensitive to blood oxygenation level dependent (BOLD) signal contrast (48 sagittal slices, 3 mm thickness; repetition time (TR) = 2400 ms, echo time (TE) = 25 ms, flip angle = 90°, field of view (FOV) = 224 mm, voxel size = 3 x 3 x 3 mm. To provide for equilibration effects, the first three3 volumes were discarded. T1 -weighted structural images were acquired at a resolution of 1 x 1 x 1 mm.
Image pre-processing
SPM5 software (www.fil.ion.ucl.ac.uk/spm/) was used for data analysis. The EPI images were sinc interpolated in time for correction of slice timing differences and realignment to the first scan by rigid body transformations to correct for head movements. Field maps were estimated from the phase difference between the images acquired at the short and long TE and unwrapped, employing the FieldMap toolbox. Field map and EPI imaging parameters were used to establish voxel displacements in the EPI image. Application of the inverse displacement to the EPI images served the correction of distortions. Utilising linear and non-linear transformations, and smoothing with a Gaussian kernel of full-width-half-maximum 8 mm EPI and structural images were co-registered and normalized to the T1 standard template in Montreal Neurological Institute space (International Consortium for Brain Mapping). Global changes were removed by high-pass temporal filtering with a cut-off of 128 s to remove low-frequency drifts in signal.
Statistical analysis
After pre-processing, statistical analysis was performed using the General Linear Model. A first-level analysis was carried out to establish each participant's voxel-wise activation during ‘Advice Stage’ (i.e. when the subject’s advice was accepted minus rejected) and the ‘Outcome Stage’ epochs (i.e. whether the advisee won or lost). Our first-level regressors included the condition for Advice Stage as follows: (i) accept, (ii) reject, (iii) accept both Advisors. For the Outcome Stage, we modelled wins when (iv) Advisor A was accepted and win outcome, (v) Advisor A was rejected and win outcome and (iv) both Advisors A and B were accepted and win outcome. For the loss conditions, (vi) Advisor A was accepted and loss outcome and (vii) Advisor A was rejected and loss outcome and (ix) both A and B accepted with loss outcome. The pre-programmed Advisee did not reject both Advisors A and B, therefore no regressors for these contrasts were entered into the model. Further to these nine regressors were six head-motion parameters defined by the realignment and added to the model as regressors of no interest. Ninety trials were presented with 15 in each Outcome Stage condition. Multiple linear regression was then run to generate parameter estimates for each regressor at every voxel. A second-level random effects analysis (one-sample t-test) was performed to analyse data at a group level. A family wise error + small volume correction (SVC) was used on a priori regions of interest (ROIs 8 mm), including ventral striatum (Yu et al., 2010) and MPFC (Izuma et al., 2010). These ROIs were chosen based on the idea that the MPFC ROI used by Izuma et al. (2010) would better reflect a region involved in self-monitoring or reputation processing, while the ventral striatum ROI would reflect basic reward processes. Activations are reported if they survive P < 0.05 SVC, with a cluster size k > 30.
RESULTS
We tested two core hypotheses: (i) that it would be rewarding to have one’s advice accepted and (ii) it will be rewarding to see the Advisee win based on our (Advisor A’s) good advice compared with Advisor B’s advice. With these two hypotheses in mind, our analysis focused on examining neural activity during the Advice (Figure 1, highlighted in red) and Outcome Stage (Figure 1, highlighted in purple) of each trial. The rationale behind examining the Advice Stage was to see if subjects found it rewarding to be accepted, while focusing on the Outcomes Stage allowed us to examine whether outcomes based on one’s advice will result in the recruitment of brain regions involved in self-referential processing, namely the MPFC. Analysis of the Outcome Stage was based on the idea that acceptance or rejection of one’s advice would modulate how people engage with the outcome of the Advisee’s decision. For example, having one’s advice accepted should lead to self-interest in the outcome.
