Abstract
Diminished responsivity to reward incentives is a key contributor to the social-communication problems seen in autism spectrum disorders (ASDs). Social motivation theories suggest that individuals with ASD do not experience social interactions as rewarding, leading to negative consequences for the development of brain circuitry subserving social information. In this study, we examined neural responses to social and non-social reward anticipation in 35 typically developing young adults, examining modulation of reward sensitivity by level of autistic traits. Using an Event-related potential incentive-delay task incorporating novel, more ecologically valid forms of reward, higher expression of autistic traits was associated with an attenuated P3 response to the anticipation of social (simulated real-time video feedback from an observer), but not non-social (candy), rewards. Exploratory analyses revealed that this was unrelated to mentalizing ability. The P3 component reflects motivated attention to reward signals, suggesting attenuated motivation allocation specific to social incentives. The study extends prior findings of atypical reward anticipation in ASD, demonstrating that attenuated social reward responsiveness extends to autistic traits in the range of typical functioning. Results support the development of innovative paradigms for investigating social and non-social reward responsiveness. Insight into vulnerabilities in reward processing is critical for understanding social function in ASD.
Keywords: autism spectrum disorder, broad autism phenotype, reward, ERPs, social motivation
Dysfunction in reward processing has been implicated in the neural bases of autism spectrum disorders (ASD). Reduced drive for social interaction is observed in many individuals with ASD and is hypothesized to contribute to atypical social development. Social motivation theories of ASD posit that aberrant responsivity to reward incentives associated with social rewards may be a developmental precursor to the social deficits and communication problems seen in the disorder (Dawson et al., 2005; Schultz, 2005; Chevallier et al., 2012; Kohls et al., 2012). If children with ASD experience social interaction as less rewarding, they may be less likely to attend to information related to other people, leading to further derailment of social performance and failure in specialization for brain circuitry subserving social information (Mundy and Neal, 2000; McPartland et al., 2004, 2011).
Given the hypothesized importance of social reward in the development of children with ASD, a growing literature examines behavioral and neural facets of reward system function in this population. Behavioral studies have revealed attenuated responsiveness to social incentives, including verbal praise (Garretson et al., 1990), pictures of smiling faces (Scott-Van Zeeland et al., 2010) and pictograms depicting social reciprocity (Demurie et al., 2011). These studies are consistent with social motivation theories in indicating that individuals with ASD do not properly anticipate and appreciate the reward value of social stimuli (Dawson et al., 2005). Convergent neurobiological evidence indicates that deficits in reward processing in ASD are associated with abnormal patterns of brain activity in dopaminergic mesocorticolimbic circuitry comprising, among other regions, the ventral striatum (including the nucleus accumbens/NAcc), amygdala and prefrontal cortex (including the anterior cingulate cortex/ACC; Kohls, et al., 2013b). Functional magnetic resonance imaging (fMRI) studies reveal reactivity of these regions, particularly the striatum (Baez-Mendoza and Schultz, 2013; Bhanji and Delgado, 2014), in typically developing individuals during the anticipation of positive social feedback (Spreckelmeyer et al., 2009; Rademacher et al., 2010), with activity correlating to social reward magnitude (Lin et al., 2012). Among individuals with ASD, aberrant brain responses have been observed in the ACC and NAcc during the anticipation of monetary reward (Dichter et al., 2012a,b). Activity in reward regions correlates with social functioning (Schmitz et al., 2008), linking atypical reward system activity in ASD to the social-communicative difficulties that define the clinical phenotype.
Several studies have directly contrasted patterns of neural activity in the context of social vs non-social incentives. In the first study of this kind, Scott-Van Zeeland et al. (2010) compared a social reward, pictures of smiling faces combined with written praise, with monetary rewards. Relative to typically developing peers, children with ASD exhibited attenuated activation in the ventral striatum in response to social rewards; this response was not, however, associated with social behavior. Using similar social and non-social reinforcers, Kohls et al. (2013b) found that individuals with ASD showed hypoactivation in mesocorticolimbic circuitry, particularly the NAcc, amygdala and ACC, in response to both social and monetary incentives. Although the exact nature of reward processing difficulties in ASD remains unclear, such studies have provided preliminary evidence of a role for reward circuitry dysfunction in the neuropathology of ASD (Dichter et al., 2012).
Taken together, fMRI research has revealed reward network dysfunction in ASD. However, hemodynamic imaging methods lack the temporal resolution to precisely delineate the chronology of reward-related brain activity (Goldstein et al., 2006). Event-related potentials (ERPs) are an alternative approach to measuring brain activity with millisecond resolution. In this way, ERPs enable the measurement of both processing efficiency and the opportunity to isolate neural responses at distinct processing stages corresponding to specific cognitive events. For example, this temporal precision enables ERP studies to contrast reward anticipation vs reward outcome. Prior research has successfully applied ERPs to demonstrate preserved reward outcome monitoring for non-social information in ASD (Larson et al., 2011; McPartland et al., 2012). ERPs have also been effective in contrasting the neural bases of reward for social and non-social information. In a study of typically developing children, Stavropoulos and Carver (2014a) found increased responsiveness of the stimulus preceding negativity (SPN) component when food reward was delivered in a social context (smiling faces) compared with a non-social context (scrambled faces).
