Abstract
Currently, the developmental trajectories of neural circuits implicated in autism spectrum disorders (ASD) are largely unknown. Here, we specifically focused on age-related changes in the functional circuitry of the posterior superior temporal sulcus (pSTS), a key hub underlying social-cognitive processes known to be impaired in ASD. Using a cross-sectional approach, we analysed resting-state functional magnetic resonance imaging (fMRI) data collected from children, adolescents and adults available through the autism brain imaging data exchange repository [n = 106 with ASD and n = 109 typical controls (TC), ages 7–30 years]. The observed age-related changes of pSTS intrinsic functional connectivity (iFC) suggest that no single developmental pattern characterizes ASD. Instead, pSTS circuitry displayed a complex developmental picture, with some functional circuits showing patterns consistent with atypical development in ASD relative to TC (pSTS-iFC with fusiform gyrus and angular gyrus) and others showing delayed maturation (pSTS-iFC with regions of the action perception network). Distinct developmental trajectories in different functional circuits in ASD likely reflect differential age-related changes in the socio-cognitive processes they underlie. Increasing insight on these mechanisms is a critical step in the development of age-specific interventions in ASD.
Keywords: autism spectrum disorders, posterior superior temporal sulcus, intrinsic functional connectivity, developmental changes, autism brain imaging data exchange
INTRODUCTION
Neuroimaging has increasingly provided insights into the age-related changes in the brain’s structural (Lenroot and Giedd, 2006; Raznahan et al., 2011) and functional organization that occur over the course of development (Durston et al., 2006; Marsh et al., 2008; Uddin et al., 2013a; Vasung et al., 2013). More recently, resting-state fMRI (rs-fMRI) approaches [measuring fluctuations in the blood oxygenation level-dependent (BOLD) signal during rest that correspond to functional brain networks; for example, Damoiseaux et al., 2006] have proven effective in capturing the processes of typical functional brain development, by bypassing the challenge of selecting tasks appropriate for a wide range of ages and abilities (Fair et al., 2007; Kelly et al., 2009; Dosenbach et al., 2010; Uddin et al., 2010; Craddock et al., 2013). The absence of task demands has enabled investigations to examine increasingly earlier stages of development (Lin et al., 2008; Gao et al., 2009; Hoff et al., 2013; Di Martino et al., 2014b). It has also facilitated the rapid growth of rs-fMRI studies of neurodevelopmental disorders (Uddin et al., 2010; Kelly et al., 2012; Castellanos et al., 2013), such as autism spectrum disorder (ASD), a prototypically early-onset psychiatric disorder that has been linked to altered brain circuitry (Just et al., 2004; Minshew and Keller, 2010).
Initial rs-fMRI studies have proven useful in substantiating the dysconnection model of autism (Just et al., 2004; Minshew and Keller, 2010; Muller et al., 2011; Vissers et al., 2012). Yet, only a handful of these studies have directly examined developmental trajectories of functional circuits in ASD (Di Martino et al., 2011; Shih et al., 2011; Wiggins et al., 2011, 2012; Padmanabhan et al., 2013; Washington et al., 2013). Most of these studies have focused on relatively narrow age ranges (e.g. from late childhood to adolescence), have surveyed different regions of interest and have used disparate analytical approaches. Nevertheless, as a whole, they suggest that ASD affects age-related trajectories of brain functional circuitry and that rs-fMRI is able to capture these changes. For example, intrinsic functional connectivity (iFC) between regions of the default mode network (DMN) has been shown to exhibit atypical development in ASD (Wiggins et al., 2011, 2012). Individuals with ASD exhibited age-related decreases where typical controls (TC) showed age-related increases in iFC between posterior and anterior regions of the DMN (e.g. iFC between posterior cingulate cortex and superior frontal gyrus; Wiggins et al., 2011). However, the opposite pattern (age-related increases in iFC in ASD but age-related decreases in TC) was observed in another circuit (between posterior DMN regions and left temporal lobe and parahippocampal gyrus; Wiggins et al., 2011). Similar atypical developmental patterns have been reported in some subcortical-cortical circuits (e.g. iFC between dorsal caudate and fusiform gyrus), but not in others (e.g. iFC between ventral striatum and fusiform gyrus) where a pattern consistent with arrested development has been observed (Padmanabhan et al., 2013).
Building on these initial efforts, this study aimed to apply a developmental perspective to the examination of ASD-related abnormalities in the functional circuitry of the posterior superior temporal sulcus (pSTS). This region’s circuitry is of particular relevance to ASD. It represents a social information processing ‘hub’ (Lahnakoski et al., 2012) that connects distinct social brain networks underlying ‘Theory of Mind’ (e.g. amygdala-orbitofrontal network; Allison et al., 2000), action understanding (i.e. fronto-parietal action perception network or mirror neuron system; Iacoboni and Dapretto 2006) and self-referential processing (DMN; Buckner, 2008, 2012). Functionally, pSTS (more often right pSTS) has been implicated in a number of social processes such as biological motion perception, speech perception, audio-visual integration, perception of gaze and face processing (Redcay, 2008; Carrington and Bailey 2009); all these processes are known to be affected by ASD. To date, several structural and functional neuroimaging studies have provided consistent evidence that pSTS dysfunction plays a role in ASD (e.g. von Dem Hagen et al., 2011; Philip et al., 2012), with a majority of studies reporting aberrant pSTS iFC (Kana et al., 2012; Mueller et al., 2013; Alaerts et al., 2014). Finally, a recent examination of an unprecedentedly large rs-fMRI dataset (360 rs-fMRI scans of individuals with ASD and 403 controls) revealed substantial degrees of hypo-connectivity of lateral temporo-cortical connections in ASD, underscoring the importance of this circuitry (Di Martino et al., 2014a).
To date, little is known about age-related changes in pSTS functional circuitry and whether they are affected by ASD despite its critical role in this neurodevelopmental disorder. One initial study suggested atypical functional network differentiation (indexed as temporal and spatial consistency in BOLD responses) of pSTS subregions in ASD (Shih et al., 2011). Although generally, differentiation increased linearly with age in both groups, non-statistically significant steeper increases in network differentiation with increasing age were observed in TC, compared with ASD (Shih et al., 2011). These tentative results were obtained within a relatively narrow age range (8–19 years) and moderately sized samples (ASD, n = 21; TC, n = 26). Accordingly, we sought to further investigate group-by-age effects to distinguish diagnostic from age-related effects and to explore whether ASD-related differences in pSTS-iFC are fixed from early in life or change as a function of age.
