Abstract
Despite being one of the healthiest developmental periods, morbidity and mortality rates increase dramatically during adolescence, largely due to preventable, risky behaviors. Heightened reward sensitivity, coupled with ineffective cognitive control, has been proposed to underlie adolescents’ risk taking. In this study, we test whether reward sensitivity can be redirected to promote safe behavior. Adolescents completed a risk-taking task in the presence of their mother and alone during fMRI. Adolescents demonstrated reduced risk-taking behavior when their mothers were present compared with alone, which was associated with greater recruitment of the ventrolateral prefrontal cortex (VLPFC) when making safe decisions, decreased activation in the ventral striatum following risky decisions and greater functional coupling between the ventral striatum and VLPFC when making safe decisions. Importantly, the very same neural circuitry (i.e. ventral striatum) that has been linked to greater risk-taking can also be redirected toward thoughtful, more deliberative and safe decisions.
Keywords: adolescence, risk taking, rewards, fMRI, influence, family
INTRODUCTION
Despite being one of the healthiest developmental periods, morbidity and mortality rates increase 300% from childhood to adolescence (National Center for Health Statistics, 2013; Kann et al., 2014) with over 70% of adolescent deaths each year due to risk-taking behaviors, such as reckless driving (Centers for Disease Control and Prevention, 2012). Evidence from developmental neuroscience suggests that risk-taking behavior increases during adolescence partly due to relatively early functional development of brain regions involved in reward sensitivity (e.g. ventral striatum) compared with more protracted functional maturation of brain regions supporting cognitive control (e.g. the prefrontal cortex [PFC]; Somerville et al., 2010). Heightened reward sensitivity, coupled with ineffective cognitive control, has largely been thought to underlie adolescents’ orientation toward risky behavior (Steinberg, 2008; Casey et al., 2011). While most prior work has focused on how this heightened reward sensitivity creates vulnerabilities for adolescents, leading to increases in risk taking, scholars have begun to question these dual systems models of adolescent brain development (Crone and Dahl, 2012; Pfeifer and Allen, 2012). Recent evidence suggests that rewards can play an adaptive role, facilitating cognitive control. For example, rewarding cues (e.g. monetary rewards; happy faces) enhance inhibitory control (Hardin et al., 2009; Kohls et al., 2009; Teslovich et al., 2014), perhaps by serving to motivate adolescents to engage in effortful control. Moreover, the ventral striatum may itself serve a regulatory function (Pfeifer et al., 2011). Thus, heightened reward sensitivity, which has largely been proposed to underlie adolescent risk taking (Steinberg, 2008; Casey et al., 2011), may also shape adolescents’ motivations to engage in greater cognitive control. In this study, we test whether adolescents’ heightened reward sensitivity can be redirected to promote cognitive control and safe behavior during risk-taking. In other words, can the heightened reward response we typically see during risk taking be reduced (i.e. risky behavior is less rewarding) and redirected to promote cognitive control?
Because neural regions involved in motivation and cognitive processes undergo significant reorganization during adolescence (Nelson et al., 2005), the adolescent brain is thought to be highly flexible and malleable (Crone and Dahl, 2012) and therefore particularly sensitive to social influences. While prior work has focused on the social contexts which may increase risk taking, for instance peer presence (Chein et al., 2011), social contexts can also decrease risk taking, such as parental presence. Indeed, parents represent one of the most direct and proximal sources of influence over teenagers. Although parents tend to adjust their supervisory practices to allow their adolescent children to be more independent, adolescents tend to engage in more maladaptive, risky behaviors during unsupervised time (Richardson et al., 1993; Beck et al., 2001; Borawski et al., 2003), highlighting the important role of parents in decreasing adolescent risk taking. While parents may decrease adolescent risk-taking merely by serving as gatekeepers and limiting adolescents’ opportunities to make poor decisions, we propose that parents may actually change the ways in which adolescents think and reason about risks during both active/deliberative as well as more automatic decision-making processes.
Parental influence on adolescent risk taking may occur in several ways. First, due to the relative immaturity of their prefrontal cortex, adolescents may not yet have the cognitive resources to effectively avoid risky behaviors and so parents may play an important scaffolding role, helping their children to regulate their behaviors and engage in more adaptive decision making. Thus, parents may facilitate improved cognitive control and thereby reduce their adolescents’ risk taking. Second, parents may reduce the affective and rewarding nature of engaging in risky behavior. That is, risk taking may be comparatively less rewarding and more aversive in the presence of family, and so the typical heightened reward response following risky behaviors may be attenuated. Lastly, parents may serve to redirect adolescents’ reward sensitivity toward safe behavior, such that reward and cognitive control systems interact to promote safe choices. Most prior work has focused on the negative contexts (e.g. peer presence) in which heightened reward sensitivity and immature cognitive control interact to lead to maladaptive behavior (Chein et al., 2011). However, several studies have demonstrated that rewards can also lead to improvements in cognitive control through bottom-up processes that increase activation in brain regions involved in regulation (e.g. ventrolateral prefrontal cortex [VLPFC]; Geier et al., 2010; Smith et al., 2011). We therefore examined whether parental presence functions through bottom-up processing, whereby the parent elicits a reward-related response that boosts adolescents’ motivation to regulate their behavior. Thus, we tested whether parents increase neural coupling between the ventral striatum and PFC to facilitate safe behavior during risk taking.
METHODS
Participants
Thirty adolescent-mother dyads participated. Two participants were excluded due to excessive movement (>3 mm), two participants did not complete the scan, and one participant’s behavioral responses were not recorded due to technical issues. Our final sample included 25 fourteen-year-old adolescents and their mother (adolescents: Mage = 14.43 years; 15 males; mothers: Mage = 43.89 years). Adolescent participants were predominantly from European-American (n = 18) or African-American (n = 5) backgrounds with the remaining from Asian-American (n = 1) or Central-American (n = 1) backgrounds. Mothers reported their highest levels of education as high school (n = 1), some college (n = 8), college (n = 3) and graduate, medical or law school (n = 13). All participants provided written assent and consent in accordance with the Institutional Review Board.
