Abstract
Media violence exposure causes increased aggression and decreased prosocial behavior, suggesting that media violence desensitizes people to the emotional experience of others. Alterations in emotional face processing following exposure to media violence may result in desensitization to others’ emotional states. This study used scalp electroencephalography methods to examine the link between exposure to violence and neural changes associated with emotional face processing. Twenty-five participants were shown a violent or nonviolent film clip and then completed a gender discrimination stop-signal task using emotional faces. Media violence did not affect the early visual P100 component; however, decreased amplitude was observed in the N170 and P200 event-related potentials following the violent film, indicating that exposure to film violence leads to suppression of holistic face processing and implicit emotional processing. Participants who had just seen a violent film showed increased frontal N200/P300 amplitude. These results suggest that media violence exposure may desensitize people to emotional stimuli and thereby require fewer cognitive resources to inhibit behavior.
Keywords: media violence, ERPs, emotion processing, inhibition, desensitization
The media is saturated with violence and aggression and decades of media violence research has suggested a strong association between viewing media violence and increased aggressive thoughts and expectations, and decreased prosocial behavior (Anderson et al., 2010). Exposure to media violence also causes increased hostile expectations (Bushman and Anderson, 2002), decreased sympathy for victims of real life violence (Fanti et al., 2009), and decreased helping behaviors towards those in need (Bushman and Anderson, 2009). Taken together, these findings have led researchers to theorize that desensitization to emotion following media violence exposure is a key mechanism underlying decreases in empathy and prosocial behavior (Bushman and Anderson, 2009). However, the cognitive changes associated with emotional processing following media violence exposure are not well understood.
Preliminary research suggests that desensitization to emotion following media violence exposure may result from autonomic and central nervous system changes. Carnagey et al. (2007) found that participants displayed a decrease in heart rate variability and skin conductance when viewing violent images after exposure to violent films, suggesting habituation, or a physiological desensitization to violence. Likewise, shooting other characters in a violent video game was associated with decreased amygdala activity, a part of the brain important for processing and responding to fear-related emotional information in an individual’s environment (Weber et al., 2006). Using scalp electroencephalography (EEG), Bartholow et al. (2006) found that playing a violent video game resulted in decreased resources allocated to processing violent emotional images, as indexed by decreased amplitude in the P300 event-related potential (ERP) as compared to playing a nonviolent video game. While this research suggests that violent media exposure can cause nervous system changes in emotional processing, it remains unclear how desensitization to emotion interacts with more top-down cognitive processes, such as inhibitory control, a capacity likely critical in regulating aggressive behavior. This study explores how media violence exposure may change bottom-up and top-down attentional processes related to emotion processing during a task that requires inhibitory control.
EMOTIONAL FACE PROCESSING
Humans analyze facial expressions to interpret and respond to the emotional experiences of others. Interpreting and reacting to emotional facial expressions and nonverbal human gestures is crucial to effectively negotiate day-to-day life experiences. Processing facial expressions is one of the first skills that infants learn (McClure, 2000) and it is essential for survival, allowing people to perceive and interpret potential threat and avoid adverse experiences (Anderson et al., 2003). Abnormalities in interpreting facial expressions have been observed in individuals chronically exposed to violence, abuse, or neglect (Pollak, 2008). However, we know very little about how media violence exposure influences emotional face processing.
Preliminary evidence suggests that playing violent video games delays identification of happy faces (Kirsh and Mounts, 2007) and that chronic exposure to violent media was related to more quickly and accurately identifying angry faces from faces that were mixed between happy and angry faces (Kirsh et al., 2006). It is possible that media violence exposure leads to an emotionally anesthetized, habitualized response, such that less cognitive resources are needed to process negatively valenced stimuli, including facial expressions. From this perspective, emotional anesthetization may contribute to moral disengagement, contributing to feelings of justification in committing aggressive acts as well as an increasing enjoyment and pleasure during violent video game play (Hartmann and Vorderer, 2010). Equally disturbing, violent-video gamers show evidence of denying the humanness of others and report victims of violence as less human (Greitemeyer and McLatchie, 2011).
In healthy individuals, processing facial emotions is an automatic process involving visuospatial and attentional processes relying on subcortical (i.e. thalamus, basal ganglia, amygdala, hippocampus) and cortical (i.e. dorsolateral prefrontal, anterior cingulate, fusiform gyrus, occipital) brain regions (Palermo and Rhodes, 2007). Humans begin processing faces almost immediately after exposure, initially engaging the primary and secondary visual cortex (Vuilleumier and Driver, 2007). Using event-related potentials (ERPs) derived from EEG recordings, researchers have argued that fear may be differentially processed as early as 100 ms after exposure to emotional faces in the primary visual cortex (Eimer and Holmes, 2007; Vuilleumier and Driver, 2007). However, this very early processing likely does not involve explicit emotional processing (Holmes et al., 2006). Instead, explicit processing of emotional information is indexed by a right lateralized frontocentral P200 ERP component that is observed around 200 ms after exposure to an emotional face (Ashley et al., 2004; Holmes et al., 2006; Eimer and Holmes, 2007). However, existing studies suggest that this ERP does not distinguish between emotional expressions (Eimer and Holmes, 2007). Bertsch et al. (2009) found that inducing aggression in the lab could alter ERP components associated with emotional face processing. Participants were provoked to behave aggressively and their brain activity was recorded while viewing emotional faces (happy, angry, fearful, and neutral). Participants who were provoked to behave aggressively displayed increased P200 amplitude in response to angry and fearful faces compared to nonprovoked participants, suggesting that state aggression can alter the neural correlates of emotional facial processing. Yet, it remains unclear how exposure to media violence interacts with emotional face processing.
INHIBITORY CONTROL PROCESSES
Top-down inhibitory control processes recruit frontocingulate brain networks, including the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) to regulate a dominant response in favor of a competing response (Morrison et al., 2004; Krawczyk et al., 2008; Silton et al., 2010). When individuals are asked to inhibit a dominant response, various midfrontal ERPs are observed, including the frontocentral N200 and P300 components that are likely generated from the ACC approximately 200 and 300 ms after inhibiting an automatic response, respectively (Kiefer et al., 1998; Badzakova-Trajkov et al., 2009). This ERP N200/P300 complex is reflective of brain activity associated with inhibitory control (Enriquez-Geppert et al., 2010). Intact inhibitory control processes are important for preventing aggression and violent behavior (Raaijmakers et al., 2008). For example, an individual may experience an impulse to respond to a social situation with aggression, but intact inhibitory control would allow this individual to overcome aggressive impulses and engage in more thoughtful and socially appropriate behaviors.