The temporal dynamics of social compared with non-social reward in ASD remain largely unexplored. In a study comparing SPN response in children with and without ASD to the anticipation of incidental social (face) and non-social (non-face) rewards, Stavropoulos and Carver (2014b) found a diminished neural response to the anticipation of social rewards among ASD participants only. SPN response to non-social incentives was intact in both groups, suggesting that children with ASD have deficits in the context of social, but not non-social, reward anticipation. In another ERP study, Kohls et al., (2011) utilized a cued incentive-delay task to compare typically developing children and those with ASD in their P3 response to social (pictures of smiling faces) and monetary incentives. In the context of reward processing, the P3 component is thought to reflect motivated attention to reward signals (Pineda et al., 1989). Manifesting as a positive deflection peaking between 300 and 600 ms post-stimulus onset most prominently at cento-parietal sites (Kok, 2001), P3 amplitude is thought to correspond to motivation for both social and non-social incentives. Kohls et al. (2011) found that children with ASD displayed an attenuated P3 response during anticipation in the context of both social and monetary incentives. Moreover, P3 activity in response to both incentive types negatively correlated with social symptom severity, suggesting a generic relationship between reward system function and social function in ASD.
In sum, behavioral and brain imaging studies have demonstrated atypical reward system function in ASD, though effects specific to social reward and the relationship to autistic symptomatology have varied across studies. Discrepant findings may reflect variability in experimental designs associated with cognitive load (Kohls et al., 2013b), as both the subjective value of and the brain’s response to reward vary inversely with the effort required to obtain it (Botvinick et al., 2009). Variability in study results, particularly with respect to social factors, may also relate to the social reward stimuli employed; most utilized static or dynamic smiling faces, which though person-related, are distinct from real-life social rewards (Risko et al., 2012; Gossens et al., 2014; Kohls et al., 2013a). Likewise, the most common non-social reward employed in previous research, money, can be argued to have social value (Demurie et al., 2011). These shortcomings highlight the need for real-world social and non-social reward paradigms.
The current study aimed to implement novel, more ecologically valid forms of social and non-social rewards in an ERP study designed to isolate neural response associated with reward motivation and its relationship to social behavior. In advancing previous studies of social and non-social reward, we contrasted primary non-social reinforcers (i.e. candy) with a realistic, dynamic social reinforcer (i.e. an observer offering simulated real-time positive feedback regarding the participant’s task performance). Video clips possess greater motivational value than static images (Blatter and Schultz, 2006) and elicit more robust responses in neural reward circuitry (Fox et al., 2009). These stimuli were applied in the context of an ERP cued incentive-delay task. As an extension of prior work examining ERP response to social and non-social reward in children with and without an ASD diagnosis (Kohls et al., 2011), neural mechanisms of social and non-social reward anticipation were examined in the context of P3 modulation, a marker of reward responsiveness both in typical and clinical populations (Goldstein et al., 2006).
To examine the relationship of reward system function to social behavior, we assessed traits associated with the broader autism phenotype in adults without an ASD diagnosis. The broader autism phenotype refers to characteristics that parallel autistic symptomatology in a milder but qualitatively similar form (Hurley et al., 2007). Several studies indicate that autistic disorders may represent the upper extreme of a constellation of deficits in social and communicative behavior that are continuously distributed in population-based samples (Constantino and Todd, 2006; Hoekstra et al., 2007), presenting the possibility that even mild variations of autistic traits may be responsible for incurring social impairment (Constantino and Todd, 2003), including disturbances in reward functioning. No ERP study to date has investigated modulation of reward sensitivity by levels of autistic traits in a non-clinical sample. Therefore, it remains to be determined the extent to which deficits in reward processing extend beyond ASD to autistic traits in the range of typical functioning.
We hypothesized that individuals with high levels of autistic traits (as measured by the Social Responsiveness Scale—Adult; SRS-A; Constantino and Todd, 2005) would display an attenuated P3 amplitude to social and non-social incentives than individuals with low levels of autistic traits, with the largest effect seen for social reward. Additionally, given that mentalizing ability (the ability to represent and attribute mental states to others and oneself; Fonagy and Bateman, 2008) is hypothesized to influence social reward processing (Krach et al., 2010) and social dysfunction in ASD (Baron-Cohen, 1989, 1991; Lombardo et al., 2011), we also explored mentalizing ability (as measured by the Reading the Mind in the Eyes Test—Adult Revised (RMET; Baron-Cohen et al., 2001) as a mediator of the relationship between autistic traits and neural response to social incentives.
METHODS
Participants
Our initial sample consisted of 43 graduate and undergraduate students (aged 18–35) from Yale University. Exclusion criteria included neurological or psychiatric disorder, history of serious head injury, intellectual or learning disability, medications that could affect cognitive processes, and sensory or motor impairments that could impede the study protocol. Two participants were excluded due to recent brain injury resulting from concussion, and six participants were excluded due to failure to provide adequate artifact-free ERP data [see electroencephalography (EEG) processing]. The final sample consisted of 35 participants (16 males; 33 right handed; mean age = 24 years), categorized into groups of high (n = 17) and low (n = 18) levels of autistic traits based upon a median score of 37 on the SRS-A. The groups did not differ with respect to age, gender or handedness (Table 1). All participants had normal or corrected to normal vision and were not taking any psychotropic medication. Participants were compensated $40 and candy for their participation in this study. The Human Investigative Committee at Yale University School of Medicine approved all procedures prior to recruitment.
Table 1.