Risk-taking task
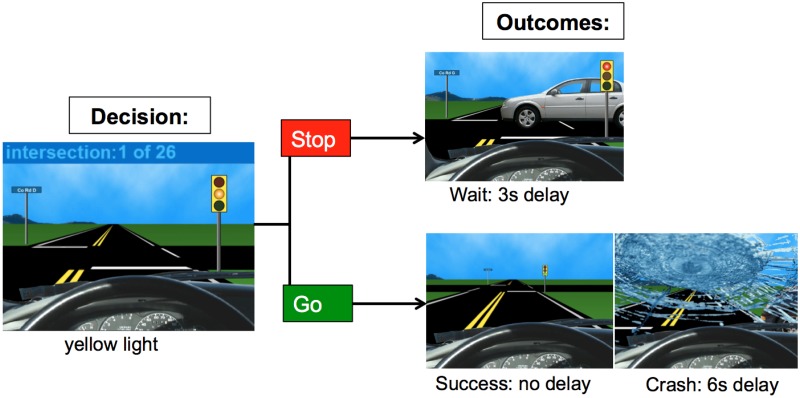
The stoplight task measures risk taking at the behavioral and neural level (Gardner and Steinberg, 2005; Chein et al., 2011). Adolescents completed a simulated driving course in which they encountered a number of stoplights and had to decide whether to ‘stop’ or ‘go’ by pressing one of two buttons (see Figure 1). A decision to ‘go’ through the intersection is the fastest option, but participants risk the possibility of crashing, which causes a 6 s delay. If they choose to ‘stop’, participants do not risk crashing, but it results in a short, 3 s delay. Participants were told that the goal is to get through the driving course in as short of a time as possible to win more money. The monetary incentive was used to encourage risk taking (Chein et al., 2011).
Fig. 1.
The stoplight task.
Adolescents completed two rounds of the stoplight task during two functional brain scans. Similar to the manipulation used by Steinberg and colleagues with peers (Chein et al., 2011), adolescents played one round of the game while their mother was watching and one round in which they were alone. During the mother condition, the participant’s mother was instructed to speak into the intercom naturally and authentically, informing their child that they would be watching during the whole scan. Instructions were given to avoid any comments that might explicitly or intentionally bias their child’s behavior. During the alone condition, the researcher spoke into the microphone and informed the participant that nobody would be watching during the round. Run order was counterbalanced across participants.
During each round of the task, participants were presented with 26 intersections. The probability of crashing was kept constant at 30% (i.e. eight intersections out of the 26 total intersections had cars approaching on the cross street, resulting in a crash if the participant made a ‘go’ decision), but this was not explicitly revealed to participants. The timing of traffic signals and the presence of a car on the cross street varied so as to be unpredictable by the participant and to introduce variable ITIs. At the end of each round, participants were presented with their overall time and the number of crashes. Due to learning effects, such that participants show higher behavioral risk performance during initial rounds of the task followed by decreased and more stable risk patterns thereafter (Peake et al., 2013), participants played two practice rounds of the task, each with 26 intersections, prior to entering the scanner.
Behaviorally, we examined the percent of decisions that are risky and safe and examined differences based on maternal presence. In terms of reaction time differences, the task is not optimized to collect reaction time. Because of the long inter-trial interval (12 s between stoplights), participants often make their decision prior to seeing the yellow light. Although they are instructed to make the decision and subsequent button response only after seeing the yellow light, many participants press the button before the yellow light appears. Such button responses are not recorded. Unfortunately, this precludes our ability to examine reaction time differences.
fMRI data acquisition and analysis
fMRI data acquisition
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The stoplight task included T2*-weighted echoplanar images (EPI) (slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; voxel size 2.5 × 2.5 × 3 mm3). Structural scans consisted of a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4 s; TE = 64 ms; FOV = 230; matrix = 192 × 192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.3 ms; FOV = 230; matrix = 256 × 256; sagittal plane; slice thickness = 1 mm; 192 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
fMRI data preprocessing and analysis
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing for each participant’s images included spatial realignment to correct for head motion (no participant exceeded 2 mm of maximum image-to-image motion in any direction). The realigned functional data were coregistered to the high resolution MPRAGE, which was then segmented into cerebrospinal fluid, grey matter and white matter. The normalization transformation matrix from the segmentation step was then applied to the functional and T2 structural images, thus transforming them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. The normalized functional data were smoothed using an 8 mm Gaussian kernel, full-width-at-half maximum, to increase the signal-to-noise ratio.
Statistical analyses were performed using the general linear model (GLM) in SPM8. Each trial was convolved with the canonical hemodynamic response function. High-pass temporal filtering with a cutoff of 128 s was applied to remove low-frequency drift in the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1.
In each participant’s fixed-effects analysis, a GLM was created with four regressors of interest each for the alone condition and mother condition, modeled as events: two decision regressors (Stop and Go) and two outcome regressors (Crash and Pass). In addition, the wait time after stop decisions were modeled as well as the final ‘Game Over’ period at the end of each run, to remove these from the implicit baseline. This resulted in 12 conditions, six each for alone and mother: StopMother, GoMother, PassMother, CrashMother, WaitMother, GameOverMother, StopAlone, GoAlone, PassAlone, CrashAlone, WaitAlone and GameOverAlone.