To enable the examination of a broad developmental range, extending from childhood to adulthood in ADS and TC, we took advantage of the availability of a large cross-sectional rs-fMRI dataset comprising children, adolescents and adults (106 with ASD and 109 TCs, ages 7–30 years) from the Autism Brain Imaging Data Exchange (ABIDE) repository (Di Martino et al., 2014a). Our main objective was to examine whether pSTS iFC varies with age, and specifically, whether age-related differences in pSTS circuitry are similar in individuals with ASD and TC. Consistent with previous studies (Kana et al., 2012; Alaerts et al., 2014), we predicted that, independent of age, individuals with ASD would show hypo-connectivity of the pSTS relative to TC. Given the limited availability of prior evidence, we could not specify a directional hypothesis regarding the nature of the effect of ASD on the developmental trajectory of pSTS-iFC (i.e. diagnostic group-by-age interactions). On one hand, pSTS hypo-connections might already be evident at the earliest age tested (6 years). A key question would then be whether such alterations persist from childhood to adulthood, or whether circuitry maturation in ASD is initially delayed but gradually normalizes over the course of development. Alternatively, pSTS hypo-connectivity might emerge later in life. In the latter case, deviation from typical maturation in ASD may emerge from failing to follow the course of normative social development, or indicate development of compensatory connections.
While our primary analyses focused on iFC, we also explored diagnosis, age and group-by-age effects on three regional rs-fMRI measures that can reveal aspects of ASD-related dysfunction not detectable by standard seed-based iFC correlation approaches (Paakki et al., 2010; Shukla et al., 2010; Anderson et al., 2011; Dinstein et al., 2011; Di Martino et al., 2014a). Specifically, we included measures of (i) regional homogeneity (ReHo) (Zang et al., 2004), assessing ‘local' functional connectivity within pSTS; (ii) voxel-wise mirrored homotopic connectivity (VMHC; Zuo et al., 2010b), assessing inter-hemispheric connectivity between right pSTS and its left hemisphere counterpart and (iii) fractional Amplitude of Low Frequency Fluctuations (fALFF; Zuo et al., 2010a), assessing the normalized magnitude of intrinsic low-frequency oscillations within pSTS. To date, an increasing number of studies have shown the utility of these measures to index inter-individual differences in cognition or behavior (e.g. Mennes et al., 2011), and their sensitivity to typical developmental patterns (for review see Di Martino et al., 2014b; Zuo and Xing, 2014) and psychopathology, including ASD (Paakki et al., 2010; Shukla et al., 2010; Anderson et al., 2011; Dinstein et al., 2011; Di Martino et al., 2014b). However, research exploring age-related changes in these intrinsic measures in ASD is currently missing. Therefore, we conducted an exploratory analysis providing a first characterization of these intrinsic rs-fMRI measures in relation to pSTS and their age-related changes.
METHODS
Participants
To explore age-related differences in iFC, we selected two large datasets from the ABIDE repository (Di Martino et al., 2014a) that included rs-fMRI data collected from children, adolescents and adults (New York University Langone Medical Center (NYU) and the University of Utah - School of Medicine (USM)]. Analyses were limited to (i) males, because females were underrepresented and only included in one of the two datasets; (ii) individuals aged between 7 and 30 years, which represents ∼1.5 standard deviations (s.d.) above and below the overall mean age across the NYU and USM datasets (17.5 ± 7.5 years); and (iii) individuals with mean frame-wise displacement (FD) (head micro-movements) (Power et al., 2012) <2 s.d. above the sample mean (0.17 ± 0.13 mm). These criteria yielded rs-fMRI datasets of 106 individuals with ASD and 109 TCs (Table 1). Detailed information on the diagnostic protocols at each site are publicly available on the ABIDE website (http://fcon_1000.projects.nitrc.org/indi/abide/). Briefly, all participants with ASD had a clinician's DSM-IV-TR diagnosis of Autistic Disorder, Asperger's Disorder, or Pervasive Developmental Disorder Not-Otherwise-Specified, confirmed by Autism Diagnostic Observation Schedule (ADOS) modules 3 or 4 (Lord et al., 1999) and the Autism Diagnostic Interview–Revised (Lord et al., 1994). Estimates of intelligence were obtained using the four subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). As a secondary measure of autism severity, we also examined the total raw scores of the Social Responsiveness Scale (SRS)-Child Version (Constantino et al., 2004) and the SRS-Adult Version (Constantino and Todd 2005) completed by parents or an informant identified by the participants, respectively.
Table 1.
Group characteristics for the included ASD and TC participants (NYU and USM ABIDE sites combined)
| ASD (n = 106) |
TC (n = 109) |
χ2 | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | All males | All males | — | — | ||||||
| Handedness (right/left)a | 86/19a | 105/4 | 11.60 | <0.001 | ||||||
| Eye status (open/closed) | 98/8 | 97/12 | 12.36 | <0.001 | ||||||
| DSM-IV-TR diagnosis (autism/asperger/PDD-NOS) | 83/17/6 | — | — | — | ||||||
| Medication status (n on meds) | 15 | — | — | — | ||||||
| Medication status (n on meds at scan)b | 5b | — | — | — | ||||||
| Comorbidityc | 36c | — | — | — | ||||||
| Mean | s.d. | Min | Max | Mean | s.d. | Min | Max | t-value | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| ADOS scoresd | ||||||||||
| Total score (social + communication) | 12.1 | 4.1 | 5.0 | 22.0 | — | — | — | — | — | — |
| Social | 4.0 | 1.6 | 0.0 | 8.0 | — | — | — | — | — | — |
| Communication | 8.1 | 2.9 | 2.0 | 14.0 | — | — | — | — | — | — |
| RRB | 2.2 | 1.7 | 0.0 | 8.0 | — | — | — | — | — | — |
| SRS raw total scores | 90.2 | 31.4 | 6.0 | 164.0 | 19.1 | 13.4 | 0.0 | 56.0 | 19.57 | <0.0001 |
| Age at scan (years) | 16.0 | 6.2 | 7.1 | 30.0 | 16.8 | 5.8 | 6.5 | 30.0 | −0.98 | 0.33 |
| Full-scale IQ | 105.3 | 16.7 | 73.0 | 148.0 | 113.9 | 13.7 | 80.0 | 148.0 | −4.14 | <0.0001 |
| VIQ | 102.0 | 17.0 | 55.0 | 137.0 | 113.0 | 13.4 | 80.0 | 141.0 | −5.29 | <0.0001 |
| PIQ | 107.9 | 17.4 | 72.0 | 149.0 | 111.6 | 14.0 | 67.0 | 155.0 | −1.72 | 0.09 |
| Head Motion (mean FD) | 0.16 | 0.09 | 0.05 | 0.41 | 0.13 | 0.07 | 0.05 | 0.37 | 2.99 | 0.003 |
Note: PDD-NOS, pervasive developmental disorder not otherwise specified; ADOS, autism diagnostic observation schedule; RRB, restricted repetitive behaviors; IQ, intelligence quotient; mean FD, mean framewise displacement; χ2, chi-square; min, minimum; max, maximum.