It is not clear from previous research what effect media violence exposure has on inhibitory control processes. Violent action video game training studies have found improved inhibitory and motor control (Ferguson, 2010); while other researchers have argued that exposure to violent video games is related to both transient and enduring patterns of disinhibition (Gentile et al., 2011). Perhaps the conflicting literature is a result of decoupling bottom-up emotional processing from more top-down inhibitory control in the presence of emotion. Recent research suggests that top-down and bottom-up cognitive processes are much less distinct than previously thought, and particularly in the presence of emotion, they interact (Dennis and Chen, 2007). Given the unique, but related contributions of top-down and bottom-up processes during emotion processing, studying how emotional processing contributes to inhibitory control following media violence exposure will characterize these phenomena in a manner that has previously been neglected. Past behavioral studies have consistently shown that media violence exposure can desensitize participants to the emotional experiences of others, this study uses EEG methods to examine the brain activity associated with neural processes that occur when completing an implicit emotional face-processing task. As such, this study was designed to examine the influence of short-term exposure to film violence on emotional facial processing (P100, N170 and P200 ERPs) and inhibitory control (N200/P300 ERP complex).
METHOD
Overview
On separate testing sessions participants viewed either a violent or nonviolent film clip before performing a gender identification stop-signal task with emotional faces (see Figure 1). On each trial, participants saw either a male or female face that exhibited either a fearful or happy face. They were instructed to push one button if the face was male and another if it was female (go trials). There was no mention in the instructions about the expression on the faces. On 50% of trials a striped box appeared around the face indicating that the participant was to withhold their response (stop trials). The time between face onset and stop-signal onset (stop-signal delay) was adjusted on each trial to adjust task difficulty for each participant. On go trials, we recorded participant accuracy (whether they correctly identified the gender of the face) and response time (RT) of this decision on correct trials. On stop trials, we recorded participant accuracy (whether they were successful in withholding their response) and mean stop-signal delay. We also recorded EEG throughout the testing session and calculated stimulus locked ERPs to both face onset on go trials and stop-signal onset on stop trials.
Fig. 1.
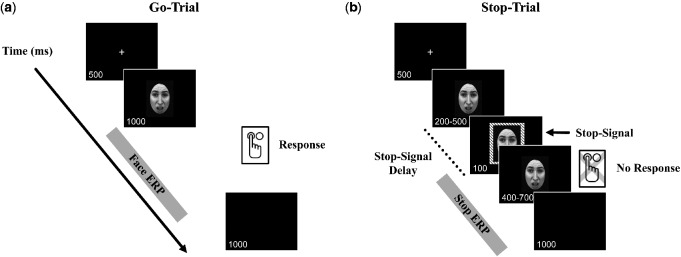
Stop-Signal Task Paradigm. (a). Go trials begin with a fixation screen followed by the presentation of the face for 1 s while the participants indicate the gender of the face by pressing one of two buttons on an electronic response box. (b). On stop trials, a stop signal was displayed for 100 ms after a 200–500 ms delay. The stop-signal delay was adjusted based on participant performance so that better withholding of responses on the previous two trials resulted in an increased delay between the presentation of the face and the stop signal, thereby making the task more difficult. On go trials, the ERP epoch was time-locked to face onset (Face ERP), while for stop trials, the ERP epoch was time-locked to the stop-signal onset (Stop ERP).
Participants
Twenty-eight undergraduate students per paid $40 for their participation in the study. Four participants were eliminated from further analysis because of poor task performance (<60% accuracy) resulting in a final sample of 25 undergraduate students (M = 21.4 years old, 14 female) for the go trials and 24 for the stop trials. Participants were recruited from, an urban, midwestern university. All participants reported that they were right-handed and did not have any known neurological disorders. The university Institutional Review Board approved all recruiting and experimental methods.
Materials and procedure
Violence manipulation
Participants visited the lab for two experimental sessions. During the first session each participant was assigned to watch either a violent or nonviolent film clip prior to completing a 30-min implicit emotion stop-signal task (Sagaspe et al., 2011). Each clip was approximately 10 min in duration. They then watched the other film clip during a second testing session several weeks later. Coyne et al. (2008) previously selected the film clips and found them to be equally engaging and arousing. Order of the film presentation was counterbalanced to eliminate any potential order effects. In the violent film condition, two female leads engage in a physical altercation including hitting, kicking, punching, hitting with objects, shooting and a fatal stabbing. In the nonviolent film, condition participants watched two female leads engage in a séance with a ghost. After the séance, one of the female leads has an interaction with the ghost. Uhlmann and Swanson (2004) found that behavioral effects from media violence on aggression and emotional processing lasted at least 30-min in a lab setting. Likewise, Anderson et al. (2010) found the effects of media violence on aggression, empathy and prosocial behavior to be greatest within 45 min of exposure. Thus, the emotional state effects that result from exposure to media violence are expected to last the duration of the stop-signal task (30 min) used in this study.
Mood measurement
Participants completed the Positive and Negative Affect Scale (PANAS; Watson et al., 1988) before and after watching each film clip. The PANAS is a 20-item scale that measures the distinct constructs of positive and negative affect. Participants answer on a five-point Likert scale how accurately words describe their current mood. For example, negative affect words include ‘afraid, nervous, and guilty’ and positive affect words include ‘active, enthusiastic, and interested.’ Individual items are averaged to create separate positive and negative affect scores.
Face stimuli
Digitalized photographic images of 30 male and 30 female Caucasian faces displaying either fear or happiness were selected from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998). Face stimuli were converted to grayscale and balanced for luminance and contrast in Adobe Photoshop. Images were cropped with an oval mask to remove hair to minimize nonfacial gender cues. Stimuli were adjusted to four degrees of visual angle wide.