Because the task was self-paced, the duration of the decision trials (stop or go) represented the time from which the traffic light appeared until the participant made a response, and the duration for the outcome (pass or crash) was 1 s. The onset of the crash event corresponded to another car crashing into the participant’s car. The pass and wait events had no specific onset time. However, because the crash events happened at most 2 s after the yellow light, we modeled the pass and wait events as being 2 s after the yellow light, the point at which the outcome of the risky decision was clear. Each was modeled with a 1 s duration. Null events, consisting of the jittered intertrial intervals, were not explicitly modeled and therefore constituted an implicit baseline.
The parameter estimates resulting from the GLM were used to create linear contrast images comparing each of four event conditions (decisions: Go, Stop; outcomes: Crash, Pass) during the Alone round to the corresponding conditions in the Mother round. The individual subject contrasts were then submitted to random-effects, group-level analyses. The following analyses were run at each voxel across the entire brain volume: StopMother-StopAlone, GoMother-GoAlone and PassMother-PassAlone. Because of the low frequency of crash events (i.e. there were only eight possible crash events per run), we had restricted power to test for CrashMother-CrashAlone. We present this contrast for the 17 participants who had at least four crash trials per condition.
We conducted psychophysiological interaction (PPI) analyses (Friston et al., 1997) to examine functional coupling between the ventral striatum and cognitive control regions. We used the ventral striatum as the seed region, as the striatum has been consistently linked with the experience of rewards. The striatum was defined structurally using the WFUpickatlas (Tzourio-Mazoyer, et al., 2002; Maldjian et al., 2003, 2004). PPI analyses were run using a generalized form of context-dependent PPI. Specifically, the automated gPPI toolbox in SPM (gPPI; McLaren et al., 2012) was used to i) extract the deconvolved times series from the ventral striatum region of interest (ROI) for each participant to create the physiological variables; ii) convolve each trial type with the canonical HRF, creating the psychological regressor; and iii) multiply the time series from the psychological regressors with the physiological variable to create the PPI interaction terms. This interaction term identified regions that covaried in a task-dependent manner with the striatum. For the first-level model, one regressor representing the deconvolved BOLD signal was included alongside each psychological and PPI interaction terms for each condition type to create a gPPI model. These first levels models created gPPI models for the following contrasts: Stop Decisions, Go Decisions, and Pass Outcomes for the Mother Present and Alone conditions separately. At the group level, we conducted random effect analyses to compare functional coupling between conditions of interest.
To correct for multiple comparisons, we conducted a Monte Carlo simulation implemented using 3dClustSim in the software package AFNI (Ward, 2000). We used our group-level brain mask, which included only gray matter. Results of the simulation indicated a voxel-wise threshold of P < 0.005 combined with a minimum cluster size of 35 voxels for the whole brain, corresponding to P < 0.05, false wise error (FWE) corrected. We used the MarsBaR toolbox to extract parameter estimates from significant clusters in the group-level analyses. To ensure that the neural effects are not driven by differences in the number of trials in the analyses (e.g. more stop decisions when mothers are present then when alone), all fMRI analyses covary for the number of decisions to stop during Alone and Mother conditions.
RESULTS
Behavioral results
Adolescents made significantly more risky decisions (i.e. ‘go’) when alone than when their mothers were present [F(1, 24) = 3.9, P < 0.001; Figure 2]. In prior studies using this same task, the presence of peers increased risk-taking behavior of adolescent participants (Gardner and Steinberg, 2005; Chein et al., 2011). In contrast, we find that the presence of mothers reduces risk-taking behavior.
Fig. 2.
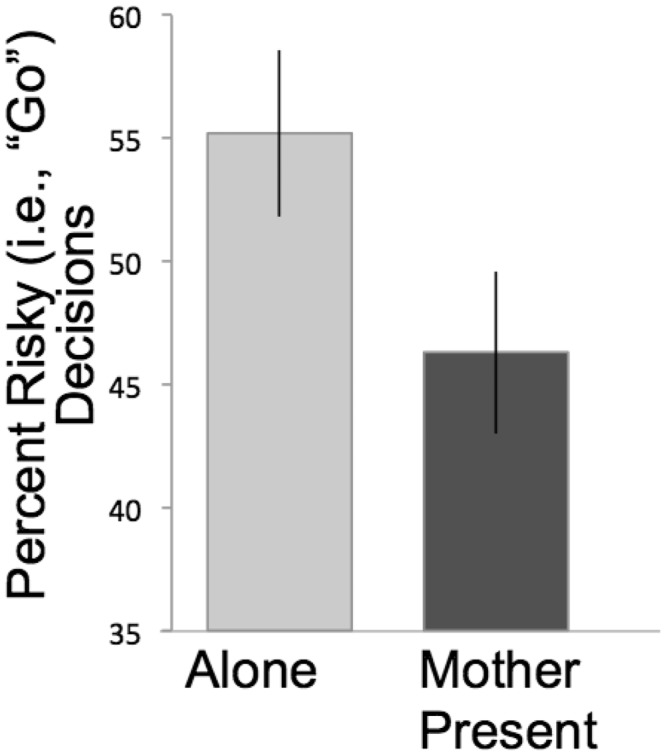
Behavioral performance. Adolescents made significantly fewer risky choices when their mothers were present than when alone. Error bars represent SEM.
fMRI results
Main effects
Our first fMRI analyses collapsed across conditions to test what regions were activated when making safe and risky choices irrespective of maternal presence. In whole brain t-tests, we examined neural activation during Stop decisions, Go decisions and Passes. When adolescents made decisions to Stop (compared with baseline), they did not show heightened activation in any region. When making Go decisions (compared with baseline), adolescents showed heightened activation in the ventral striatum, insula, dACC and SMA. In addition, we compared activation between Stop and Go decisions collapsed across conditions. Adolescents demonstrated greater activation in the insula, dACC and midbrain when making Go decisions relative to Stop decisions. The only region that was more active during Stop compared with Go decisions was the precentral gyrus. Finally, when adolescents successfully passed through an intersection without crashing following a risky decision, they demonstrated heightened activation in a large cluster encompassing the superior frontal gyrus, precuneous and extending into the left temporoparietal junction (TPJ) (Table 1).