aHandedness label was not available for one participant with ASD. bFluoxetine (n = 2), guanfacine (n = 2); methylphenidate (n = 1). Note that for one participant, information on medication status at time of scan was not available. cAttention deficit hyperactivity disorder [not otherwise specified (n = 3); hyperactive (n = 1); inattentive (n = 4) and combined (n = 4)]; specific phobia (n = 7); mood disorder (n = 6); dysthymia (n = 5); enuresis (n = 5); encopresis (n = 3); anxiety disorder not otherwise specified (n = 2); diurnal and nocturnal enuresis (n = 2); generalized anxiety disorder (n = 2); social phobia (n = 2); Tic disorder (n = 2); depressive disorder (n = 1); disruptive disorder (n = 1); separation anxiety (n = 1) and agoraphobia (n = 1). dThese scores are based on the algorithm for ADOS module 4 (n = 39), module 3 (n = 65) and module 2 (n = 2) (Lord et al., 1999). Since we included module 4, the more recent scores per Gotham et al., (2009) were not used as they are only available for modules 1, 2 and 3.
Descriptive phenotypic data for the combined sample are shown in Table 1, and separately for each site in Supplementary Table S1. Descriptive statistics were calculated with Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA). ASD and TC groups were matched on age across sites and within each site separately. Individuals with ASD showed significantly lower full-scale intelligence quotient (FSIQ) and verbal IQ (VIQ), as well as non-significantly lower performance IQ (PIQ) (Table 1). Given the cross-sectional nature of this study, and to assess potential sampling bias across age groups, we examined whether relationships existed between age and ratings of autism symptom severity indexed by the total raw SRS and ADOS total scores. Within the ASD group, age and ADOS total scores were not significantly related (β = 0.08; t(104) = 0.89; P = 0.37; the ADOS was not obtained for TC participants). Similarly, there was no significant relationship between age and total raw SRS scores, either within or across diagnostic groups (ASD: β = −0.02; t(102) = −0.16; P = 0.87; TC: β = −0.03; t(84) = −0.26; P = 0.79; all: β = 0.03; t(188) = 0.40: P = 0.69).
Data acquisition
New York University, Langone Medical Center
Imaging data were acquired using a Siemens Allegra 3.0 Tesla scanner at the NYU Center for Brain Imaging. Participants completed a 6-min rs-fMRI scan comprising 180 contiguous whole-brain functional volumes, acquired using a multiecho echo-planar imaging (EPI) sequence [repetition time (TR) = 2.0 s; effective echo time (TE) = 33 ms; flip angle = 90°; 33 slices; matrix = 80 × 80; voxel size = 3 × 3 × 4 mm]. For the rs-fMRI scan, 121 participants were instructed to rest with eyes open and 20 participants were instructed to rest with eyes closed. Eyes open/closed status was included as a covariate in group-level analyses to account for this variation. A high-resolution T1-weighted anatomical image was also acquired using a magnetization-prepared gradient-echo sequence [TR = 2530 ms; TE = 3.25 ms; inversion time (TI) = 1100 ms; flip angle = 7°; 128 slices; field of view (FOV) = 256 mm; voxel size = 1.3 × 1.3 × 1 mm].
University of Utah - School of Medicine
Imaging data were acquired using a Siemens Magnetom Trio 3.0 Tesla scanner. Participants completed an 8-min rs-fMRI scan (240 volumes) using a multiecho EPI sequence (TR = 2.0 s; TE = 28 ms; flip angle = 90°; 40 slices; matrix = 64 × 64; voxel size = 3.4 × 3.4 ×3.0 mm). During the rs-fMRI scan, all participants were instructed to rest with eyes open. A high-resolution T1-weighted anatomical image was also acquired using a magnetization-prepared gradient-echo sequence (TR = 2300 ms; TE = 2.91 ms; TI = 900 ms; flip angle = 9°; 160 slices; FOV = 256 mm; voxel size = 1 × 1 × 1.2 mm).
Image preprocessing
We employed an alpha version of the Configurable Pipeline for the Analysis of Connectomes (C-PAC, http://fcp-indi.github.com/C-PAC/) for subject-level analyses and group analyses. CPAC is a publicly available, Nipype-based, automated processing pipeline that interfaces AFNI (http://afni.nimh.nih.gov/afni/) and FMRIB software library (FSL; www.fmrib.ox.ac.uk) commands for neuroimaging analyses. Image preprocessing steps included motion correction, nuisance signal regression [including six motion parameters, five nuisance signals obtained by means of a principal components analysis of white matter and cerebrospinal fluid signals using the component-based noise correction (CompCor) approach (Behzadi et al., 2007) and linear trends), temporal filtering (0.009–0.08 Hz; except for frequency-domain analyses (i.e. fALFF)] and normalization to MNI152 stereotactic space (2 mm isotropic) using linear and non-linear registration [including boundary-based registration (Greve and Fischl, 2009)]. To compute VMHC, functional data were registered to a symmetric template obtained by averaging the MNI152 template and its left-right flipped version (Zuo et al., 2010b). Finally, we applied spatial smoothing (FWHM = 6 mm) for all analyses.