Stop-signal task
The stop-signal task was based on the paradigm used by Sagaspe et al. (2011). Faces exhibited either a fearful or happy expression; however, the task is considered an implicit attention to emotion task since participants were not asked to attend to or identify the specific facial emotions. On go trials (see Figure 1a), participants were asked to press a button to indicate whether they believed the face presented was of a male or female. Go trials began with a fixation screen followed by a face presented for 1 s while participants responded by pressing one of two buttons on the electronic response box. Participants could respond any time after the stimulus onset for up to 1 s. A black screen followed the face stimulus. On stop trials (see Figure 1b), a stop signal (a stripped box surrounding the face stimulus) was displayed for 100 ms shortly after face stimulus onset. When the stop signal appeared, participants were asked to withhold their gender discrimination response. The delay between face stimulus presentation and the stop signal was adjusted (200–500 ms) to standardize task difficulty across participants. Specifically, if a participant was successful in withholding his/her response on the two previous stop trials the next stop trial would have a 20-ms longer stop delay. This served to make the task slightly more difficult. The better the participant was at withholding his/her response the longer the delay became, thus calibrating response inhibition effort across participants (Sagaspe et al., 2011). Go and stop trials were randomly intermixed 50/50 within each block of trials. Donkers and van Boxtel (2004) found that participants who completed a 50/50 go and stop trial task had higher accuracy rates in both go and stop conditions than participants who completed a 80/20 or 20/80 task, and importantly for our study the 50/50 task mix does not generate a oddball associated P300 ERP (Campanella et al., 2002).
Participants were seated 100 cm from a 21-inch CRT monitor in a quiet room. The stimuli were presented and responses recorded using E-Prime 2.0 (Schneider et al., 2002). Participants received task instructions and then performed two sets of 20 practice trials with trial-by-trial feedback representative of the various tasks, face valence, and face genders. During testing participants completed 720 total trials divided into 12 blocks. They received 180 trials for each task and valence (go happy, go fearful, stop happy, stop fearful). Half were male and half were female faces. The same 60 face stimuli were used in each block of trials; however, they varied in their assignment to task (go, stop) across blocks. Each block was representative of the four conditions and two genders and trials were presented in random order across participants. Participants received a 20-s break between each block.
EEG recording and data reduction
EEG data were recorded from each participant using a Biosemi Active2 EEG system. Custom-designed Falk Minow caps with 64 equidistant active electrodes (Ag/AgCl) were used for data collection. CMS/DRL were placed near the vertex. Two electrodes were located on the mastoid bones. Two electrodes were lateral to each eye to monitor horizontal eye movements. Two additional electrodes were placed on the inferior edge of the orbit of each eye to monitor vertical eye movements. Data were recorded with a band pass of 0–104 Hz, and sampled at a rate of 512 Hz.
The following EEG data processing steps were performed in EMSE (Source Signal Imaging). EEG data were re-referenced to common average reference and then digitally filtered with a 0.01 Hz high-pass filter and band-stop filter from 59 to 61 Hz. All filters had a cutoff attenuation of 12 dB/octave. A polynomial detrend was applied to the data to implement a 100 ms pre-stimulus baseline adjustment for ERP averaging. A spatial PCA filter was applied to remove ocular artifacts. Muscle and other artifacts were removed via visual inspection of the raw EEG signal and a ±100 µV trial-by-trial rejection criterion during averaging. Participants who were included in ERP analyses had fewer than 15% rejected trials on every condition.
Mean amplitude and 50% area latency scores (Luck, 2005) were calculated for the occipital P100 and frontal P200 for go trials and the frontocentral N200/P300s for stop trials. Electrode sites and scoring windows were selected based on a priori scoring windows derived from other studies investigating these ERPs in similar contexts and visual inspection of the data in this study (P100: Herrmann et al., 2005; P200: Ashley et al., 2004; N200/P300: Enriquez-Geppert et al., 2010). For go trials, the P100 was measured from 70 to 130 ms post face stimulus onset, N170 was measured from 120 to 200 ms past face-stimuli onset, and P200 was measured from 230 to 290 ms post face-stimulus onset. A cluster of three right posterior electrodes was identified for the P100 analyses (see Figure 3b), the N170 was measured using multiple sites corresponding to T5, O1, T6 and O2. Three left lateral posterior electrodes were used for T5, three right lateral posterior electrodes were used for T6, two left posterior electrodes were used for O1 and two right posterior electrodes were used for O2 (see Figure 4b). A cluster of five frontocentral electrodes, located near FZ, and a cluster of two posterior electrodes near PZ, were identified for P200 analyses (see Figure 5b). For stop trials, the N200/P300 complex was measured from 140 to 400 ms post stop-signal onset. A cluster of four frontocentral electrodes was identified for N200/P300 analyses (see Figure 6b). On average, participants had 136 correct ‘Go Happy’ trials, 136 correct ‘Go Fearful’ trials, 131 correct ‘Stop Happy’ trials, 134 ‘Stop Fearful’ trials.
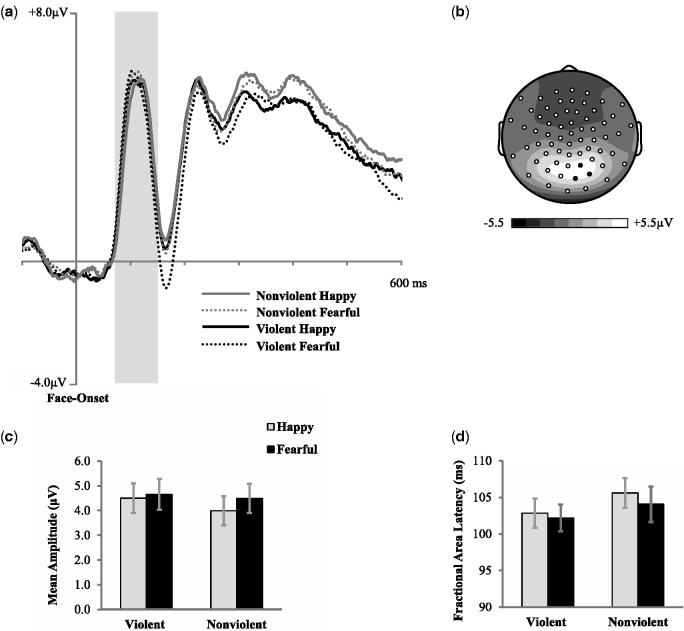
Fig. 3.
(a) Grand average ERPs (time-locked to the face onset) for correct trials averaged across three posterior electrodes indicated in black on topographic map. (b) Mean amplitude scalp topography for the average of all four conditions (70–130 ms post-face onset). (c) P100 mean amplitude (70–130 ms post face onset) for correct go trials across conditions. (d) 50% fractional area latency across conditions. Error bars represent ±1 SEM.