Table 1.
Brain regions activated during the stoplight task irrespective of maternal presence
| Contrast | Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|---|
| Stop Decisions | |||||||
| – | |||||||
| Go Decisions | |||||||
| Ventral striatum | −12 | 5 | −2 | 4.96 | 191 | ||
| R Insula | 33 | 29 | −2 | 3.48 | 110 | ||
| L Insula | −36 | 14 | 7 | 3.84 | 93 | ||
| dACC | 32/24 | 3 | 14 | 43 | 4.21 | 621 | |
| Precentral gyrus | 33 | −13 | 67 | 4.24 | 472 | ||
| SMA | −15 | −2 | 54 | 3.35 | 57 | ||
| Stop-Go Decisions | |||||||
| Precentral gyrus | −42 | −22 | 61 | 4.67 | 47 | ||
| Go-Stop Decisions | |||||||
| Insula | 39 | 5 | 10 | 5.94 | 266 | ||
| dACC | 9 | 8 | 43 | 4.73 | 1278a | ||
| SMA | 9 | −20 | 48 | 4.26 | a | ||
| Postcentral gyrus | 45 | −19 | 49 | 5.53 | a | ||
| Midbrain | 6 | −28 | −14 | 4.18 | 229 | ||
| R Precuneus | 18 | −76 | 40 | 5.32 | 165 | ||
| L Precuneus | −9 | −73 | 46 | 5.39 | 392 | ||
| Pass Outcome | |||||||
| Superior frontal gyrus | 24 | 11 | 55 | 5.97 | 2957b | ||
| R Precuneus | 9 | −73 | 43 | 4.99 | b | ||
| L Precuneus | −12 | −73 | 46 | 4.07 | b | ||
| TPJ | 54 | −42 | 34 | 5.49 | b | ||
| Cerebellum | −6 | −58 | −26 | 3.93 | 122 | ||
Note. BA refers to putative Broadman’s areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. TPJ, temporoparietal junction; SMA, supplementary motor area; dACC, dorsoanterior cingulate cortex. a,b regions are part of the same cluster; – indicates no significant clusters were identified.
Interactions between Alone and Mother present condition
Next, we examined differences in neural activation during Stop decisions, Go decisions and Passes when adolescents were alone compared with mother present (Alone vs Mother).
Decisions
When adolescents made decisions to stop when their mothers were present compared with alone (StopMother-StopAlone), they showed increased activation in the left VLPFC and the MPFC (Figure 3a, Table 2). For descriptive purposes, we extracted parameter estimates of signal intensity from the VLPFC and MPFC clusters when adolescents made Stop decisions when alone (StopAlone-baseline) and when their mother was present (StopMother-baseline). As shown in Figure 3b, adolescents demonstrated significantly greater activation in the VLPFC and MPFC when making safe decisions in the presence of their mother compared with alone. When adolescents made decisions to go through the intersection when their mothers were present compared with alone (GoMother-GoAlone), they also showed increased activation in the MPFC (Table 2).
Fig. 3.
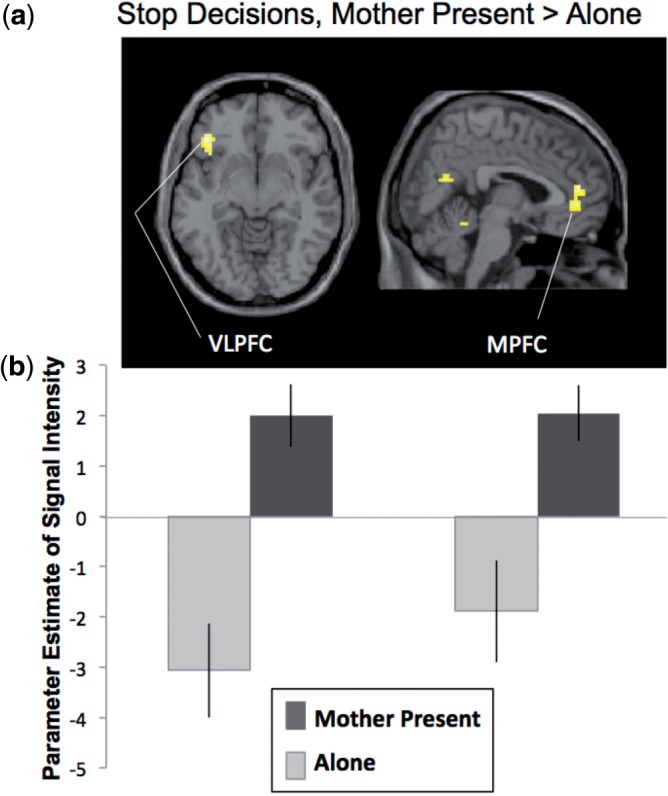
(a) Neural activation during decisions to ‘stop’ when adolescents’ mothers were present compared with alone. For descriptive purposes, parameters estimates of signal intensity were extracted from the VLPFC and MPFC cluster separately for Stop decisions when Alone and Mother Present (relative to baseline). Error bars represent SEM.
Table 2.