Given the potential confounding effects of micro-movements on rs-fMRI indices (Power et al., 2012; Van Dijk et al., 2012), we examined group differences in mean FD and its relationship with age. As expected, individuals with ASD showed greater mean FD than TC (Table 1 and Supplementary Figure S1A). However, there was no significant relationship between age and mean FD across all participants (β = −0.04; t(213) = −0.52; P = 0.60) nor within groups (ASD: β = −0.10; t(104) = −0.99; P = 0.32; TC: β = 0.07; t(107) = 0.73; P = 0.46) (Supplementary Figure S1B). Nonetheless, as recommended, to minimize the artifactual effects of even minor extents of head motion (Fair et al., 2012; Satterthwaite et al., 2013; Yan et al., 2013; Di Martino et al., 2014a), we included each participant’s mean FD as a nuisance covariate in primary analyses. Secondary analyses, in which frames with FD >0.2 mm were removed (‘scrubbing’) (Power et al., 2012), were also performed (Supplementary Figure S3).
iFC analyses
Seed selection
We performed iFC analyses using 10 mm radius spherical seed regions-of-interest (ROIs) positioned in the right and left posterior portions of the STS, centered on MNI-coordinates [±47, −60, 4]. These coordinates were based on a prior study (Alaerts et al., 2014) which identified regions activated in young adults with ASD and TC during an emotional processing task employing point light displays. The same seed in the right hemisphere was found to exhibit weaker iFC in individuals with ASD relative to TC regardless of age (Alaerts et al., 2014).
Subject-level
For each participant, we extracted the mean time series by averaging across all voxels in each seed ROI. We then computed bivariate correlation coefficients between the seed time-course and that of all other brain voxels. The resultant participant-level correlation maps were Fisher z-transformed to z-value maps.
Group-level
Following analyses confirming previously identified group differences (TC > ASD) in pSTS-iFC (Alaerts et al., 2014), we aimed to (i) assess linear and quadratic effects of age on pSTS-iFC across all participants and (ii) identify diagnostic group-by-age interaction effects. To do so, we carried out random-effects ordinary least squares group analyses, including four nuisance regressors (site, mean FD, FSIQ and eye status (open or closed)) with age as the regressor of interest. Cluster-level Gaussian random field theory was employed for multiple comparisons correction (Z > 2.3; P < 0.05, corrected).
Exploratory analyses on regional measures of intrinsic brain architecture
Beyond iFC, we explored three regional voxel-wise measures of intrinsic brain architecture within the pSTS bilaterally, i.e. ReHo (Zang et al., 2004), VMHC (Zuo et al., 2010b) and fALFF (Zuo et al., 2010a). Each measure assesses a distinct property of the intrinsic brain. Specifically, ReHo provides information about local connectivity by quantifying the similarity or synchronization between the time series of a given voxel and its 26 adjacent neighbors using Kendall’s tau coefficient of concordance (Zang et al., 2004). VMHC quantifies functional homotopy by computing iFC between each voxel in one hemisphere and its mirrored counterpart in the other (Zuo et al., 2010b). Finally, fALFF represents a frequency domain metric reflecting the ratio between the amplitude of fluctuations in the 0.01–0.1 Hz band and the total amplitude within the sampled periodogram; it represents the relative contribution of oscillations within this low-frequency band to the entire measured frequency range (Zuo et al., 2010a).
Results
Intrinsic functional connectivity
Group differences regardless of age
Consistent with prior findings from a larger sample of the ABIDE repository (Alaerts et al., 2014), we identified a main effect of diagnostic group (i.e. regardless of age) in four distinct clusters. Specifically, relative to TC, the ASD group exhibited weaker iFC between right pSTS and two large clusters centered around bilateral fusiform gyrus, as well as two large clusters centered around bilateral inferior parietal cortex (IPC), extending to premotor cortex (BA 6), insula and anterior superior temporal gyrus (STG) (see Figure 1 and Supplementary Table S2). We observed similar ASD-related decreases in iFC for the left pSTS seed albeit constrained to the left hemisphere only; the ASD group exhibited weaker iFC between left pSTS and fusiform gyrus and left anterior STG extending into insula (Supplementary Figure S2A). We did not detect any regions that exhibited increased iFC in individuals with ASD, relative to TC.
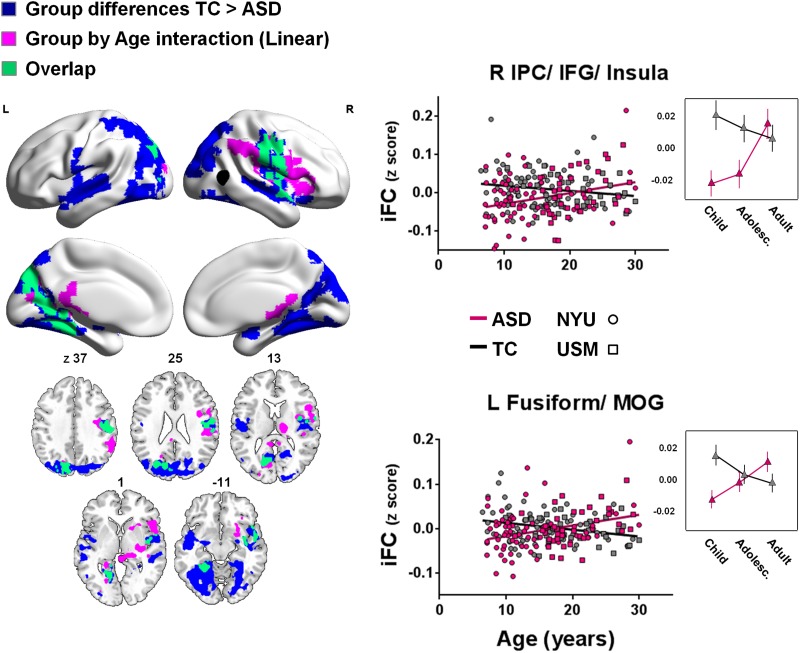
Fig. 1.