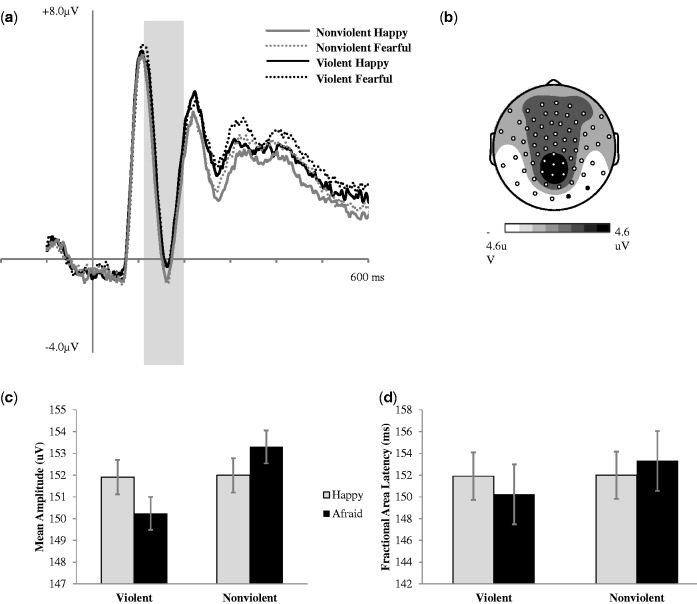
Fig. 4.
(a) Grand average ERPs (time-locked to face onset) for correct trials averaged across two right posterior electrodes indicated in black on topographic map. (b) N170 (120–200 ms post-face onset) mean amplitude scalp topography for the average of all four experimental conditions. (c) N170 mean amplitude (120–200 ms post face onset) across conditions. (d) 50% fractional area latency across conditions. Error bars represent ±1 SEM.
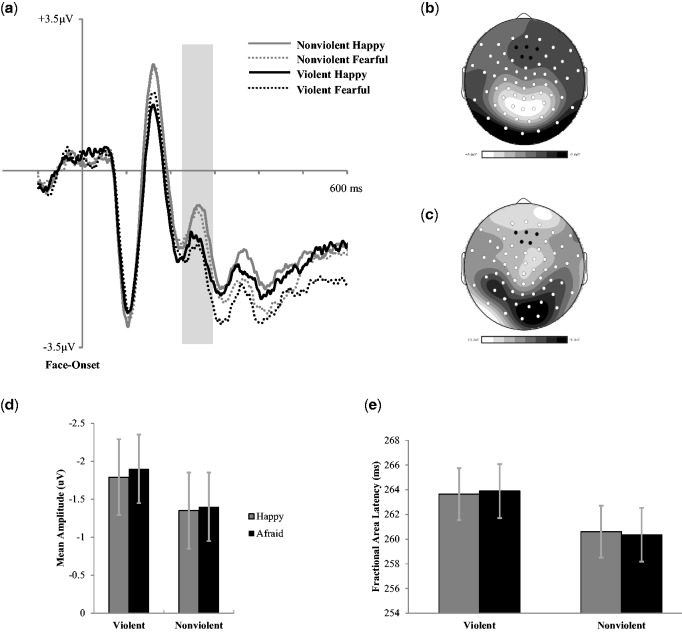
Fig. 5.
(a) Grand average ERPs (time-locked to face onset) for correct trials averaged across five frontal central electrodes indicated in black on topographic map. (b) P200 (230–290 ms post-face onset) mean amplitude scalp topography for the average of all four experimental conditions. (c) P200 (230–290 ms post-face onset) subtraction topography for violent and the nonviolent film conditions. (d) P200 mean amplitude (230–290 ms post face onset) across conditions. (e) 50% fractional area latency across conditions. Error bars represent ±1 SEM.
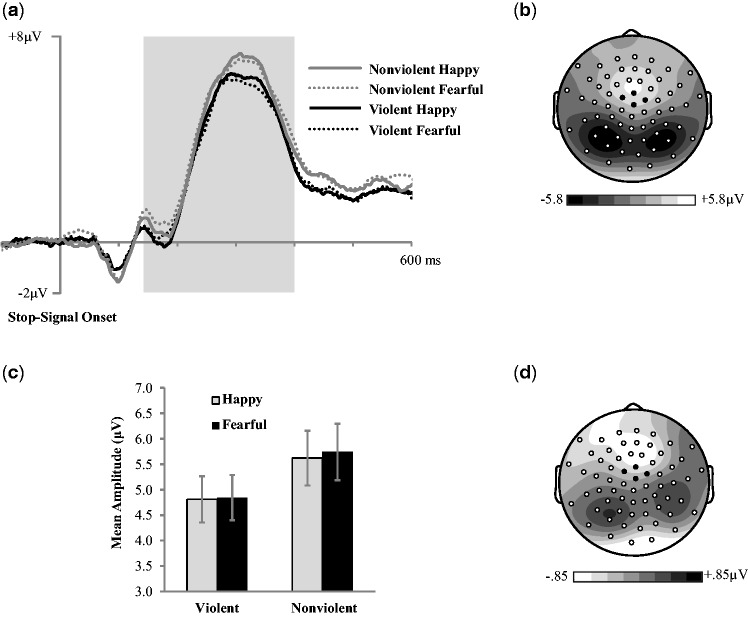
Fig. 6.
(a) Grand average ERPs (time-locked to stop-signal onset) for correct trials averaged across four central electrodes indicated in black on topographic map. (b) N200/P300 (140–400 ms post stop-signal onset) mean amplitude scalp topography for the average of all four experimental conditions. (c) N200/P300 mean amplitude (140–400 ms post stop-signal onset) across conditions. (d) N200/P300 mean amplitude (140–400 ms post stop-signal onset) subtraction topography for violent and nonviolent film conditions. Error bars represent ±1 SEM.
Two by two repeated-measure analysis of variance (ANOVAs) were conducted to examine the influence of face valence (happy and fearful) and film condition (violent and nonviolent) on emotional face processing and inhibitory control. As an estimate of effect size, partial eta square was reported for each main effect and interaction (Green and Salkind, 2008). Behavioral data were analysed by examining the effect of face valence and film condition on reaction time and accuracy for the go trials and accuracy and stop-signal delay for the stop trials. In regard to the EEG data, mean amplitude and 50% area latency was examined for the P100, N170 and P200 ERP components for the go trials and the N200/P300 component for the stop trials.