Advice stage
Post-MRI scan questioning revealed that having the Advisee accept one’s good advice was rewarding (Q3: mean 5.8 ± 2.1). On the other hand, how rewarding or how much they gloated when the Advisee lost after rejecting their advice resulted in an average rating (Q1: 3.5 ± 1.8). A direct comparison between these two questions showed that it was more rewarding to see the advisee win based on the subject’s advice compared with seeing them lose after being rejected (t-test: P < 0.0001). Furthermore, having the subject’s advice rejected by the Advisee did not evoke strong negative feelings of rejection (Q2: mean 2.5 ± 1.4). Given the low rating of how upset the subjects felt at being rejected, we chose to report only findings for the Advice (when accepted or not) and Outcome (accepted) Stages.
Advice accepted compared with rejected
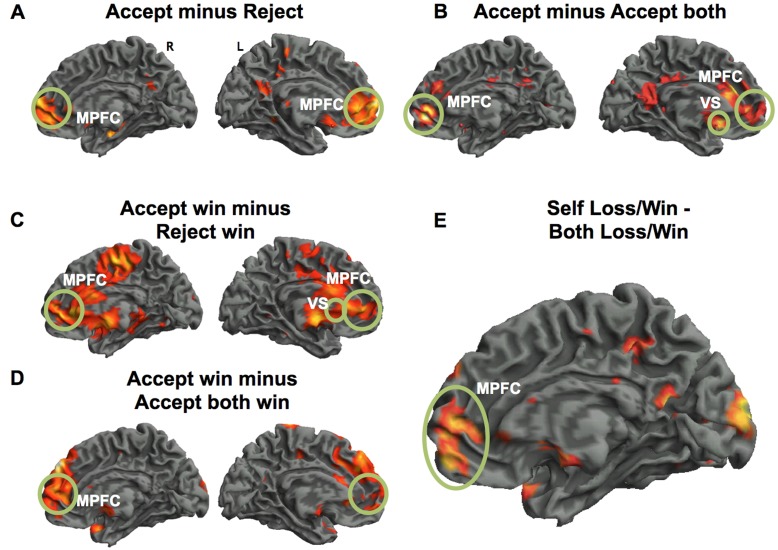
For the Functional Magnetic Resonance Imaging (fMRI) analysis, we examined the Advice Stage or the time when the Advisee accepted or rejected the subject’s advice (Figure 1, highlighted in red). We observed a main effect for the comparison of accepted minus rejected advice, which, as hypothesized, showed increased activity within the MPFC ([6, 62, 10], Z = 3.09, k = 141, P < 0.021 SVC; Figure 2A). We found no significant activations associated with rejected minus accepted advice.
Fig. 2.
fMRI results. (A) Medial PFC (MPFC) activity associated with having advice accepted compared with rejected. (B) Medial PFC (MPFC) and striatal activity associated with having advice accepted compared with when the Advisee accepted the subject (Advisor 1) and Advisor 2. (C) Activity associated with observing the Advisee win after having one’s advice accepted compared with rejected. (D) Neural activity when the Advisee won money after accepting advice from the subject versus the subject plus Advisor B. (E) Activity for the Self loss/win minus Both loss/win comparison. All images are displayed at P < 0.001uncorrected. Encircled areas reflect peak co-ordinates. MPFC = medial prefrontal cortex; VS = ventral striatum. Both the MPFC and VS regions were SVC at P < 0.05 FWE with an 8 mm sphere (Izuma et al., 2010; Yu et al., 2010).
Subject's advice uniquely accepted compared with both advisors' advice accepted
A comparison of accepted Advisor A’s advice alone (and Advisor B’s advice rejected) vs both Advisor A’s and Advisor B’s advice accepted showed increased activity in the MPFC ([12 52 −2], Z = 3.25, k = 109, P < 0.009 SVC) and ventral striatum ([−10 20 −12], Z = 3.28, k = 143, P < 0.008 SVC; Figure 2B). It is possible that the activity in these areas reflected the anticipation of winning. Therefore, we examined whether activity in the striatum and MPFC parametrically varied in accordance with the probability of the Advisee winning (i.e. 60%, 70%, 80% and 90% chance of winning upon acceptance of advice). We found no significant positive parametric modulation by probability in the MPFC or striatum (P > 0.05). Of course, absence of evidence is not evidence of absence, but this analysis certainly provides no further support for the idea that anticipation of winning is the appropriate explanation of the data.