Demographic characteristics and behavioral performance by autistic trait group
| Low SRS-Aa | High SRS-Aa | P values | |
|---|---|---|---|
| (n = 18) | (n = 17) | ||
| SRS-A score | |||
| Mean (s.d.) | 26.61 (8.02) | 65.24 (21.18) | <0.001* |
| Age (years) | |||
| Mean (s.d.) | 24.11 (4.16) | 23.94 (3.36) | 0.89 |
| Gender | 0.31 | ||
| Male | 7 | 9 | |
| Female | 11 | 8 | |
| Handedness | 1.00 | ||
| Left | 2 | 1 | |
| Right | 16 | 16 | |
| SRQ score | |||
| SR mean (s.d.) | 6.65 (1.57) | 5.78 (2.37) | 0.35 |
| CR mean (s.d.) | 6.58 (1.93) | 5.47 (2.02) | 0.66 |
| NR mean (s.d.) | 3.43 (2.09) | 3.51 (2.12) | 0.77 |
| RMET score | |||
| Mean (s.d.) | 30.61 (1.88) | 26.88 (3.33) | <0.001* |
aBased on a median split of 37. SRS-A, social responsiveness scale—adult. SRQ, subjective ratings questionnaire. RMET, reading the mind in the eyes. SR, social; CR, non-social; NR, non-reward. *, Statistically significant between group difference (P < 0.05).
Experimental procedure
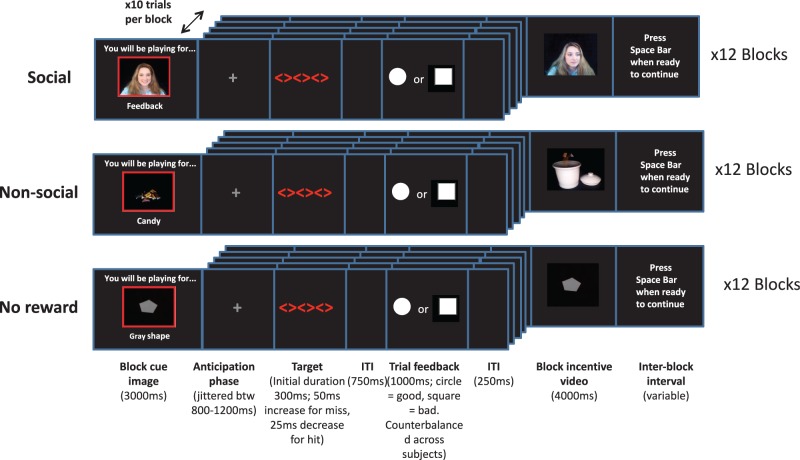
We used a modified version of a cued incentive-delay ERP task previously introduced by Kohls et al. (2011; Figure 1) with three incentive conditions: social reward (SR), non-social reward (candy; CR) and non-reward (NR). Prior to the task, participants were told that they would receive three different types of feedback based on their performance: video feedback from an observer (social reward), a video of a chosen candy (one of three) being dispensed into a jar that they would receive at the end of the task (non-social reward) and a video of a semi-static grey shape (non-reward). For the social reward condition, participants were told that the computer screen on which they performed the task is linked to another room with an unfamiliar (female) person watching, who will give feedback on task performance at the end of each block; in actuality, videos were pre-recorded to ensure consistency from participant to participant.
Fig. 1.
Illustration of the cued incentive-delay task including three different incentive conditions: social, non-social and non-reward. ITI, inter/intra-trial intervals.
Altogether, 36 experimental blocks were presented pseudorandomly (counterbalanced across participants). Each reward condition comprised 12 blocks, with each block starting with a 3000 ms image signaling the type of reward that would be obtained for ongoing good task performance. Each block consisted of 10 trials. At the onset of each trial, a grey fixation cross was presented (jittered 800–1200 ms), followed by a target stimulus of three red diamonds. Participants were instructed to respond with the index finger of their dominant hand on a response console as quickly and as accurately as possible upon seeing the target. The target was initially displayed for 300 ms, decreasing by 25 ms following a hit and increasing by 50 ms following a miss, ensuring an overall hit rate of 66% (Table 2). After the target, a fixed intra-trial interval was displayed (750 ms), followed by trial feedback (1000 ms). Trial feedback consisted of a white circle for hits and a white square for misses (counterbalanced across participants). These cues served as the reward anticipation phase. This was followed by an inter-trial interval (250 ms) and the start of the next trial. After 10 trials participants received the video reward associated with the incentive condition. If hits were achieved in the majority of trials (>5) then high positive feedback was given (e.g. statements from the observer such as ‘terrific, keep up the good work’ for social reward or a large handful of candy for non-social reward). If misses were observed in the majority of trials then low positive feedback was given (e.g. statements such as ‘good effort, better luck next time’ for social reward and a small handful of candy for non-social reward). The video of the grey shape in the non-reward condition remained the same regardless of performance. Each social and non-social reward video was distinct to maintain the illusion of real-time feedback. Reward videos were displayed for 4000 ms. Each trial had a length of ∼2850–3500 ms, and the block length was ∼35–42 s.
Table 2.
Task performance and P3 peak amplitude at electrode Pz as a function of autistic trait group and incentive type
| Low SRS-Aa | High SRS-Aa | P values | |
|---|---|---|---|
| (n = 18) | (n = 17) | ||
| Reaction time (ms) M (s.d.) | |||
| SR | 196.05 (23.57) | 191.74 (29.58) | 0.64 |
| CR | 191.08 (24.57) | 189.81 (29.23) | 0.89 |
| NR | 193.81 (27.22) | 189.05 (26.82) | 0.60 |
| Accuracy (mean %) | |||
| SR | 67.22 | 67.35 | 0.64 |
| CR | 67.31 | 67.3 | 0.89 |
| NR | 66.06 | 65.93 | 0.60 |
| P3 peak amplitude (μV) M (s.d.) | |||
| SR | 5.43 (3.16) | 3.13 (2.16) | 0.018* |
| CR | 4.57 (3.87) | 4.06 (3.34) | 0.68 |
| NR | 4.17 (2.91) | 3.74 (1.75) | 0.59 |
SRS-A, social responsiveness scale—adult. SR, social; CR, non-social; NR, non-reward. *, Statistically significant between group difference (P < 0.05).