Results
Behavioral results
Mood manipulation (PANAS)
To assess the effect of the two films on mood, we calculated mood change scores for each participant in both the violent and nonviolent film conditions by subtracting their self-reported mood score after exposure to each film from their self-reported mood score before exposure to the film. Exposure to both films resulted in an overall increase in negative mood (t(49) = −7.22, P < 0.001) and decrease in positive mood (t(49) = 5.46, P < 0.001). A one-way ANOVA was conducted to examine possible differences between groups in overall changes in negative mood. There was no difference between the two films in regards to overall changes in negative mood (F(2, 48) = 0.06, P = 0.94).
Go trials
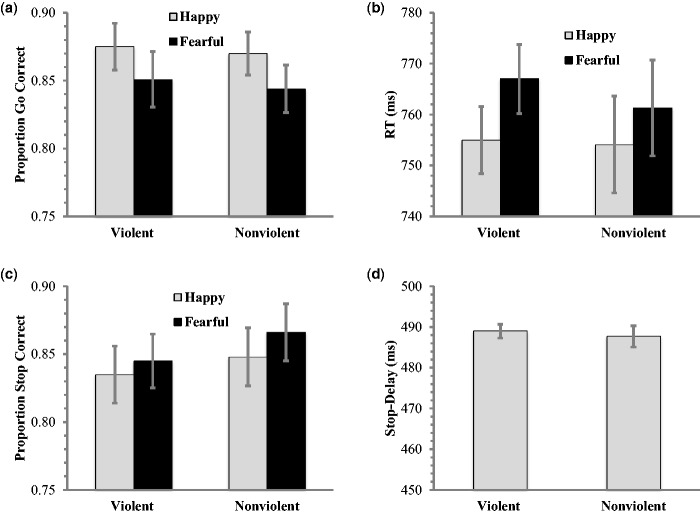
To assess potential behavioral differences in emotional-face processing after exposure to violent and nonviolent films, two repeated measures ANOVAs were run examining accuracy and RT for happy and fearful faces for the go trials only. With regard to accuracy, the main effect of film condition was not significant (F(1, 24) = .20, P = 0.67, η2p = 0.01); although, a main effect of face valence was observed (F(1, 24) = 14.48, P = 0.001, η2p = 0.38). However, the film condition by face–valence interaction was not significant (F(1, 24) = 0.03, P = 0.85, η2p = 0.001). Regardless of film condition, people were less accurate at identifying the gender of fearful faces compared to happy faces (See Figure 2a).
Fig. 2.
(a) Accuracy for go trials. Participants were significantly less accurate at identifying the gender of fearful faces. There was no effect of film condition or interaction. (b) RT for go trials. Consistent with accuracy results participants were faster to correctly identify happy than fearful faces. Once again there was no main effect of film condition; however, there was a reliable interaction such that people were slower at identifying the gender of fearful faces and this effect was exacerbated after exposure to a violent film. (c) Rate of success at stopping on stop-trials. Consistent with a longer RT for fearful faces on go trials, participants were less accurate at stopping on trials with happy than fearful trials. There was no effect of film condition or interaction. (d) There was no difference in stop-delay for participants in the violent and nonviolent film conditions. Error bars represent ±1 SEM.
Similar to the accuracy results, RT did not differ across film conditions (F(1, 24) = 0.10, P = 0.76, η2p = 0.004); however, there was a main effect of face valence (F(1, 24) = 29.06, P < 0.001, η2p = 0.55). In contrast to accuracy results, there was a film condition by face–valence interaction for RT(F(1,24) = 4.50, P = 0.04, η2p = 0.16). Regardless of film condition, people were generally slower at identifying the gender of fearful faces, and exposure to film violence further slowed RT (See Figure 2b).
Stop trials
To assess the impact of violent film exposure on inhibitory control, we conduced a two (film condition) by two (face valence) repeated measures analysis of variance (ANOVA) on stop-signal accuracy. There was no main effect of film condition (F(1, 23) = 0.06, P = 0.81, η2p = 0.002); however there was a main effect of face valence (F(1, 23) = 5.10, P = 0.03, η2p = 0.18), but no interaction (F(1, 23) = 0.58, P = 0.46, η2p = 0.02). Regardless of the film viewed, participants made fewer errors when stopping their response to fearful faces (See Figure 2c). This is consistent with the go trial results discussed above where participants were less accurate and slower to respond on trials with fearful faces. The behavioral data from the go trials suggest that it takes people longer to process features associated with fearful faces and as a result they may spend more time processing these faces. Threat-related stimuli captures attention (Vuilleumier and Schwartz, 2001) and it is difficult to disengage attention from threat stimuli (Koster et al., 2004). Because this task was an implicit measure of emotional processing, these threat-related stimuli may interfere with the gender discrimination task. This delay in processing may make it easier to recognize and withhold responding in stop trials.
In lieu of RT (as a correct response is achieved by withholding a response), the average delay (i.e. stop-signal delay) between the presentation of the face and the stop-signal was calculated. We collapsed across happy and fearful faces because the staircase is adjusted every two trials and thus a given measurement of stop-signal delay is not clearly the result of either a happy or fearful face (as they are presented in randomized order). A repeated measures ANOVA showed no effect of film condition on average stop-signal delay (F(1, 23) = 1.1, P = 0.29, η2p = 0.05; see Figure 2d).
ERP results
Go trials
P100 amplitude and peak latency
A two (film condition) by two (face valence) repeated measures ANOVA was conducted to examine the influence of media violence exposure on the occipital P100 (see Figure 3). There was no main effect of film condition (F(1, 24) = 1.94, P = 0.18, η2p = 0.08), but a significant effect of face valence (F(1, 24) = 8.17, P = 0.01, η2p = 0.25), and no interaction (F(1, 24) = 2.22, P = 0.15, η2p = 0.08). These analyses show that fearful faces resulted in an increased P100 mean amplitude in comparison to happy faces in the primary visual cortex, regardless of film condition (see Figure 3a).