Outcome stage
Post-MRI scan questionnaires were administered to assess how much the subjects enjoy giving advice in everyday situations (Q5: mean 6.4 ± 1.7) and feelings of personal responsibility for Advisee wins (Q4: mean 4.6 ± 2.0). Relating these individual differences on these questions revealed that seeing the Advisee win after giving good advice positively correlated with questionnaire measures relating to how much people enjoy giving advice in everyday situations (Pearson’s one-tailed test: r = .55, P = 0.004), and feelings of personal responsibility for the Advisee’s subsequent wins (r = .61, P = 0.002).
Advisor A minus Advisor B advice leading to an Advisee win
For the fMRI data, we next examined the outcome conditions (observing the Advisee win; Figure 1, highlighted in purple) by comparing a win based solely on the Advisee’s advice vs the Advisee taking advice from Advisor B. Despite having an average (jittered) inter trial interval (ITI) of 8 s between having one’s advice accepted and the winning outcome, we wanted to ensure that this activity was not a direct function of the previous component of the trial. We therefore exclusively masked the outcome neural activity with activity from the accepted minus rejected advice contrast (Figure 2A). We found significant activity in the striatum ([−14, 8, −8], Z = 3.00, k = 170, P < 0.025 SVC) and MPFC ([10, 54, 6], Z = 3.19, k = 121, P < 0.026 SVC), suggesting that the rewarding outcome activity is distinct from the reward regions associated with Acceptance. To further clarify the role of the MPFC, we examined if the MPFC activation parametrically increased with the probability of winning. We did not observe a significant increase in MPFC activation supporting the notion that this region is not involved in providing better advice.
Advisor A minus Advisor B advice leading to an Advisee lose
Similar to the win outcome, we found activity in the MPFC ([8, 54, 14], Z = 3.18, P < 0.015 SVC). For the opposite contrast (Advisor B minus Advisor A, loss outcome), we found significant activity in the caudate and visual cortex.
Advisor A minus (Advisor A + Advisor B) advice leading to an Advisee win
We next investigated the differences between seeing the Advisee win outcome based on the subject’s (Advisor A’s) advice alone compared with when the Advisee won based on correct advice from both Advisors (Advisor A − [Advisor A + Advisor B]). We again found significant activity in the MPFC ([12, 56, 12], Z = 3.16, k = 253, P < 0.009 SVC; Figure 2D).
Advisor A minus (Advisor A + Advisor B) advice accepted leading to an Advisee lose
We also examined the neural activity associated with outcome loss associated with Advisor A minus joint advice (the advisee accepting both Advisor A and Advisor B and losing). We observed activity in the MPFC, albeit at the liberal threshold of P < 0.0005uncorrected. For the opposite contrast, we did not find any significant voxels.
Self loss/win minus both loss/win comparison
To examine the neural basis of self-relevant vs shared advice, we conducted the analysis between Self loss/win minus Both loss/win (Figure 2E). We again found increased activation in the MPFC ([12, 56, 0], Z = 2.70, k = 182, P < 0.046 SVC) supporting the idea that the MPFC plays a role in self-relevant information.
DISCUSSION
Our results support the hypothesis that the MPFC is more active in social situations that reflect exclusively on oneself. The MPFC was active when the Advisee exclusively accepted the subject’s advice compared with when the Advisee accepted the advice of the subject and a second advisor (Advisor B), which fits with its role in self-monitoring, including thinking about what others think of us (Amodio and Frith, 2006). Furthermore, we support the supposition that, under certain conditions, having one’s advice accepted is socially rewarding. Post-MRI scan questioning suggested that the amount of reward felt when seeing the Advisee win positively correlated with the subjects’ proclivity to give advice in everyday situations. Furthermore, having one’s advice accepted results in activity in the brain’s reward circuitry. One possibility, based on the additional recruitment of the striatum for the acceptance of only Advisor A’s advice vs Advisor A and Advisor B’s advice, is that it is more rewarding to have one’s advice accepted alone than to share acceptance of advice with another (Advisor B). The recruitment of the ventral striatum, however, was only observed when the subject’s advice resulted in the Advisee winning and not when the subjects shared the glory of seeing the Advisee win. This suggests that it is more rewarding to see another win when we are exclusively, rather than jointly, responsible for that win.