To ensure that all participants understood the task instructions, the experimental procedure was preceded by one block of five practice trials in the non-social condition, with the opportunity to repeat the practice trials if needed. Participants first completed the ERP task, followed by questionnaires relating to social behavior. Following the questionnaires, participants were debriefed that there was no live observer and videos were pre-recorded.
EEG data collection and processing
Stimuli were presented in frontal view on a 17 inch LCD monitor (60-Hz, 1024 × 768 resolution) with E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) at a viewing distance of 70 cm in a sound attenuated room with low ambient illumination. EEG recordings were digitally obtained from 128 Ag/AgCL electrode net with high impedance amplifiers (NetAmps 300 amplifiers; Electrical Geodesics, Inc., Eugene, OR). The net was placed on the participant’s head and fitted according to the manufacturer’s specifications. Impedances were kept below 40 kΩ. EEG was recorded continuously at 500 Hz with data high-pass filtered at 0.1 Hz and low-pass filtered at 30 Hz offline. Data were re-referenced to an average reference, with Cz serving as the online reference point for all electrodes.
EEG data were processed using NetStation v.4.4 software. The data were segmented to epochs lasting 100 ms before to 500 ms after stimulus onset (i.e. trial feedback for accurate responses triggering outcome anticipation). Artifact detection settings were set to 200 µv for bad channels, 140 µv for eye blinks and 100 µv for eye movements. Channels with artifacts on >40% of trials were marked as bad channels and replaced through spline interpolation. Segments that contained eye blinks, eye movement or more than 10 bad channels were marked as bad and excluded. Data were baseline corrected to the 100 ms pre-stimulus epoch. Trial-by-trial data were subsequently averaged at each electrode for each condition (SR, CR, NR) separately for each individual. In line with previous studies (Goldstein et al., 2006), participants with fewer than 10 good trials per condition were excluded from analysis (n = 6). For all participants included in the analysis, an average of 40 artifact-free trials were obtained in the social condition, 41 were obtained in the non-social condition and 38 in the non-reward condition; two-tailed t-tests showed no significant difference between the high and low autistic trait groups for comparisons of the number of good trials between social, non-social and non-reward conditions (ps > 0.05). Based on maximal observed amplitude of the P3 and prior research (Kohls et al., 2011), amplitude and latency to maximal peak for the P3 were extracted at the Pz electrode (no. 62) for trial feedback resulting from accurate responses. The temporal window for analysis of the P3 was chosen by visual inspection of the grand averaged data. The resultant time window, extending from 200–400 ms post-stimulus onset, was then verified for each participant.
Behavioral measures
The SRS-A (Constantino and Todd, 2005) is a 65-item self-report questionnaire assessing the presence and extent of autistic symptoms in adult populations. Scores range from 0 (highly socially competent) to 195 (severely socially impaired), with scores between 60 and 80 indicating deficiencies in reciprocal social behavior that result in mild to moderate interference in everyday social interactions and scores above 80 suggestive of a more severe interference in everyday social interactions (Constantino and Gruber, 2005). The SRS-A has demonstrated high internal consistency (α = 0.95 and 0.94 in typical males and females, respectively, Constantino and Todd, 2005) and test–re-test reliability (r = 0.8; Constantino et al., 2000). The questionnaire was selected based on its excellent psychometric properties and its focus on deficits aligning with the social domain of ASD.
The RMET (Baron-Cohen et al., 2001) assesses first order mentalizing ability through inferring another’s mental state as expressed in the eyes. Participants are presented with a series of 36 photographs of the eye regions of actors and actresses expressing a complex mental state (e.g. playful). Photographs are presented with four descriptors (e.g. playful, comforting, irritated, bored). Participants are asked to choose which word best describes what the person in the photograph is thinking or feeling, with a glossary available if needed. RMET performance is inversely correlated with measures of autistic traits (Baron-Cohen et al., 2001), suggesting the test is a sensitive measure of adult social intelligence, indirectly indicating that it bears relation to the thoughts and feelings of others (mentalizing).
We employed a modified version of a subjective ratings questionnaire (SRQ) used in Kohls et al. (2011). Participants were asked separately for the three reward conditions (i) how rewarding they found the condition, (ii) how motivating they found the condition (iii) how important it was to succeed in each condition. Answers were reported on an 11-point visual analogue scale, from 0 (not at all) to 10 (very much). Scores were then averaged within each condition to give three total subjective ratings. A question was also included asking whether participants believed that the reward videos were occurring live. Results were ‘yes’ (20%) ‘no’(51%) or ‘not sure’ (29%).