A second repeated measures ANOVA was also conducted to examine the influence of media violence exposure on the right occipital P100 latency (50 percent area latency scores; Luck, 2005) in response to emotional faces. The main effect of film condition was not significant (F(1, 24) = 3.37, P = 0.08, η2p = 0.12) and no effect of face valence (F(1, 24) = 2.0, P = 0.17, η2p = 0.08) and, there was no interaction in P100 latency between film condition and face latency (F(1, 24)=0.41, P = 0.53, η2p = 0.02 see Figure 3d). Together, these analyses show that exposure to film violence did not significantly alter early visual processing, but that negatively valenced emotional expressions could potentially modulate early visual processing.
N170 amplitude and peak latency
In order to examine the effect of media violence exposure on the N170 amplitude, a 2X2X2 repeated measures ANOVA was run examining film condition (violent and nonviolent), face valence (happy and afraid), and lateralization (right or left hemisphere) (see Figure 4). The N170 was measured bilaterally at four posterior sites (two bilateral clusters of two electrodes each corresponding to T5/O1 and T6/O2) in order to take into account lateralized differences in holistic facial processing. There was an effect of film condition (F(1, 47) = 4.64, P = 0.04, η2p = 0.09), no effect of face valence (F(1, 47) = 0.14, P > 0.05, η2p = 0.003), an effect of lateralization (F(1, 47) = 4.50, P = 0.04, η2p = 0.09), and no interactions (F (1, 47) < 1.12, P > 0.05, η2p < 0.02). These results indicate that that N170 amplitude is larger in the right posterior portion of the brain, and that exposure to media violence is associated with a bilateral reduction in N170 amplitude (see Figure 4c).
In order to examine the effect of media violence exposure on facial encoding and processing on N170 latency, a 2 × 2 × 2 repeated measures ANOVA was run examining film condition (violent and nonviolent), face valence (happy and fearful) and lateralization (right or left hemisphere). There was no effect of film condition (F(1, 47) = .54, P > 0.05, η2p < 0.01), face valence (F(1, 47) = 0.10, P > 0.05, η2p = 0.002), no effect of lateralization (F(1, 47) = 0.02, P > 0.05, η2p < 0.001), and no significant interactions (F (1, 47) < 2.12, P > 0.05, η2p < 0.04). These results suggest that exposure to media violence, face valence, and hemisphere did not alter the time course of holistic facial processing (see Figure 4d).
P200 amplitude and peak latency
A two (film condition) by two (face valence) repeated measures ANOVA was conducted to examine the influence of media violence exposure on the frontal central P200 (see Figure 5). There was a main effect of film condition (F(1, 24) = 6.07, P = 0.02, η2p = 0.21), but no effect of face valence (F(1, 24) = 0.56, P = 0.46, η2p = 0.02), and no interaction (F(1, 24) = 0.68, P = 0.26, η2p = 0.01; see Figure 5c). These analyses show that exposure to film violence results in decreased frontal central P200 amplitude compared to the nonviolent film condition regardless of the emotion being displayed on the face.
A two (film condition) by two (face valence) repeated measures ANOVA was conducted to examine the influence of media violence exposure on the posterior P200. There was no effect of film condition (F(1, 24) = 1.24, P = 0.28, η2p = 0.05), no effect of face valence (F(1, 24) = 1.88, P = 0.18, η2p = 0.08) and no interaction (F(1, 24) = 0.07, P = 0.79, η2p = 0.001). These analyses show that exposure to film violence did not modulate the posterior P200 amplitude compared to the nonviolent film condition regardless of the emotion being displayed on the face.
A third repeated measures ANOVA was also conducted to examine the influence of media violence exposure on the frontal central P200 latency (50% area latency scores; Luck, 2005) in response to emotional faces. There was no effect of film violence on the frontal central P200 latency (F(1, 24) = 0.32, P = 0.58, η2p = 0.01), no effect of face valence (F(1, 24) = 0.001, P = 0.97, η2p = 0.001), and no interaction (F(1, 24) = 0.006, P = 0.71, η2p = 0.006 see Figure 5d). Together, these analyses show that exposure to film violence did not modulate the time course of emotional face processing.
Finally, a repeated measures ANOVA was also conducted to examine the influence of media violence exposure on the posterior P200 latency (50% area latency scores; Luck, 2005) in response to emotional faces. There was no effect of film condition (F(1, 24) = 1.85, P = .19, η2p = 0.07), no effect of face valence (F(1, 24) = 0.01, P = 0.99, η2p = 0.001), and no interaction (F(1, 24) = 0.03, P = 0.86, η2p = 0.001). Together, these analyses show that exposure to film violence did not modulate the posterior P200 amplitude or latency to emotional faces.
Stop trials
N200/P300 amplitude
A two-way repeated measures ANOVA was conducted to examine the influence of media violence exposure on the frontocentral N200/P300 mean amplitude in response to emotional faces (Figure 5). There was a main effect of film condition, with exposure to film violence resulting in less positive waveforms (F(1, 23) = 5.54, P = 0.03, η2p = 0.19), and no effect of face valance (F(1, 23) = 0.33, P = 0.57, η2p = 0.01). The film condition × face valence interaction was not significant (F(1, 23) = 0.30, P = 0.59, η2p = 0.01; see Figure 6). These results suggest that exposure to film violence resulted in decreased cognitive resources being allocated to inhibitory control processes.
DISCUSSION
Results from this study suggest that short-term exposure to film violence can lead to ‘emotional anesthetization’ or a reduction in cognitive resources allocated to processing emotional face expressions. The results also suggest that exposure to media violence can lead to alteration in the way people process human faces. Media violence also subsequently influenced the neural correlates of inhibitory control processes. The current results also suggest that media violence exposure did not modulate the early visual processing in the primary visual cortex, but did modulate early holistic processing of faces as well as processing of emotional information contained in facial expressions These results build on past research that has consistently shown that exposure to violence in the media is related to desensitization to emotion (Carnagey et al., 2007), decreased prosocial behavior (Bushman and Anderson, 2009), and increased aggressive behavior (Bushman and Anderson, 2001). The results from this study elucidate the neural processes associated with these changes in behavior observed in past media violence research.