Our results lead to the intriguing question of whether or not it is only gratifying to see an Advisee win when it reflects positively on one’s self. Studies show that lack of anonymity influences subjects’ questionnaire responses in a direction of increasing social desirability (Lautenschlager and Flaherty, 1990) and that people more readily express their true feelings when they express their opinions through the voice of a third party (Jonas et al., 2005). These observations reveal that before speaking their true feelings, people bear in mind the nature of an audience. Extending upon these observations, the same may hold true in situations of advice giving, where the quality of advice given may be seen as a direct or indirect reflection of the quality of the person giving the advice. In support of this suggestion, increases in MPFC and striatal activity were observed during watching the Advisee win after accepting the subject’s advice in preference to Advisor B’s advice. This leads to the conclusion that under certain conditions, people may give advice for self-centred reasons, although the rather controlled nature of the manipulated scenario might limit the degree to which one can generalize these findings to more naturalistic scenarios and situations. Limitations aside, after having given good advice, we feel most rewarded when we can exclusively, rather than jointly, take credit for another’s success.
Further analysis suggests that the MPFC is also involved in processing negative social outcomes that reflect directly on us. For example, comparison between self-relevant win and loss outcomes minus shared win and loss outcomes supports the hypothesis that the MPFC is associated with self-relevant processes. The MPFC is known to be involved in tasks that evoke self-relevance (Amodio and Frith, 2006). These studies include metacognitive evaluations (Schmitz et al., 2004), theory of mind or mentalizing (Fletcher et al., 1995) and trait evaluation tasks (Kelley et al., 2002). Such processes would be critical to processing the social consequence of one’s behaviour. It has previously been shown that the MPFC becomes active when thinking about one’s own reputation (Izuma et al., 2008), while the striatum activates during value representations (Hare et al., 2008). The MPFC is also evoked during positive evaluation by others (e.g. when someone likes you; Davey et al., 2010) which may reflect meta-cognitive attributions, such as thinking about what others think about us (Amodio and Frith, 2006). In light of these findings, it is intriguing that self-relevant social situations (Moran et al., 2006; Mobbs et al., 2009a,b) and enhancements in self-esteem result in increases in MPFC activity (Moran et al., 2006; Mobbs et al., 2009a,b; Somerville et al., 2010). In the context of this study, we speculate that the MPFC may form part of a reputation management network. For example, the MPFC may detect socially relevant information, particularly when it reflects directly on us. Future studies, however, should also try and examine the overlap or the distinct MPFC regions that support self-relevance, reputation and mentalizing.
We acknowledge several potential caveats to this study. For example, the relationship between behavioural and neural data is sparse thereby limiting our assumptions about the relationship between behaviour and MPFC and striatal activity. Furthermore, we cannot be certain that our findings only relate to positive feelings that are associated with having one’s advice accepted and do not also encompass the motivations behind giving advice. Although our findings indicate that giving good advice may be one method by which we enhance our reputation, other explanations do exist. For example, ego-inflation or increases in self-esteem, and anticipated positive social feedback are equally plausible explanations. Egotism, the high regard for oneself, provides a salient mechanism by which advice giving results in self-focus. Presumably, many of these alternative explanations are part and parcel of processing self-relevant processes in the social context.
Navigating the social environment successfully depends on the implementation of several operations including the ability to detect self-relevant information and act in an appropriate manner. Our results support the idea that the MPFC plays a key role in this process, while the ventral striatum is active when self-relevant information is perceived to be positive (e.g. advice acceptance and reflected glory Cialdina et al., 1976). More broadly, while advice giving often falls under the rubric of altruism, the ‘egocentric’ perspective proposes that giving advice affords us opportunities for reputation enhancement by having others believe that we are knowledgeable, trustworthy, benevolent and indispensible (Mayer et al., 1995; Sniezek and Buckley, 1995; Yaniv and Kleinberger, 2000; Helm and Salminen, 2010). This leads to the intriguing speculation that while giving advice is often relayed in the context of benevolent help, it may also serve more selfish motivations such as increasing our value within a group, which in turn minimizes the risk of social rejection.