Main effects of group and linear group-by-age interaction effects for right pSTS iFC. Shown are clusters for which group differences (TC > ASD) (blue) or linear group-by-age interactions (purple) were observed (min Z > 2.3; cluster significance: P < 0.05, corrected). The right pSTS seed is illustrated in black. The scatter plots and adjacent line plots show the linear group-by-age interactions (i.e. individual participants’ iFC-scores for the clusters shown in purple). In the line plots, age ranges are grouped for childhood: 7–12.8 years; adolescence: 12.9–18.6 years and adulthood: 18.7–30.0 years. Relationships with age are plotted separately for the ASD (pink) and TC groups (gray). NYU participants are displayed as circles; USM participants as squares. Significant clusters are overlaid on inflated surface maps generated using BrainNet Viewer (http://www.nitrc.org/projects/bnv/), as well as on axial images generated with MRIcroN (http://www.mricro.com/). L, left hemisphere; R, right hemisphere. Vertical lines in the line plots denote SEM.
Linear and quadratic relationships with age across participants
Across subjects, we observed a positive linear relationship between age and right pSTS-iFC in five distinct clusters: a cluster in mid-cingulate gyrus, two clusters in bilateral fusiform gyrus and two fronto-parietal clusters centered around bilateral insula (Figure 2A and Supplementary Table S2). We also identified local age-related decreases in right pSTS-iFC within the right posterior STS/STG itself (local iFC), as well as between right pSTS and left STG (Figure 2A and Supplementary Table S2).
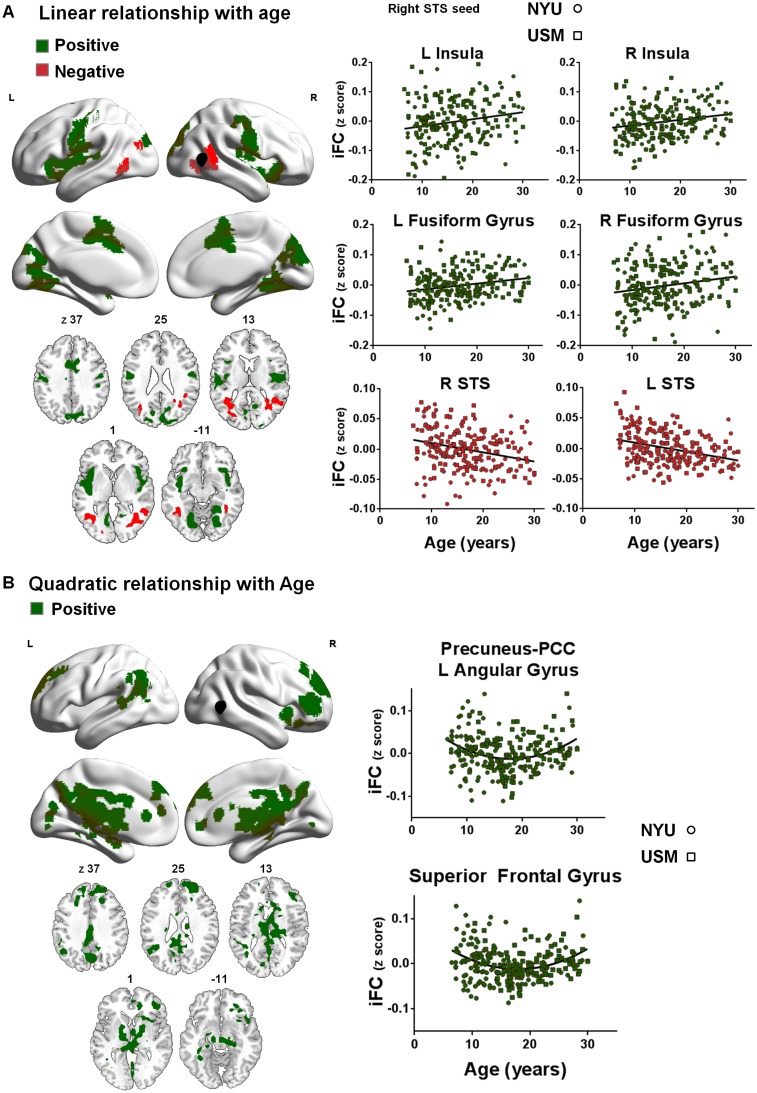
Fig. 2.
Linear (A) and quadratic (B) effects of age (7–30 years) for right pSTS iFC. Panel A depicts clusters in which iFC increased (green) or decreased (red) linearly as a function of age. Panel B depicts clusters in which iFC increased (green) quadratically as a function of age (min Z > 2.3; cluster significance: P < 0.05, corrected). The right pSTS seed is illustrated in black. The scatter plots show the positive (green) or negative (red) relationships with age across all participants (ASD and TC groups combined). NYU participants are displayed as circles; USM participants as squares. Significant clusters are overlaid on inflated surface maps generated using BrainNet Viewer (http://www.nitrc.org/projects/bnv/), as well as on axial images generated with MRIcroN (http://www.mricro.com/). L, left hemisphere; R, right hemisphere.
Examination of quadratic effects across subjects revealed a positive quadratic relationship (U-shaped) between age and right pSTS-iFC with several regions of the DMN, including the precuneus, posterior cingulate cortex, left angular gyrus, anterior cingulate cortex and superior frontal gyrus. This U-shaped relationship indicates a decrease in pSTS-DMN iFC during adolescence/young adulthood (16–18 years) across groups relative to both childhood and middle adulthood (Figure 2B and Supplementary Table S2). For left pSTS-iFC, similar positive quadratic relationships with age were observed across all participants, although the clusters obtained were less extensive (Supplementary Figure 2C and D). No negative quadratic relationships were observed.
At the cluster-level, we determined the best model fit (linear or quadratic) using the Akaike’s information criterion (AIC) (Akaike, 1974) to test whether both linear and quadratic effects of age are revealed in the same cluster. This analysis confirmed best linear fits (over quadratic) for the clusters in Figure 2A [linear fit, AIC <−490.9; P < 0.001] and best quadratic fits (over linear) for the clusters in Figure 2B [quadratic fit AIC <−727.3; P < 0.01].