Brain regions activated during the stoplight task when adolescents’ were alone compared with mother present
| Contrast | Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|---|
| Decisions | |||||||
| StopMother-StopAlone | |||||||
| R MPFC | 32/10 | 9 | 47 | 10 | 4.34 | 53 | |
| L VLPFC | 47/11 | −42 | 35 | −8 | 3.01 | 46 | |
| Precuneus | 0 | −61 | 19 | 4.10 | 60 | ||
| L Postcentral gyrus | −42 | −19 | 34 | 4.19 | 75 | ||
| R Postcentral gyrus | 51 | −7 | 34 | 3.95 | 44 | ||
| StopAlone-StopMother | |||||||
| – | – | ||||||
| StopMother | |||||||
| VLPFC | 47 | −39 | 23 | −8 | 3.33 | 36 | |
| StopAlone | |||||||
| – | |||||||
| GoMother-GoAlone | |||||||
| R MPFC | 32/10 | 9 | 41 | −8 | 4.15 | 62 | |
| L MPFC | 10 | −9 | 44 | −5 | 3.97 | 44 | |
| GoAlone-GoMother | |||||||
| – | |||||||
| GoMother | |||||||
| – | – | ||||||
| Go Alone | |||||||
| Midbrain | 6 | −22 | −5 | 4.02 | 117 | ||
| R ACC | 24/32 | 12 | −1 | 46 | 3.52 | 417 | |
| R Precentral gyrus | 45 | −19 | 49 | 3.90 | 157 | ||
| Outcomes | |||||||
| PassAlone-PassMother | |||||||
| VS | 3 | 5 | −11 | 4.50 | 52 | ||
| L Amygdala | −26 | 5 | −24 | 3.26 | 36 | ||
| PassMother-PassAlone | |||||||
| – | – | ||||||
| PassMother | |||||||
| R Precentral gyrus | 27 | −22 | 52 | 3.70 | 54 | ||
| PassAlone | |||||||
| L Amygdala | −27 | 5 | −24 | 3.56 | 38 | ||
| VS | −18 | 17 | −5 | 3.78 | 40 | ||
| R Insula | 44 | 20 | −8 | 3.64 | 53 | ||
| R TPJ | 40 | 48 | −40 | 43 | 4.14 | 186 | |
| L Middle frontal gyrus | −33 | 20 | 40 | 4.97 | 41 | ||
| R Middle frontal gyrus | 6 | 27 | 11 | 55 | 4.70 | 114 | |
| Posterior Cingulate Cortex | −3 | −28 | 34 | 4.54 | 439 | ||
| R Caudate | 16 | −3 | 16 | 3.55 | 43 | ||
| PPI with VS | |||||||
| StopMother-StopAlone | |||||||
| R VLPFC | 47/11 | 30 | 29 | −14 | 4.64 | 47 | |
| StopAlone-StopMother | |||||||
| – | |||||||
| StopMother | |||||||
| R VLPFC | 47 | 30 | 29 | −14 | 4.20 | 37 | |
| L VLPFC | −20 | 62 | 0 | 5.61 | 52 | ||
| R DLPFC | 46 | 45 | 41 | 22 | 3.76 | 54 | |
| MPFC | 32/10 | 0 | 50 | 10 | 3.76 | 55 | |
| Precentral gyrus | 51 | 2 | 16 | 3.45 | 47 | ||
| SMA | 0 | −25 | 52 | 3.45 | 103 | ||
| Caudate | 12 | 5 | 10 | 5.45 | 199 | ||
| Insula | 30 | 20 | −11 | 3.28 | 37 | ||
| StopAlone | |||||||
| MPFC | 32/10 | 3 | 59 | 1 | 5.79 | 63 | |
| Caudate | 6 | 22 | 6 | 4.46 | 78 | ||
| GoMother-GoAlone | |||||||
| – | |||||||
| GoAlone-GoMother | |||||||
| – | – | ||||||
| GoMother | |||||||
| – | – | ||||||
| GoAlone | |||||||
| dACC | 3 | 41 | 13 | 3.17 | 41 | ||
| L Amygdala | −22 | 0 | −14 | 3.88 | 53 | ||
| STS | 51 | −22 | −14 | 3.95 | 40 | ||
| PassMother-PassAlone | |||||||
| – | – | ||||||
| PassAlone-PassMother | |||||||
| – | – | ||||||
| PassMother | |||||||
| Temporal pole | 21 | −51 | −4 | −23 | 4.53 | 99 | |
| Posterior cingulate cortex | −6 | −40 | 1 | 4.14 | 175 | ||
| PassAlone | |||||||
| R Insula | 54 | 14 | −8 | 4.06 | 54 | ||
| R Amygdala | 27 | −4 | −14 | 5.29 | 40 | ||
| R TPJ | 51 | −28 | 16 | 4.25 | 132 | ||
| L TPJ | −51 | 26 | 23 | 3.86 | 104 | ||
| Posterior cingulate cortex | −12 | −40 | 4 | 4.38 | 530 | ||
| SMA | 3 | −7 | 49 | 3.55 | 38 | ||
Note. BA refers to putative Broadman’s areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. MPFC, medial prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; VS, ventral striatum; SMA, supplementary motor area; TPJ, temporoparietal junction; STS, superior temporal sulcus; dACC, dorsoanterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; – indicates no significant clusters were identified.
To further probe these effects, we extracted parameter estimates of signal intensity from the clusters in the MPFC and left VLPFC for the four conditions relative to baseline (StopAlone, GoAlone, StopMother and GoMother). We used these parameters in SPSS to conduct interaction analyses. For the VLPFC, we find a significant main effect of context [Mother vs Alone; F(1,23) = 20.66, P < 0.0001] as well as a significant interaction [F(1,23) = 6.5, P < 0.01], such that the VLPFC is recruited significantly more within the Mother condition for Stop (M = 2.14, SEM = 0.96) than Go (M = 0.41, SEM = 0.54) decisions [t(24) = 2.2, P < 0.05]. In contrast, when alone, adolescents tend to recruit the VLPFC more when making Go (M = − 1.64, SEM = 0.43) than Stop (M = −3.06, SEM = 0.62) decisions [t(24) = 2.07, P = 0.05]. Importantly, the VLPFC is activated significantly more than baseline only in the Mother condition [t(24) = 2.1, P < 0.05] when making safe decisions. For the MPFC, we find a significant main effect of context [Mother vs Alone; F(1,23) = 19.44, P < 0.0001], but do not find an interaction.