Acknowledgments
We thank Jason Stretton for his help with data analysis. This work was funded by the UK Medical Research Council (MC_US_A060_0017).
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Cialdini RB, Borden RJ, Thorne A, Walker MR, Freeman S, Sloan LR. Basking in reflected glory: three (football) field studies. Journal of Personality and Social Psychology. 1976;34:366–75. [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2009;31(4):660–8. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes CT, Fowler JH, Johnson T, McElreath R, Smirnov O. Egalitarian motives in humans. Nature. 2007;446:794–6. doi: 10.1038/nature05651. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Science. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- O’Doherty J. Reward representations and reward-related learning in the human brain: insights from human neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. fMRI responses relate to differences in real-world social experience. Emotion. 2007;7:745–54. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–5. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm S, Salminen R. Basking in reflected glory: using customer reference relationships to build reputation in industrial markets. Industrial Marketing Management. 2010;39(5):737–43. [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. The roles of the medial prefrontal cortex and striatum in reputation processing. Social Neuroscience. 2010;5(2):133–47. doi: 10.1080/17470910903202559. [DOI] [PubMed] [Google Scholar]
- Izuma K. The social neuroscience of reputation. Neuroscience Research. 2012;72:283–8. doi: 10.1016/j.neures.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Jonas E, Schulz-Hardt S, Frey D. Giving advice or making decisions in someone else’s place: the influence of impression, defense, and accuracy motivation on the search for new information. Personality and Social Psychology Bulletin. 2005;31:977–90. doi: 10.1177/0146167204274095. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Lautenschlager GJ, Flaherty VL. Computer administration of questions: more desirable or more social desirability? Journal of Applied Psychology. 1990;75:310–4. [Google Scholar]
- Lippitt R. Dimensions of the consultant’s job. Journal of Social Issues. 1959;15:5–12. [Google Scholar]
- Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF. Subjective Socioeconomic Status Predicts Human Ventral Striatal Responses to Social Status Information. Current Biology. 2011;21(9):794–7. doi: 10.1016/j.cub.2011.03.050. [DOI] [PubMed] [Google Scholar]
- Mayer RC, Davis JH, Schoorman FD. An integrative model of organizational trust. Academy of Management Review. 1995;20(3):709–34. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Hassabis D, Seymour B, et al. Choking on the money: incentive-induced performance decrements in a reward pursuit task. Psychological Science. 2009a;20(8):955–62. doi: 10.1111/j.1467-9280.2009.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, et al. A key role for similarity in vicarious reward. Science. 2009b;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by explicit and implicit self-relevance. Social Neuroscience. 2009;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortony A, Clore GL, Collins A. 1990 The cognitive structure of emotion. University of Cambridge Press, Cambridge, UK. [Google Scholar]
- Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proceedings of the National Academy of Science of the United States of America. 2010;107:13099–104. doi: 10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rosen S, Tesser A. On reluctance to communicate undesirable information: the MUM effect. Sociometry. 1970;33:253–63. [Google Scholar]
- Sniezek JA, Buckley T. Cueing and cognitive conflict in judge-advisor decision making. Organizational Behavior and Human Decision Processes. 1995;62(2):159–74. [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20:3005–13. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv I, Kleinberger E. Advice taking in decision making: egocentric discounting and reputation formation. Organizational Behavior and Human Decision Processes. 2000;83:260–81. doi: 10.1006/obhd.2000.2909. [DOI] [PubMed] [Google Scholar]
- Yu R, Mobbs D, Seymour B, Calder AJ. Insula and striatum mediate the default bias. Journal of Neuroscience. 2010;30(44):14702–7. doi: 10.1523/JNEUROSCI.3772-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]