Behavioral data demonstrated that participants were less accurate and slower in gender discrimination when fearful faces were presented even though the emotion of the faces was irrelevant to the gender discrimination task (i.e. it was an implicit emotion processing task). Past research has shown threat-related stimuli captures attention relative to neutral or nonthreat-related stimuli (Pourtois et al., 2004). Thus, the attentional capture by fearful stimuli may make it more difficult to disengage in order to adeptly respond to the task at hand. In this study, slower behavioral responses when fearful stimuli were present likely resulted from difficulties disengaging attention to threat-related information. Likewise, consistent with earlier studies (Vuilleumier and Driver, 2007; Eimer and Holmes, 2007) we found that early visual processing as measured by the P100 ERP was also sensitive to face valence with fearful faces evoking a larger amplitude than happy faces. In addition, exposure to film violence further slowed responses on trials that involved fearful faces. This finding is consistent with past behavioral research that shows that exposure to short-term media violence results in a bias towards negatively valenced information and hostile expectations (Bushman and Anderson, 2002). Processing fearful faces may have triggered the vigilance surveillance system (Nitschke and Heller 2002), thus better preparing participants to quickly inhibit their response when the stop-signal appeared. Participants were significantly better at inhibiting their response to fearful faces as compared to happy faces. While exposure to film violence did not alter early visual attention as measured by the P100 ERP component, exposure to film violence was associated with a bilateral reduction in the N170 ERP component. The N170 ERP component has been associated with holistic face processing (Sagiv and Bentin, 2001). Regardless of film condition, the N170 was larger in the right posterior region and this finding is consistent with past research that has demonstrated right lateralization for human face processing (McCarthy et al., 1997). Previous media violence researchers have found that exposure to violence in the media results in denying the ‘humanness in others’ (Greitemeyer and McLatchie, 2011). For example, participants who played a violent video game were more likely to assign less human attributes to people than people who played a nonviolent video game and were less likely to view people as unique (Greitemeyer and McLatchie, 2011). Perhaps the observed reduction in the N170 component after exposure to violence is reflective of the cognitive processes associated with these changes in behavior associated with dehumanization of others.
Similarly, previous research that has shown that participants begin to process emotional aspects of facial expressions around 200 ms in frontal brain regions (Holmes et al., 2006). In this study, brief exposure to film violence resulted in decreased frontal central P200 amplitude in response to both happy and fearful faces compared to exposure to these same faces after watching a nonviolent film. Decades of research on the effects of media violence have found that exposure to media violence results in desensitization to real-life violence (Carnagey et al., 2007). Bushman and Anderson (2009) stated that exposure to violence in the media leaves people ‘comfortably numb’ to the pain and suffering of others. Perhaps this desensitization and numbness to others is a result of decreased processing and attention to emotional information, even the relatively automatic processing of emotional faces.
With regard to inhibitory control, after exposure to film violence, participants displayed changes in the N200/P300 complex. Specifically, participants exposed to a violent film showed decreased N200/P300 amplitude when inhibiting behavior than after exposure to a nonviolent film clip. The frontocentral N200/P300 ERP complex is indicative of motor/behavioral inhibition (Enriquez-Geppert et al., 2010). This study suggests that exposure to film violence resulted in decreased cognitive resources needed for inhibiting behavioral responses as indicated by decreased N200/P300 amplitude. Given the decreased P200 amplitude following film violence exposure, it is possible that participants who watched the violent film spent less cognitive resources processing the emotional information contained in faces, and subsequently needed less cognitive resources to successfully inhibit motor behavior. Past research has shown that it is more difficult to inhibit behavior in the presence of emotion (Chan et al., 2008) because it is more difficult to disengage from emotional information (Schaefer et al., 2003). The results of this study suggest that exposure to media violence leads to desensitization and decreased processing of emotional information and thus less cognitive resources are needed to inhibit behavior.
This study offers a first step towards identifying how violent media influences the neural correlates of emotional face processing and subsequent inhibitory control functions. However, the manipulation was only a short-term experimental manipulation and cannot address the long-term effects of media violence exposure on emotional and cognitive processes. Future researchers should examine the potential long-term effects of media violence exposure on emotion processing and how this may interact with acute exposure to violence. Likewise, this study compared the processing of happy and fearful faces in order to assure that the facial stimuli were equally engaging (Goeleven et al., 2008). However, this manipulation did not allow for a neutral condition. Therefore, it is possible that exposure to film violence modulates facial processing in general, regardless of the emotional information. This seems unlikely because early visual attention was not modulated by film condition, but future research should address this limitation and compare the effects of exposure to violence in the media on emotional face processing compared to a neutral face condition. This study focused on emerging adults who are a major consumer of media violence. Future studies should evaluate the effect on children or adolescents who are exposed to large amounts of violence in the media. Children and adolescents are still developing scripts and schemas regarding aggression and violence and thus may be at increased risk from violent media.
The media is saturated with violence and aggression and people are spending more time with the media today than ever before. The findings of this study support the idea that exposure to media violence leads to emotional anesthetization, particularly with regard to desensitization of the emotional experiences of others as reflected in their facial emotions. Given the accumulating empirical evidence supporting these finding, parents and policy makers should seriously evaluate the costs and consequences of media violence exposure on children and society.
CONFLICT OF INTEREST
None declared.
Acknowledgments
The authors thank Krishna Bharani for assistance in programming the stop-signal task, and Robert Palumbo and Callie Short for help with data collection and recruitment. American Psychological Association Scott Mesh Honorary Grant for Research in Psychology and the Provost and The Carroll and Adelaide Johnson Scholarship Funds for their generous support.