Outcomes
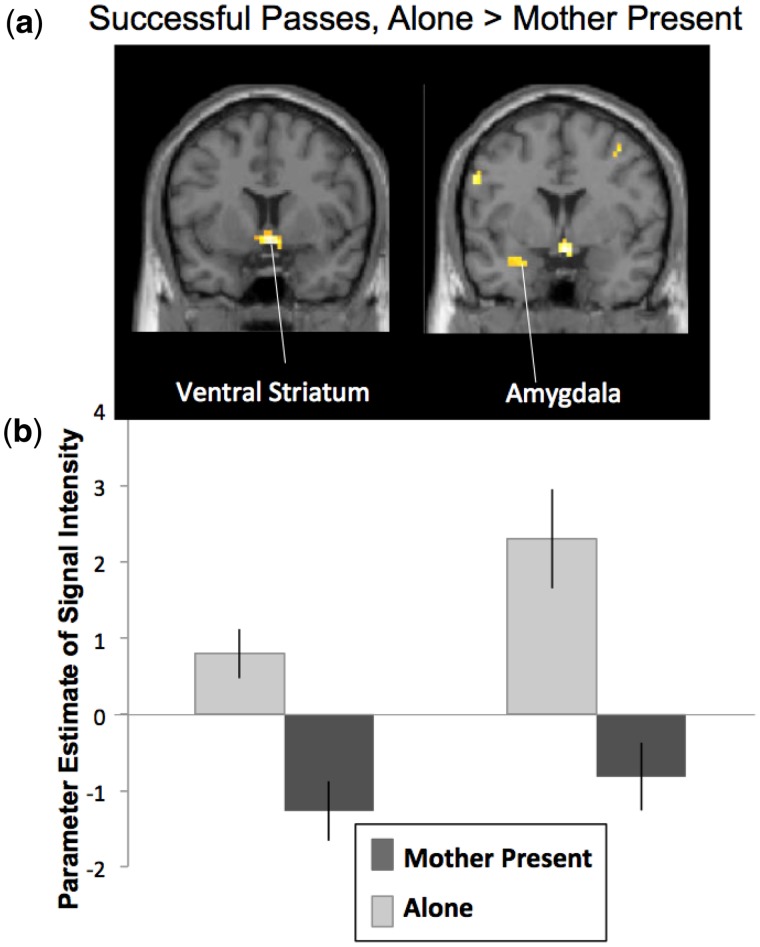
When adolescents successfully passed through an intersection without crashing following a risky decision (PassMother-PassAlone), they showed significantly less activation in the amygdala and ventral striatum when their mothers were present compared with alone (Figure 4a; Table 2). For descriptive purposes, we extracted parameter estimates of signal intensity from the ventral striatum and amygdala clusters for successful passes when adolescents were alone (StopAlone-baseline) and when their mother was present (StopMom-baseline). As shown in Figure 4b, adolescents demonstrated significantly greater activation in the ventral striatum and amygdala following a successful risky decision when alone than when their mother was present. We do not find any significant effects for Crash outcomes (CrashMother-CrashAlone). However, because most participants had too few crash events for statistical tests, we have limited power for this analysis. Therefore this null result should be interpreted with caution.
Fig. 4.
(a) Neural activation during successful passes when adolescents were alone compared with mother present. For descriptive purposes, parameters estimates of signal intensity were extracted from the ventral striatum and amygdala clusters separately for Passes when Alone and Mother Present (relative to baseline). Error bars represent SEM.
PPI analysis
Using the ventral striatum as the seed region, we first extracted parameter estimates of signal intensity during Stop and Go decisions and Pass outcomes during the Alone and Mother Present conditions and ran paired-samples t-tests. Adolescents demonstrated greater ventral striatum activation when making stop decisions when their mother was present (M = 2.09, SEM = 1.19) than when alone [M = −0.76, SEM = 0.46; t(24) = 2.44, P < 0.05], but did not differ when making go decisions when their mother was present (M = 0.99, SEM = 0.83) compared with alone [M = 0.23, SEM = 0.79; t(24) = 0.6, ns]. For Pass outcomes, adolescents showed significantly greater activation when alone (M = 0.58, SEM = 0.38) compared with when their mother was present [M = −1.10, SEM = 0.40; t(24) = 4.56, P < 0.0001].