Diagnostic group-by-age interactions
Pronounced linear group-by-age interaction effects were observed for iFC between right pSTS and left fusiform gyrus, as well as a cluster encompassing right IPC, extending to premotor cortex (BA 6)/inferior frontal gyrus (BA 44), insula and thalamus (Figure 1 and Supplementary Table S2). In these regions, pSTS-iFC remained either relatively constant with increasing age (right IPC/IFG/insula) or decreased with age (left fusiform gyrus) in the TC group. In contrast, in the ASD group pSTS-iFC linearly increased with age for both clusters (Figure 1). As shown in Figure 1, the areas exhibiting group-by-age interactions largely overlapped with regions that showed a main group effect. For these clusters, ASD-related hypo-connectivity of pSTS was most pronounced at the younger ages and diminished with increasing age. The line plots on the right side of Figure 1 visualize the age effects for ASD and TC, divided in three age groups: childhood: 7–12.8 years; adolescence: 12.9–18.6 years and adulthood: 18.7–30.0 years (age boundaries were determined using terciles to ensure equal numbers of participants in each age group–childhood, n = 72; adolescence, n = 72 and adulthood, n = 71). Post-hoc comparisons exploring ASD-TC group effects separately within each age-group indicated that for the cluster in right IPC/iFG/insula, hypo-connectivity was most pronounced in children (ASD-TC group difference, P < 0.001 (Fisher’s post-hoc)), diminished (but still significantly different) in adolescents (12.9–18.6 years) (P < 0.05) and was not significantly different in adults (18.7–30 years) (P > 0.05; Figure 1, top right figure). For the left fusiform gyrus cluster, ASD-related hypo-connectivity that was evident in children (P < 0.01) was not significant in adolescents and adults (P > 0.05) (Figure 1, lower right figure).
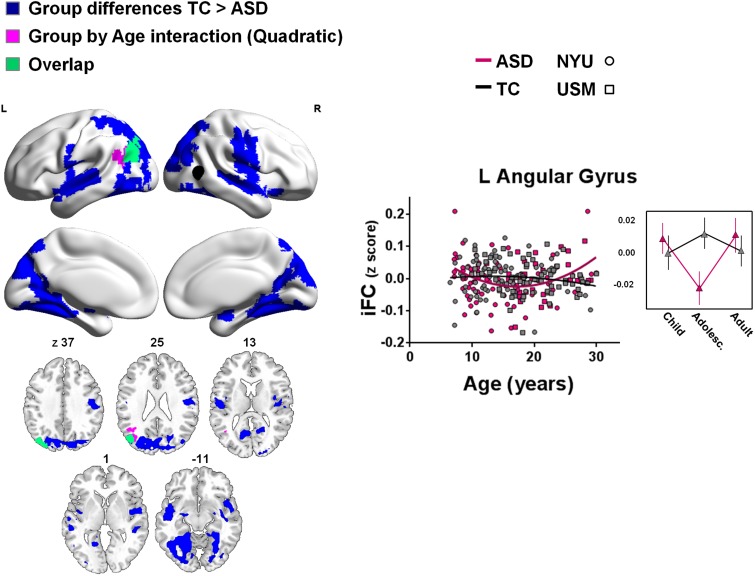
Analyses of quadratic group-by-age effects revealed one region within the left angular gyrus that exhibited a significant interaction. In this region, iFC with right pSTS remained constant over age in TC, but showed a positive quadratic (U-shaped) relationship with age in ASD. ASD-related decreases in iFC were more pronounced during adolescence/young adulthood, relative to childhood and middle adulthood (Figure 3 and Supplementary Table S2).
Fig. 3.
Effects of group and quadratic group-by-age interaction effects for right pSTS iFC. Shown are clusters for which group differences (TC > ASD) (blue) or group-by-age (quadratic) interaction effects (purple) were observed (min Z > 2.3; cluster significance: P < 0.05, corrected). The right pSTS seed is illustrated in black. The scatter plot and adjacent line plot show the quadratic group-by-age interaction (i.e. individual participants’ iFC-scores for the cluster shown in purple). In the line plot, age ranges are grouped for childhood: 7–12.8 years; adolescence: 12.9–18.6 years and adulthood: 18.7–30.0 years. Relationships with age are plotted separately for the ASD (pink) and TC groups (gray). NYU participants are displayed as circles; USM participants as squares. Significant clusters are overlaid on inflated surface maps generated using BrainNet Viewer (http://www.nitrc.org/projects/bnv/), as well as on axial images generated with MRIcroN (http://www.mricro.com/). L, left hemisphere; R, right hemisphere. Vertical lines in the line plots denote SEM.
Analysis of linear group-by-age interaction effects for the left pSTS seed identified similar clusters in left fusiform gyrus and right thalamus (Supplementary Figure S2E). No quadratic group-by-age interactions were observed for left pSTS-iFC.
Regional measures of intrinsic brain architecture
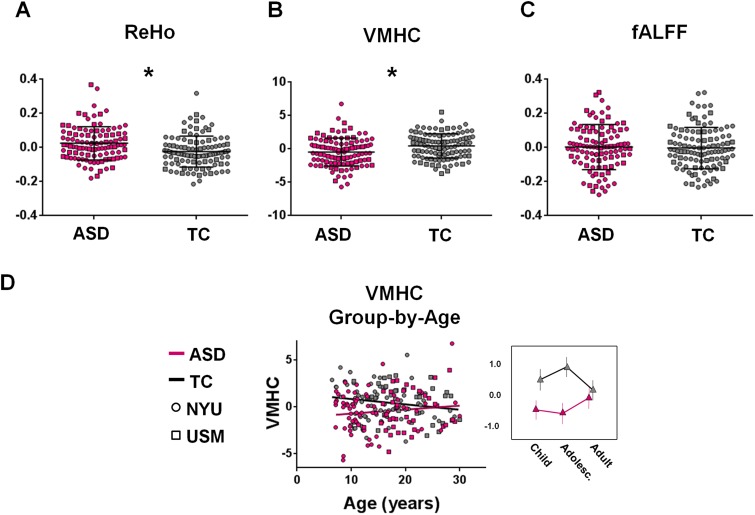
For each participant, regional measures (ReHo, fALFF and VMHC) were calculated across the whole-brain as described previously (Di Martino et al., 2014a). We extracted these metrics from the two pSTS ROIs (left and right) and averaged them across the volume of each ROI. Regardless of age, relative to TC, participants with ASD showed significantly increased local connectivity (ReHo) (Figure 4A) and decreased inter-hemispheric iFC (VMHC) (Figure 4B) in left and right pSTS. No group differences were observed for fALFF in the pSTS seeds (Figure 4C).