Data analysis
Amplitudes and latencies to peak for the P3 ERP component were analyzed separately using 2 × 3 repeated measures analyses of variances (ANOVAs), with incentive type (SR, CR, NR) as the within subjects factor and autistic trait group (high, low) as the between subjects factor. Planned comparisons utilized paired sample t-tests to contrast P3 amplitudes among conditions and independent sample t-tests used to compare P3 amplitudes between groups. Pearson correlations were used to examine the relationship between P3 amplitude and autistic traits. A mediation analysis was conducted to examine P3 amplitude to social incentives in the context of the relationship between autistic traits and mentalizing (according to criteria outlined by Baron and Kenny, 1986), with continuous levels of autistic traits as the independent variable, mentalizing as the mediator and P3 peak amplitude to social incentives as the dependent variable. For all analyses, significance level was set at α < 0.05. The Greenhouse-Geisser correction for non-sphericity was used when necessary and is indicated by epsilon (e). Effect size is presented as either partial eta-squared (ηρ2) or Pearson’s correlation coefficient (r).
RESULTS
Subjective ratings
The post-test questionnaire (SRQ) revealed a significant main effect of incentive type on subjective ratings [F(2,68) = 38.95, ε = 0.80, P > 0.001, η2ρ = 0.53], with the highest ratings found in the social condition, followed by the non-social condition, and the lowest ratings in the non-reward condition. Significant differences were found between social and non-reward incentives [t(34) = 6.95, P < 0.001, r = 0.59], and non-social and non-reward incentives [t(34) = 6.72, P < 0.001, r = 0.60], but not between social and non-social incentives [t(34) = 0.76, ns, r = 0.02], suggesting that both social and non-social incentives were rated as significantly and equivalently more rewarding than non-reward. These data demonstrate that reward manipulation within the experimental paradigm was successful. We did not find a main effect of group [F(1,33) = 1.27, ε = 0.86, ns, η2ρ = 0.04], or a significant incentive × group interaction [F(2,66) = 1.64, ε = 0.86, ns, η2ρ = 0.05], suggesting that participants with lower levels of autistic traits did not significantly differ in their subjective ratings of the ERP task compared with participants with higher levels of autistic traits for social, non-social or non-reward incentives (Table 1).
Task performance
Analysis of reaction time for accurate responses revealed a main effect of incentive type [F(2,68) = 3.56, P = 0.034, η2ρ = 0.095], with pairwise comparisons revealing significantly faster reaction times in the non-social incentive condition compared with non-reward [t(34) = 2.54, P = 0.016, r = 0.39]. However, differences in reaction time between social and non-social incentives, and social and non-reward incentives were not significant (ps > 0.05). We did not find a main effect of group [F(2,66) = 0.384, ns, η2ρ = 0.04], or a significant incentive × group interaction [F(2,66) = 1.00, ns, η2ρ = 0.03]. Taken together, these results suggest that reaction time did differ between conditions but was not differentially affected by levels of autistic traits. As expected, all interaction effects with regards to task accuracy (incentive type, group, incentive × group) were found to be non-significant, indicating that both groups performed equally in terms of number of hits across conditions (66%). Task performance is summarized in Table 2.
P3 amplitude
Across all participants, we found no significant main effect of incentive type on P3 amplitude [F(2,70) = 0.39, ns, η2ρ = 0.01]. However, we found a significant incentive × group interaction [F(2,66) = 3.40, P = 0.039, η2ρ = 0.09]. Individuals with high autistic traits displayed a significantly attenuated P3 amplitude to social incentives than individuals with low levels of autistic traits [t(33) = 2.49, P = 0.018, r = 0.39]. In contrast, we found no significant differences in P3 amplitude between high and low autistic trait groups for non-social incentives [t(33) = 0.42, P = 0.68, r = 0.07], or for non-reward [t(33) = 0.53, P = 0.59, r = 0.18]. Thus, between group differences existed during the anticipation of social incentives only. This interaction effect can be seen in Figures 2 and 3. Planned comparisons within the low autistic trait group indicated significantly higher P3 amplitudes to social incentives than non-reward incentives (P = 0.02), but no significant differences in P3 amplitudes between social and non-social incentives, or non-social incentives and non-reward (ps > 0.05). Within the high autistic trait group, there were no differences in P3 amplitude across the conditions (ps > 0.05). Results are summarized in Table 2.
Fig. 2.
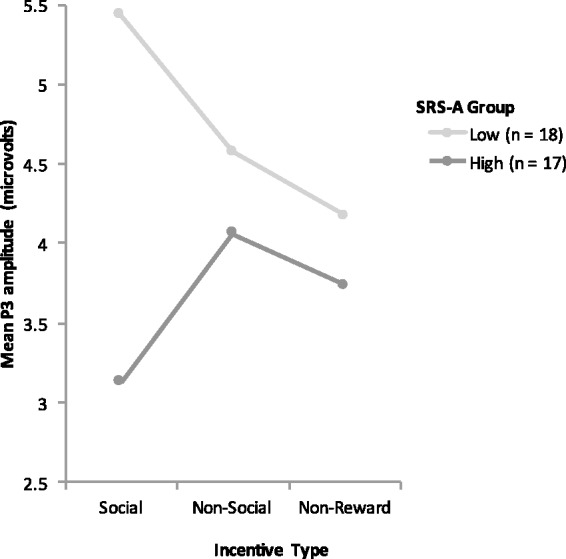
Significant incentive × autistic trait group interaction at electrode Pz; F(2,66) = 3.40, P = 0.039, η2ρ = 0.09.
Fig. 3.