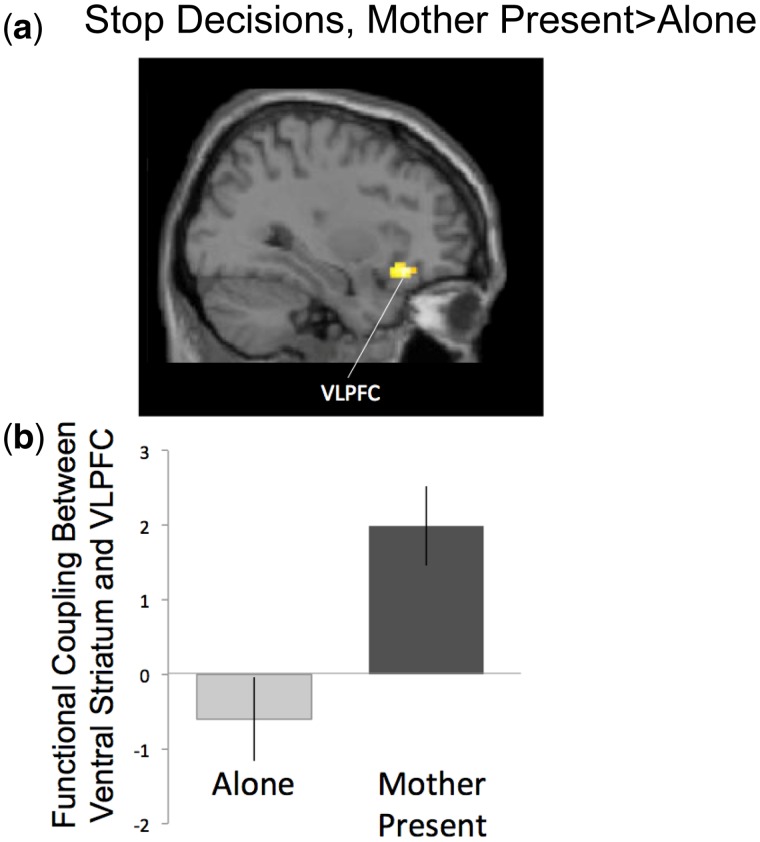
Next, we conducted PPI analyses to test for functional connectivity between the ventral striatum and cognitive control regions. When adolescents made a decision to stop (StopMother-StopAlone), we found a significant interaction between the ventral striatum and VLPFC. For descriptive purposes, we extracted parameter estimates of signal intensity from the VLPFC cluster that showed a significant interaction with the VS and plotted the effects separately for StopMother and StopAlone (vs baseline). As shown in Figure 5, adolescents show VS-VLPFC coupling significantly greater when their mothers are present than when alone (Table 1). To further probe this effect, we ran PPI analyses separately for the Mother and Alone condition for Stop decisions. Adolescents do not show significant functional coupling between the VS and VLPFC when alone but do show significant coupling between these regions when their mother was present (Table 1). No brain regions differentially functionally interacted with the VS during decisions to Go (GoMother-GoAlone) or during successful passes (PassMother-PassAlone; see Table 1 for Mother and Alone conditions separately).
Fig. 5.
(a) PPI analysis during stop decisions when mothers were present compared with alone. Significant activation represents positive coupling with the ventral striatum. (b) For descriptive purposes, parameters estimates of signal intensity were extracted from the VLPFC cluster separately for Stop decisions when Alone and Mother Present (relative to baseline). Error bars represent SEM.
DISCUSSION
Adolescence is a developmental period marked by exquisite changes in functional brain development. Heightened reward sensitivity, coupled with relatively less developed cognitive control, has largely been thought to underlie adolescents’ engagement in risky behavior (Steinberg, 2008; Casey et al., 2011). In this study, we test whether the presence of parents alters adolescent risk taking via changes in reward sensitivity and cognitive control.
Our findings suggest that maternal presence has a large impact on adolescents’ risky behavior and brain function. When mothers were present, adolescents engaged in significantly less risky behavior. Surprisingly, prior research showing that parental supervision is associated with lower adolescent risk taking has all been correlational (Richardson et al., 1993; Beck et al., 2001; Borawski et al., 2003). This is the first study to experimentally demonstrate the protective role that parental presence plays on reducing adolescent risk taking. Importantly, we identified the neural mechanisms by which mothers decrease their adolescent’s risk taking. First, adolescents showed greater recruitment of the VLPFC when making safe decisions in the presence of their mothers. The VLPFC is involved in self-control and the ability to regulate and control one’s prepotent thoughts and behaviors (Baker et al., 1997; Gray et al., 2002) and plays a causal role in goal-directed inhibitory control, including the braking of motor responses (Wessel et al., 2013). These results suggest that mothers boost self-control by increasing PFC activation, facilitating more deliberative and safe decisions. In addition, adolescents showed heightened activation in the MPFC when making both safe and risky decisions when their mother was present. The MPFC is involved in monitoring and computing the reward value of ongoing cognitive tasks (Pochon et al., 2002), as well as the subjective value of rewards (Kable and Glimcher, 2007; Levy et al., 2010). Thus, when their mothers are present, adolescents may evaluate the relative reward of running the stoplight vs stopping. The MPFC is also involved in self-related processing and thinking about close others (Mitchell et al., 2006), and so adolescents may be incorporating their mothers’ perspective to inform their own behavior and decide whether to engage in risky decisions.
Second, adolescents showed decreased activation in the ventral striatum and amygdala following a risky decision in the presence of their mothers. The ventral striatum has been consistently linked with hedonic rewards, tends to be more active in adolescents than children or adults during reward and risk-taking tasks and predicts greater engagement in real-life risk taking behavior (Galvan et al. 2006, 2007). The amygdala codes for emotionally salient stimuli and tends to be more active in adolescents than adults during emotion (particularly positive emotions) and reward processing (Ernst et al., 2005; Hare et al. 2008; for reviews see Somerville et al., 2011, Nelson et al., 2014). Together, our findings suggest that mothers reduce the rewarding and salient nature of engaging in risk taking, perhaps taking the fun out of being risky. Thus, when adolescents make risky decisions in the presence of their mothers, they respond to risky outcomes with decreased amygdala and ventral striatum activation than when making the same risky decisions alone.
Finally, and most novel, maternal presence facilitated functional coupling between neural regions involved in reward and cognitive control processing when adolescents made safe but not risky decisions, suggesting that mothers increased the rewarding nature of engaging in cognitive control. These functional connectivity results suggest that within adolescents, those who engage the ventral striatum to a greater extent also engage the VLPFC more when making safe decisions, but this only occurs when adolescents’ mothers are present. Theories of adolescent risk-taking propose that heightened reward sensitivity largely underlies increased risk-taking during adolescence (Steinberg, 2008), and most prior work has focused on the contexts in which reward sensitivity leads to maladaptive, risky behavior, for example, in the presence of peers (Chein et al., 2011). Here, we demonstrate that safe behavior occurs when the ventral striatum is functionally coupled with the VLPFC when mothers are present. Thus, heightened coupling between these regions is associated with safer behavior, suggesting that maternal presence may promote a reward response that boosts adolescents’ engagement of cognitive control and promotes more safe decision making. Importantly, the very same neural circuitry (i.e. ventral striatum) that has been linked to greater risk taking may also be related to thoughtful, more deliberative and safe decisions when mothers are present. Therefore, maternal presence may serve to redirect adolescents’ reward sensitivity away from risk (i.e. reduced ventral striatum activation during risky outcomes) and toward more safe behavior (i.e. increased VS-PFC coupling during safe decisions). Because PPI analyses are not directional, an alternative explanation for functional coupling between the ventral striatum and VLPFC is that engaging in cognitive control in the presence of their mother is a rewarding process. In other words, recruitment of cognitive control facilitates safe decisions in the presence of their mother which results in a heighted sense of reward.