Fig. 4.
Exploration of regional measures of intrinsic brain architecture within the right pSTS. Panel A shows mean ReHo for participants with ASD (pink) and TC (gray). Participants with ASD exhibited significantly higher ‘local’ (ReHo) connectivity than the TC group. Panel B shows mean VMHC for participants with ASD (pink) and TC (gray). Participants with ASD exhibited significantly lower inter-hemispheric ‘mirrored’ (VMHC) intrinsic connectivity than the TC group. Panel C shows fALFF connectivity for participants with ASD (pink) and TC (gray). No group differences were observed in fALFF. The scatter plot and line plot in panel D show the linear group-by-age interaction obtained for VMHC, which indicates that group differences in VMHC were more pronounced at the younger ages and diminished with increasing age. In the line plot, age ranges are grouped for childhood: 7–12.8 years; adolescence: 12.9–18.6 years and adulthood: 18.7–30.0 years. VMHC is plotted as a function of age, separately for the ASD (pink) and TC groups (gray). NYU participants are displayed as circles; USM participants as squares. Vertical lines in panel A–C denote s.d. *P < 0.05 (corrected).
A negative linear relationship with age was observed across all participants for ReHo in right pSTS, indicating that local connectivity within right pSTS decreased with increasing age (β = −0.15; t(213) = −2.27; P = 0.02). No relationships with age across participants were observed for ReHo in left pSTS, nor were there any relationships observed for fALFF or VMHC in either right or left pSTS.
A group-by-age interaction was identified for pSTS VMHC, indicating that ASD-related decreases in pSTS interhemispheric homotopic connectivity were more pronounced at younger ages and diminished with increasing age (Figure 4D). Outlier detection [exceeding Q3+ 1.5 (Q3–Q1) or beneath Q3− 1.5 (Q3–Q1) where Q1 and Q3 are the first and third quartiles, respectively] revealed 6 outliers in the ReHo measure, 1 outlier in the VMHC measure and 3 outliers in the fALFF measure. Secondary analyses, excluding these participants, revealed a similar pattern of results as in the primary analyses including all participants (i.e. all effects remained significant, P < 0.05; data not shown).
Secondary analyses of potential confounds
Analysis of ‘scrubbed’ data
To determine the extent to which our findings were robust to motion correction, we computed pSTS-iFC using data ‘scrubbed’ at FD >0.2 mm and verified consistency in the pattern of group-by-age interaction effects within the clusters identified in primary analyses. Group-by-age interaction effects remained significant on the scrubbed data (P < 0.05) (Supplementary Figure S3).
Site-specific analysis
As primary findings were identified across two of the sites from the ABIDE repository (NYU and USM), we examined group-by-age interaction analyses within the clusters identified in primary analyses to determine the extent to which the main group-by-age interactions were detectable within each site. As shown in Supplementary Figure S4, similar group-by-age interaction effects were identified in each site (P < 0.05).
Analyses using seeds with different radii
Primary findings were identified using a 10 mm radius spherical seed. Secondary analyses, using seeds with smaller radii (4, 6 and 8 mm) showed results similar to those emerging from primary analyses (Supplementary Figure S5; group-by-age interaction effects, P < 0.05).
Analyses on subsamples pair-wise matched on age and IQ
Primary findings were identified using a sample of ASD participants with significantly lower FSIQ and VIQ but non-significantly lower PIQ (Table 1). To verify that group differences in IQ did not affect our main results, we selected subsamples of TC and ASD participants pair-wise matched on both age and IQ scores and examined age-by-group interactions in the two clusters identified in primary analyses (Supplementary Figure S6). Overall, the pattern of results obtained was consistent with our main findings (Supplementary Figure S6). In another secondary analysis, we excluded four participants identified as outliers based on their full-scale IQ. The results were unchanged (group-by-age interaction effects, P < 0.05 in both clusters; data not shown).
Relationship with symptom severity in the ASD group
Although not the primary aim of this study, we explored whether measures of symptom severity were related to rs-fMRI indices independent of age. To do so, we computed multiple regressions to test whether symptom severity as assessed by ADOS subscale scores (communication, social interaction, restricted and repetitive behaviors; Lord et al., 1999) were predicted by iFC of the right pSTS with the clusters identified by group-by-age interactions (i.e. right pSTS-iFC with the right fronto-parietal cluster and the left fusiform gyrus cluster, for which linear group-by-age interactions were observed) as well as local connectivity (ReHo), and mirrored connectivity (VMHC). We did not use the more recent ADOS calibrated severity scores (Gotham et al., 2009) since at the time of ABIDE aggregation the algorithm to generate them was available for all ADOS modules except for module 4, which was used for most of the adults in the sample.
ADOS scores were however not significantly related to any of the rs-fMRI measures; none of the examined relationships survived correction for multiple comparisons. Similarly, only non-significant relationships were revealed between SRS total scores and rs-fMRI measures (within or across groups).
DISCUSSION
In a substantial cross-sectional sample of the ABIDE repository spanning ages 7–30 years, we investigated age-dependent changes in the intrinsic functional properties of pSTS in individuals with and without ASD. We focused on pSTS circuitry due to its key role in social cognitive processes known to be affected by ASD (Zilbovicius et al., 2006; Redcay, 2008; Shih et al., 2010, 2011; Pelphrey et al., 2011; Kaiser and Pelphrey, 2012; Kana et al., 2012; Mueller et al., 2013; Alaerts et al., 2014). Analyses revealed linear and non-linear effects of age that were common to both groups, as well as age effects that were dependent on diagnosis. Age-related patterns varied across functional circuits. Specifically, while linear age-related changes were observed for the action perception network (Rizzolatti and Craighero, 2004; Caspers et al., 2010; Molenberghs et al., 2012), quadratic age-related changes were observed for pSTS iFC with the DMN (Buckner et al., 2008). ASD-specific developmental patterns also varied across circuits; with some circuits pointing toward patterns of delayed maturation in ASD, whereas other circuits showed ‘atypical’ developmental trajectories for ASD and TC. Below, we discuss our most salient findings.