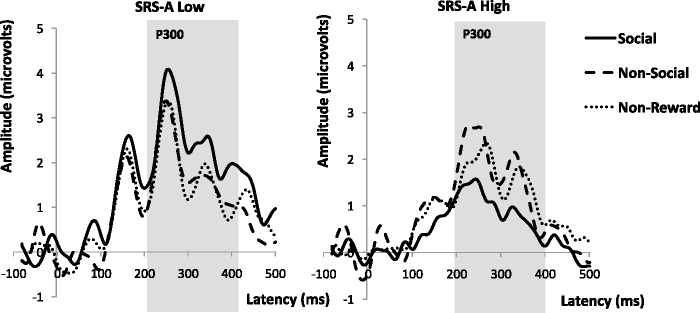
Grand mean ERP waveforms for trial hits shown separately for high and low autistic trait groups as measured by the SRS-A. (Pz electrode). Solid line social reward, Dashed line non-social reward, Dotted line non-reward. Gray bar indicates time window for statistical analysis of P3 component.
P3 latency
No significant P3 latency differences were found as a function of incentive type [F(2,70) = 1.83, ns, η2ρ = 0.50] or group [F(2,68) = 1.72, ns, η2ρ = 0.05].
P3 amplitude and autistic trait correlations
We found a significant negative relationship between autistic traits and P3 peak amplitude to social incentives [r(35) = − 0.35, P = 0.04], suggesting that as levels of autistic traits increased, P3 peak amplitude to social incentives decreased. In the non-social condition, we found no significant relationship between levels of autistic traits and P3 peak amplitude [r(35) = −0.08, P = 0.67]. Taken together, this indicates autistic traits are associated with neural response to social, but not non-social, incentives.
Correlations: mentalizing
Pearson’s (r) correlation coefficients revealed significant negative relationship between mentalizing ability and levels of autistic traits [r(35) = −0.63, P < 0.001], suggesting that as mentalizing ability increased levels of autistic traits decreased. We found a positive relationship in the predicted direction between mentalizing ability and P3 peak amplitude in the social [r(35) = 0.30, P = 0.08], but not non-social [r(36) = −0.07, P = 0.68], condition. This indicates a marginal relationship between mentalizing ability and P3 peak amplitude for social, but not non-social, incentives.
Mediation model: mentalizing
A series of regression analyses were conducted to assess whether the relationship between autistic traits and P3 peak amplitude in the social condition was mediated by mentalizing ability. Analyses revealed that autistic traits significantly predicted both P3 amplitude [B = −0.04, t(33) = −0.211, P = 0.04], and mentalizing ability [B = −0.08, t(33) = −0.466, P < 0.001]. However, mentalizing ability was not found to be a significant predictor of P3 amplitude [B = 0.27, t(33) = 1.81, P = 0.08], suggesting that mentalizing did not mediate the effect of autistic traits on P3 amplitude to social incentives.
DISCUSSION
The current study applied a cued incentive-delay task to explore the differential effects of social and non-social reward anticipation on the P3 ERP component in typically developing young adults, examining modulation of reward sensitivity by levels of autistic traits. A further aim was to advance previous studies of social and non-social reward processing by incorporating more ecologically valid reward. As predicted, individuals with high autistic traits exhibited attenuated P3 amplitudes under social reward conditions than those with low levels of autistic traits. These findings concord with Kohls et al. (2011) and Stavropoulos and Carver (2014b), who found diminished P3 and SPN response to the anticipation of social incentives in children with ASD. No significant differences in P3 amplitude were observed between individuals with high and low autistic traits for non-social incentives as well as the non-reward condition, suggesting that neural reward sensitivity is modulated by autistic traits only in the context of social reward anticipation. Similarly, higher levels of autistic traits were inversely correlated with P3 amplitude to social incentives only, indicating an association between attenuated neural responsiveness specifically to social reward and deficits in social behavior.
The P3 component is thought to be the electrophysiological manifestation of activity of the Locus ceruleus-norepinephrine (LC-NE) system, a widespread cluster of norepinephrine containing neurons that functions concurrently with dopaminergic reward circuitry to evaluate the salience of incoming stimuli (Nieuwenhuis et al., 2005; Bennaroch, 2009). As P3 amplitude may correspond to reward motivation, the lower P3 amplitude observed in those with high autistic traits during the anticipation of social reward may reflect an attenuated state of motivation allocation toward social incentives. This resonates with social motivation theories linking social dysfunction in ASD to the decreased reward value placed on social stimuli (Dawson et al., 2005), and behavioral evidence indicating that those with ASD do not find social stimuli rewarding (Garretson et al., 1990; Demurie et al., 2011). Our findings are also consistent with fMRI data demonstrating a negative correlation between activity in the ventral striatum in response to social, but not non-social incentives, and scores of social reciprocity in typically developing children (Scott Van-Zeeland et al., 2010). The results of this study thus add to the growing body of evidence that the social-communicative dysfunction in the autism phenotype is characterized by atypical motivation for social reward at the neural level.
As an exploratory analysis, we investigated mentalizing ability as a potential mediating factor between levels of autistic traits and P3 response to social incentives. Although mentalizing ability was found to negatively correlate with levels of autistic traits, it did not predict P3 amplitude. This suggests that autistic traits modulate the P3 response to social incentives irrespective of mentalizing abilities. These data are consistent with accounts characterizing mentalizing difficulties as a developmentally emergent symptom of autism rather than a contributing factor, with the two items in a neurotypical population likely tapping a similar latent construct (Frith, 1989; Klin et al.,1992).