Our findings contribute to conceptualizations of adolescent neurodevelopment. While dual systems models (Steinberg, 2008; Somerville et al., 2010; Casey et al., 2011) have gained considerable attention, several alternative perspectives have been proposed (Crone and Dahl, 2012; Pfeifer and Allen, 2012). For example, striatal reactivity is not always associated with greater adolescent risk taking, as suggested by dual system models, and, depending on the context, striatal reactivity can result in lower risk taking behavior (Pfeifer et al., 2011; Telzer et al., 2013). We build upon these findings and show that ventral striatum activation is associated with safe decision making when it occurs in tandem with cognitive control-related neural activation in the presence of mothers. Together, our findings underscore the importance of understanding the social context in which adolescent decision making occurs and suggests that the striatum plays a diverse function, sometimes promoting risky behavior but at other times deterring risky behavior depending upon the motivational context.
While our task did not have a condition to test for the effect of the person on adolescent risk taking and neural reactivity, our findings are the opposite of those found by Chein et al. (2010) who used the same manipulation with peer presence and found that peers increase risk taking and ventral striatum activation during risky decisions. Therefore, our effects are likely not due to someone watching vs not watching, as peers’ presence alters risk taking and related neural patterns in a different way than does parents’ presence. However, without directly comparing peer and parent influence in the same paradigm, we cannot be sure that these contexts have opposite effects on adolescents, and our findings could be due to other factors that differ between the studies (e.g. characteristics of the samples, age, etc.). Thus, future work should examine how both parents and peers impact adolescent decision making and neural processing during risk taking as well as the differential role of parental and authority figures to test whether adults decrease adolescent risk taking or whether our patterns are specific to parents.
Mothers in our study did not vocally encourage or discourage any kind of behavior, actively regulate their child’s decisions or provide feedback. Instead, mothers simply told their child they would be present and watching. Our findings suggest that parental influence does not have to be explicit. The mere presence of a parent in the room or car may help adolescents to slow down, think twice and make more deliberative and safe decisions. Parents are in a position to regulate their teenagers’ decision making capacities in ways that allow their children to engage in more mature cognitive processes. Importantly, our findings suggest that parents do not just serve as gatekeepers but actually change the ways in which adolescents think and reason about risks.
Conflict of Interest
None declared.
References
- Baker SC, Frith CD, Dolan RJ. The interaction between mood and cognitive function studied with PET. Psychological Medicine. 1997;27:565–78. doi: 10.1017/s0033291797004856. [DOI] [PubMed] [Google Scholar]
- Beck KH, Shattuck T, Raleigh R. Parental predictors of teen driving risk. American Journal of Health and Behavior. 2001;25(1):10–20. doi: 10.5993/ajhb.25.1.2. [DOI] [PubMed] [Google Scholar]
- Borawski EA, Ievers-Landis CE, Lovegreen LD, Trail ES. Parental monitoring, negotiated unsupervised time, and parental trust: the role of perceived parenting practices in adolescent health risk behaviors. Journal of Adolescent Health. 2003;33(2):60–70. doi: 10.1016/s1054-139x(03)00100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Science of the United States of America. 2011;108(36):14998–5003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Web-based Injury Statistics Query and Reporting System (WISQARS) 2012 National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–35. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–29. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Journal of Child Psychology and Psychiatry. 2009;50(12):1550–8. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychology. 2008;63(10):927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA. Morbidity and Mortality Weekly Report. Surveillance Summaries; 2014. Youth risk behavior surveillance – United States, 2013. (Washington, D.C.: 2002), 63(Suppl 4), 1–168. [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. 2009;12(4):614–25. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. Neural representation of subjective value under risk and ambiguity. Journal of neurophysiology. 2010;103(2):1036–47. doi: 10.1152/jn.00853.2009. [DOI] [PubMed] [Google Scholar]
- Maldijian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21(1):450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–39. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae. CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2013. US Department of Health and Human Services, Centers for Disease Control and Prevention. Hyattsville, MD; 2013. [Google Scholar]
- Nelson EE, Lau JY, Jarcho JM. Growing pains and pleasures: how emotional learning guides development. Trends in Cognitive Sciences. 2014;18(2):99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Peake S, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. NeuroImage. 2013;82:23–4. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–9. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, III, et al. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–36. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, et al. The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences. 2002;99(8):5669–74. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JL, Radziszewska B, Dent CW, Flay BR. Relationship between after-school care of adolescents and substance use, risk taking, depressed mood, and academic achievement. Pediatrics. 1993;92(1):32–8. [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011;56:1693–704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Developmental neuropsychology. 2011;36(4):408–28. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich T, Mulder M, Franklin NT, et al. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Developmental Science. 2014;17(1):59–70. doi: 10.1111/desc.12092. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the mni mri single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ward BD. 2000 Simultaneous inference for fMRI data. Available: http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf (accessed 24 March 2015) [Google Scholar]
- Wessel JR, Conner CR, Aron AR, Tandon N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. Journal of Neuroscience. 2013;33(50):19611–9. doi: 10.1523/JNEUROSCI.3468-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]