References
- Anderson AK, Christoff K, De Rosa E, Gabrieli JDE. Neural correlates of the automatic processing of threat facial signals. The Journal of Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CA, Shibuya A, Ihori N, et al. Violent video game effects on aggression, video games, empathy, and prosocial behavior in Eastern and Western countries: A meta-analytic review. Psychological Bulletin. 2010;136:151–173. doi: 10.1037/a0018251. [DOI] [PubMed] [Google Scholar]
- Ashley V, Vuilleumier P, Swick D. Time course and specificity of event-related potentials to emotional expression. NeuroReport. 2004;15:211–216. doi: 10.1097/00001756-200401190-00041. [DOI] [PubMed] [Google Scholar]
- Badzakova-Trajkov G, Barnett KJ, Waldie KE, Kirk IJ. An ERP investigation of the Stroop task: The role of the cingulate in attentional allocation and conflict resolution. Brain Research. 2009;1253:139–148. doi: 10.1016/j.brainres.2008.11.069. [DOI] [PubMed] [Google Scholar]
- Bartholow BC, Bushman BJ, Sestir MA. Chronic violent video game exposure and desensitization to violence: Behavioral and event-related potential data. Journal of Experimental Social Psychology. 2006;42:532–539. [Google Scholar]
- Bushman BJ, Anderson CA. Comfortably numb: Desensitizing effects of violent media on helping others. Psychological Science. 2009;20:273–277. doi: 10.1111/j.1467-9280.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Böhnke R, Kruk MR, Naumann E. Influence of aggression on information processing in the emotional stroop task–an event-related potential study. Frontiers in Behavioral Neuroscience. 2009;3:28–31. doi: 10.3389/neuro.08.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman BJ, Anderson CA. Violent video games and hostile expectations: A test of the general aggression model. Personality and Social Psychology Bulletin. 2002;28:1679–1686. [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelink M, Guerit JM. Discrimination of emotional facial expressions in a visual oddball task: An ERP study. Biological Psychology. 2002;59:171–186. doi: 10.1016/s0301-0511(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Carnagey NL, Anderson CA, Bushman BJ. The effect of video game violence on physiological desensitization to real-life violence. Journal of Experimental Social Psychology. 2007;43:489–496. [Google Scholar]
- Chan RCK, Shum D, Toulopoulou T, Chen EYH. Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Coyne SM, Nelson DA, Lawton F, et al. The effects of viewing physical and relational aggression in the media: Evidence for a cross-over effect. Journal of Experimental Social Psychology. 2008;44:1551–1554. [Google Scholar]
- Dennis TA, Chen C. Neurophysioloigcal mechanisms in the emotional modulation of attention: The interplay between threat sensitivity and attentional control. Biological Psychology. 2007;76:1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage. 2010;51:877–887. doi: 10.1016/j.neuroimage.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Fanti KA, Vanman E, Henrich E, Avraamides MN. Desensitization to media violence over a short period of time. Aggressive Behavior. 2009;35:179–187. doi: 10.1002/ab.20295. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ. Blazing angels or resident evil? Can violent video game be a force for good? Review of General Psychology. 2010;14:68–81. [Google Scholar]
- Gentile DA, Choo H, Liau A, et al. Pathological video game use among youths: A two-year longitudinal study. Pediatrics. 2011;127:319–329. doi: 10.1542/peds.2010-1353. [DOI] [PubMed] [Google Scholar]
- Goeleven R, De Raedt R, Leyman L, Verschuere B. The Karolinska directed emotional face: A validation study. Cognition and Emotion. 2008;22:1094–1118. [Google Scholar]
- Green SB, Salkind NJ. Using SPSS for Windows and Macintosh: Analyzing and Understanding Data. Upper Saddle River, NJ: Pearson Prentice Hall; 2008. [Google Scholar]
- Greitemeyer T, McLatchie N. Denying humanness to others: A newly discovered mechanism by which violent video games increase aggressive behavior. Psychological Science. 2011;22:659–665. doi: 10.1177/0956797611403320. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Vorderer P. It's okay to shoot a character: Moral disengagement in violent video games. Journal of Communication. 2010;60:94–119. [Google Scholar]
- Herrmann MJ, Ehlis AC, Ellgring H, Fallgatter AJ. Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs) Journal of Neural Transmission. 2005;112:1073–1081. doi: 10.1007/s00702-004-0250-8. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kiss M, Eimer M. Attention modulates the processing of emotional expression triggered by foveal faces. Neuroscience Letters. 2006;394:48–52. doi: 10.1016/j.neulet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbroad M, Scherg M, Spitzer M. The time course of brain activations during response inhibition: Evidence from event-related potentials in a go/no go task. Cognitive Neuroscience and Neuropsychology. 1998;19:765–770. doi: 10.1097/00001756-199803090-00037. [DOI] [PubMed] [Google Scholar]
- Kirsh SJ, Mounts JRW. Violent video game play impacts facial emotion recognition. Aggressive Behavior. 2007;33:353–358. doi: 10.1002/ab.20191. [DOI] [PubMed] [Google Scholar]
- Kirsh SJ, Mounts JRW, Olczak PV. Violent media consumption and the recognition of dynamic facial expressions. Journal of Interpersonal Violence. 2006;21:571–584. doi: 10.1177/0886260506286840. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Morrison RG, Viskontas I, et al. Distraction during relational reasoning: The role of prefrontal cortex in interference control. Neuropsychologia. 2008;46:2020–2032. doi: 10.1016/j.neuropsychologia.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- Lundqvist, D., Flykt, A., Öhman, A. (1998). The Karolinska Directed Emotional Faces-KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. ISBN 91-630-7164-9.
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Morrison RG, Krawczyk D, Holyoak KJ, et al. A neurocomputational model of analogical reasoning and its breakdown in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. 2004;16:260–271. doi: 10.1162/089892904322984553. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W. The neuropsychology of anxiety disorders: Affect, cognition, and Neural circuitry. In: D'Haenen H, den Boer JA, Westenberg H, Wilner P, editors. Biological Psychiatry. Chichester, England: John Wiley and Sons Ltd; 2002. pp. 975–988. [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MA, Smidts DP, Sergeant JA, et al. Executive functions in preschool children with aggressive behavior: Impairments in inhibitory control. Journal of Abnormal Child Psychology. 2008;36:1097–1107. doi: 10.1007/s10802-008-9235-7. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Schwartz S, Vuilleumier P. Fear and stop: A role for the amygdala in motor inhibition by emotional signals. NeuroImage. 2011;55:1825–1835. doi: 10.1016/j.neuroimage.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Sagiv N, Bentin S. Structural encoding of human and schematic faces: Holistic and part-based processes. Journal of Cognitive Neuroscience. 2001;13:937–951. doi: 10.1162/089892901753165854. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Collette F, Philippot P, et al. Neural correlates of “hot” and “cold” emotional processing: A multilevel approach to the functional anatomy of emotion. NeuroImage. 2003;18:938–949. doi: 10.1016/s1053-8119(03)00009-0. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Silton RL, Heller W, Towers DN, et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. NeuroImage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann E, Swanson J. Exposure to violent video games increases automatic aggressiveness. Journal of Adolescence. 2004;27:41–52. doi: 10.1016/j.adolescence.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Biological Sciences. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Beware and be aware: Capture of spatial attention by fear-related stimuli in neglect. NeuroReport. 2001;12:1119–1122. doi: 10.1097/00001756-200105080-00014. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weber R, Ritterfeld U, Mathiak K. Does playing violent video games induce aggression? Empirical evidence of a functional magnetic resonance imaging study. Media Psychology. 2006;8:39–60. [Google Scholar]