To improve ecological validity relative to prior studies comparing neural responses to social and non-social reward, we contrasted a primary non-social reinforcer (candy) with a realistic, dynamic social reinforcer (positive video feedback from a feigned live observer). Behavioral data revealed that incentive salience was higher under social relative to non-social conditions. This contrasts with a previous study yielding higher ratings for non-social rewards (money) relative to social rewards (pictures of smiling faces; Kohls et al., 2011). This pattern of results suggests our effort to increase the realism of social reward was effective, consistent with prior studies indicating that a combination of facial rewards with verbal praise (Scott-Van Zeeland et al., 2010) elicited greater NAcc responsivity than static faces alone (Dichter et al., 2012a). The observed difference between social and non-social reward may also reflect our use of candy, rather than money, as a non-social reward. Monetary incentives used in previous studies may be exceptionally motivating and may also have social elements (Scott-Van Zeeland et al., 2010; Kohls et al., 2011; Dichter et al., 2012a). We chose food as a non-social incentive because it is a primary reinforcer (i.e. directly satisfying evolved appetitive mechanisms; Marsgall and Magruder, 1960; McClure et al., 2007), yielding similar non-social value across contexts. Finally, our behavioral results may reflect the differences in sample in that is the first study to focus on autistic traits in the range of typical functioning, rather than the autism phenotype, per se.
Refining the social vs non-social reward dissociation likely improves the ability to detect subtle differences in incentive salience of social and non-social stimuli between differing levels of autistic traits (Kohls et al., 2013a). However, group differences were only evident at the neural level. This adds to a body of evidence highlighting the sensitivity of ERPs to detect differences in cognitive processes that are not evident in behavior (Pelphrey and McPartland, 2012), or in processes not accessible to conscious awareness or evaluation (Dichter et al., 2012b).
By demonstrating differences in social reward processing across the broader autism phenotype, results from this study inform understanding of the role of reward-related motivation deficits in the social dysfunction in ASD. As ASD may represent the upper extreme of social and communicative deficits that are within the same distribution as typical functioning, understanding the neural mechanisms underlying specific domains of reward functioning associated with non-clinical levels of autistic traits may also offer clinically relevant information regarding ASD. Diagnostic classification frameworks for mental disorders such as the Research Domain Criteria (RDoC) intend to implement neuroscience based psychiatric classification, adding neurobiological parameters to augment clinical symptoms in brain disorders such as ASD. Taking such an approach may provide opportunity for more personalized treatment formulations tailored to specific areas of vulnerability (Insel et al., 2010).
Results from this study also highlight the importance of taking into account the heterogeneity of control groups in ASD studies; results suggest that natural, subclinical variety in social reward processing could contribute to the presence or absence of ASD group differences. Future studies comparing individuals with clinical levels of ASD to typically developing comparison groups should consider evaluating the influence of levels of autistic traits in control group participants. This will help to ensure more between group variability in autistic symptom severity, leading to more nuanced comparisons between clinical and non-clinical phenotypes.
The present study should be considered in light of its limitations. Because of a limited sample size and our focus on autistic traits within the range of typical functioning, our high and low autistic trait groups were based on a median split of scores on the SRS-A, rather than identified thresholds for deficiencies in reciprocal social behavior. Future studies of social deficits in the broad autism phenotype should dichotomize samples based on predetermined, phenotypically significant cut-offs, which will help to ensure more between group variability in autistic symptom severity. It is notable that we observed significant differences in a sub-clinical sample, but additional study is required to specify the relationship between social reward and clinically significant levels of autistic traits. Moreover, our power to detect within group effects for social vs non-social reward was also likely weakened by the relatively small group sizes and substantial neural heterogeneity within each of the groups. This is consistent with the medium to large effect sizes observed for within group differences, despite failing to meet criteria for statistical significance. Although data indicate that social videos were an effective experimental manipulation, over half of participants reported not believing that the social incentive videos were occurring in real-time. Future studies could improve the validity of social stimuli by incorporating actual live social feedback. Although we screened for psychiatric illness, our specific focus on autistic traits does not allow us to rule out the influence of other intra-individual factors, such as personality attributes, anxiety or impulsiveness.
In summary, results of the current study are consistent with prior research demonstrating attenuated neural responsiveness during the anticipation of social reward in individuals with ASD. Attenuated neural responsiveness was observed in those with high autistic traits for social, but not non-social incentives, revealing for the first time that social reward processing deficits extend beyond clinical levels of autism to autistic traits in the range of typical functioning. Although preliminary these findings support social motivation theories linking autism symptomatology to decreased reward value placed on social stimuli (Dawson et al., 2005). The current study was the first to incorporate simulated real-time performance feedback from a feigned live observer as a social incentive, which appeared to make social reward more rewarding. Future studies should consider utilizing similar paradigms in investigations of social and non-social reward, applying them to clinical populations to better understand neural deficits associated with problematic social behavior. If social motivation deficits are indeed a developmental precursor to the clinical phenotype of ASD then targeted interventions that increase motivational salience of social information (such as use of oxytocin, Bartz et al., 2011; Bartz and Hollander, 2008) may prove beneficial in improving the social-communication skills of individuals with the disorder.
Conflicts of Interest
None declared.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of the Yale Developmental Electrophysiology Laboratory, Dr. Giulia Righi, Gordon Moseley and Max Rolison. This research was supported by NIMH R01 MH100173 (JM), NIMH K23 MH086785 (JM), the NARSAD Atherton Young Investigator Award (JM), CTSA Grant Number UL1 RR024139 (JM, LCM), Autism Speaks Translational Postdoctoral Fellowship (AN). This work was submitted in partial fulfilment of the requirement of the degree of MSc Psychodynamic Developmental Neuroscience by Anthony Cox. The work was supported by the Anna Freud Centre—University College London—Yale Child Study Center program (UK).
REFERENCES
- Baez-Mendoza R, Schultz W. The role of the striatum in social behavior. Frontiers in Neuroscience. 2013;7:233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The autistic child’s theory of mind: a case of specific developmental delay. Journal of Child Psychology and Psychiatry. 1989;30:285–98. doi: 10.1111/j.1469-7610.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Do people with autism understand what causes emotion? Child Development. 1991;62:385–95. [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘reading the mind in the eyes’ test revised version: a study with normal adults, and adults with asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–51. [PubMed] [Google Scholar]
- Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Progress in Brain Research. 2008;170:451–62. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bennaroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73(20):1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Bhanji JP, Delgado MR. The social brain and reward: social information processing in the human striatum. Wiley Interdisciplinary Reviews: Cognitive Science. 2014;5(1):61–73. doi: 10.1002/wcs.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Experimental Brain Research. 2006;168:541–6. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale manual. Los Angeles: Western. Psychological Services. 2005 [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Development and Behavioural Pediatrics. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60:524–30. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57(6):655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2006;57:655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52(11):1164–73. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. Journal of Neurodevelopmental Disorders. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012a;7(2):160–72. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012b;42:147–60. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Bateman A. The development of borderline personality disorder—a mentalizing model. Journal of Personality Disorder. 2008;22(1):4–21. doi: 10.1521/pedi.2008.22.1.4. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJS. Defining the face processing network: optimization of the functional localizer in fMRI. Human Brain Mapping. 2009;30(5):1637–51. doi: 10.1002/hbm.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Autism and “theory of mind”. In: Gillberg C, editor. Diagnosis and Treatment of Autism. New York: Plenum Press; 1989. pp. 33–44. [Google Scholar]
- Garretson HB, Fein D, Waterhouse L. Sustained attention in autism. Journal of Autism and Developmental Disorders. 1990;20(1):101–14. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. International Journal of Psychophysiology. 2006;62:272–9. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossens A, Groppe SE, Winkler L, et al. Neural evidence for an association between social proficiency and sensitivity to social reward. Social cognitive and affective neuroscience. 2014;9(5):661–70. doi: 10.1093/scan/nst033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability of autistic traits in the general population. Archives of Pediatric and Adolescent Medicine. 2007;161:372–7. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- Hurley RES, Losh M, Parlier M, Reznik JS, Piven J. The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders. 2007;37:1679–90. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Klin A, Volkmar F, Sparrow S. Autistic social dysfunction: some limitations of the theory of mind hypothesis. Journal of Child Psychology and Psychiatry. 1992;33(5):861–76. doi: 10.1111/j.1469-7610.1992.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: neurobiological underpinnings and treatment implications. Journal of Neurodevelopmental Disorders. 2012;4:10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Ruther M, et al. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41(11):1523–33. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013b;51:2062–9. doi: 10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Rüther M, Nehrkorn B, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2013a;8(5):565–72. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–77. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Krach S, Paulus FM, Bodden M, Kircher T. The rewarding nature of social interactions. Frontiers in Behavioural Neuroscience. 2010;4:1–3. doi: 10.3389/fnbeh.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, South M, Krauskopf E, Clawson A, Crowley MJ. Reward and feedback processing in high-functioning autism. Psychiatry Research. 2011;187:198–203. doi: 10.1016/j.psychres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience. 2012;7:274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. 2011;56:1832–8. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Marsgall HR, Magruder L. Relations between parent money education practices and children’s knowledge and use of money. Child Development. 1960;31:253–84. doi: 10.1111/j.1467-8624.1960.tb04964.x. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. Journal of Neuroscience. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Crowley MJ, Perszyk DM, et al. Preserved reward outcome processing in ASD as revealed by event-related potentials. Journal of Neurodevelopmental Disorders. 2012;4(1):16–24. doi: 10.1186/1866-1955-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Wu J, Bailey CA, Mayes LC, Schultz RT, Klin A. Atypical neural specialization for social percepts in autism spectrum disorder. Social Neuroscience. 2011;6(5–6):436–51. doi: 10.1080/17470919.2011.586880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Neal AR. Neural plasticity, joint attention, and a transactional social-orienting model of autism. International Review of Research in Mental Retardation. 2000;23:139–68. [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–32. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, McPartland JC. Brain development: neural signature predicts autism’s emergence. Current Biology. 2012;22(4):R127–8. doi: 10.1016/j.cub.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Foote SL, Neville HJ. Effects of locus coeruleus lesions on auditory event-related potentials in monkey. Journal of Neuroscience. 1989;9:81–93. doi: 10.1523/JNEUROSCI.09-01-00081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer K. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Risko EF, Laidlaw KEW, Freeth M, Foulsham T, Kingstone A. Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience. 2012;6:143. doi: 10.3389/fnhum.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DGM. Neural correlates of reward in autism. The British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdale and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2–3):125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer S. Reward processing in autism. Autism Research. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos KKM, Carver LJ. Reward sensitivity to faces versus objects in children: an ERP study. Social Cognitive and Affective Neuroscience. 2014a;9(10):1569–75. doi: 10.1093/scan/nst149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos KKM, Carver LJ. Reward anticipation and processing of social versus non-social stimuli in children with and without autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2014b;55(12):1398–408. doi: 10.1111/jcpp.12270. [DOI] [PubMed] [Google Scholar]