Across groups, we observed linear age-related decreases in iFC between pSTS and proximal cortical areas (local iFC and ReHo), and linear age-related increases in iFC between pSTS and more intermediate (>40 mm) as well as distal (>80 mm) areas, within and between hemispheres. This is consistent with prior reports of typical developmental shifts in iFC strength from short- to long-range connections (Fair et al., 2007, 2009; Kelly et al., 2009; Supekar et al., 2009). As in prior work (Fair et al., 2012; Satterthwaite et al., 2013), these findings were robustly detectable across motion-correction approaches (i.e. group-level correction and ‘scrubbing’), alleviating concerns that they may be artifactual in nature.
Our primary goal was to explore the impact of diagnosis on age-related changes in pSTS intrinsic (diagnostic group-by-age interaction). Our findings of diagnosis-related differences in age-related changes were suggestive of two ASD-related maturational patterns in pSTS iFC: delayed and atypical maturation. As defined elsewhere (Shaw et al., 2010; Di Martino et al., 2014b), delayed maturation represents abnormalities in maturational timing; for example a deceleration in the progression of age-related, but otherwise typical, changes. Atypical maturation on the other hand, refers to more profound changes in the maturational pattern beyond timing. The co-emergence of both delayed and atypical maturation highlights a complex profile of age-related iFC changes in ASD that vary depending on the circuits involved, as described below.
Consistent with delayed maturation, portions of the action perception network were relatively stable in their iFC from age 7 to 30 years in TC but not in ASD. Specifically, while iFC between pSTS and right IPC, premotor cortex, dorsal posterior insula and ventro-lateral thalamus changed minimally over the age range examined in TC, iFC increased with age in ASD. Accordingly, the group differences in pSTS iFC within this circuit that were apparent in children (hypo-connectivity in ASD, compared with TC) were absent in the adult groups. This pattern was also observed for pSTS homotopic iFC (indexed by VMHC). These novel findings parallel the observation that the social cognition processes supported by the pSTS action-perception network emerge early in life in TC (Marshall and Meltzoff, 2011). We speculate that ASD-related impairments in some aspects of social-cognitive processing reflect underlying delays in the neural maturation of pSTS iFC with regions of the action perception network.
Findings of delayed developmental maturation in the action perception network were accompanied by patterns pointing toward atypical age-related changes in ASD. Atypical age-related changes were both linear (iFC between right pSTS and left fusiform gyrus) and quadratic (iFC between right pSTS and left angular gyrus). In these cases, the age-dependent trajectories exhibited by ASD were opposite to those observed in TC (i.e. increases in iFC strength with increasing age in ASD vs age-related decreases in TC). Similar increases in iFC strength with increasing age in ASD and age-related decreases in TC have been reported in two prior studies that examined age-related changes from childhood to adolescence in iFC of several nodes of the DMN (Wiggins et al., 2011, 2012) and in one study that examined changes in striatal circuitry from childhood to young adulthood (Padmanabhan et al., 2013).
Taken together with the larger literature (Di Martino et al., 2011; Wiggins et al., 2011, 2012; Padmanabhan et al., 2013; Washington et al., 2013), our findings of both ‘delayed’ and ‘atypical’ developmental trajectories in ASD are suggestive of an increasingly complex developmental picture. No single developmental phenomenon appears to be sufficient to explain the large range of findings emerging from both age-dependent and age-independent studies comparing ASD and TC. Beyond the patterns of delayed and atypical development noted in our work, another recently reported pattern is that of arrested development (i.e. development that halts prematurely), previously shown for iFC between ventral striatum and fusiform gyrus (Padmanabhan et al., 2013) and in DMN (Washington et al., 2013). These findings emphasize that distinct developmental patterns characterize distinct brain circuits and likely the specific cognitive and affective processes they support. Thus, our findings suggest the importance of accounting for age, as well as the circuit examined when interpreting group differences between ASD and TC.
Irrespective of age, a predominant pattern of pSTS hypo-connectivity in ASD was observed with bilateral fusiform gyrus and several nodes of the action perception network. These findings are consistent with prior observations of hypo-connectivity between pSTS and DMN regions (Mueller et al., 2013), as well as between pSTS and nodes of the fronto-parietal action observation network (Kana et al., 2012; Alaerts et al., 2014). Another study on pSTS (Shih et al., 2010) and others examining different circuits have instead reported ASD-related hyper-connectivity (e.g. Uddin et al., 2013b). This apparent inconsistency (i.e. hypo- vs hyper-connectivity) has been increasingly highlighted in the literature (Muller et al., 2011; Vissers et al., 2012, Uddin et al., 2013a; Kana et al., 2014) and has been attributed to methodological differences across studies (e.g. head motion, use of global signal regression, examination of task-based and intrinsic signal correlations) or a reflection of ASD-related processes (Uddin et al., 2013a; Di Martino et al., 2014a; Hahamy et al., 2015). For example, whole-brain analyses in the larger ABIDE sample yielded evidence of both hypo- and hyper-connectivity, with the pattern obtained varying as a function of the circuit involved (Di Martino et al., 2014a). Subcortical-based regions showed mostly hyper-connections in ASD relative to TC, whereas cortico-cortical circuits showed ASD-related hypo-connectivity. While findings of ASD-related hypo-connectivity of the pSTS, an associative cortical region, are consistent with the circuit-related variation model, other factors have been suggested to contribute to variability of findings, including the age of the sample examined (Uddin et al., 2013a) or the idiosyncratic topological distribution of iFC (Nair et al., 2014). As the field is progressing, careful reporting of methods and sample characterization, as well as data sharing for future replications with independent approaches are critical to resolve this open issue.
Results of this study must be interpreted in light of several limitations. First, it relies on a cross-sectional design, which can be subject to biases in participant recruitment. Although individuals in our sample exhibited similar autism severity regardless of age, a longitudinal design can capture developmental patterns associated with ASD. Such studies are critical to identify time sensitive windows for intervention on specific skills. Although studies tend to examine trajectories in late childhood and adolescence, the greatest developmental changes occur earlier, paralleling the onset of ASD in the first 3 years of life. It is likely that other measures of intrinsic brain architecture are affected in ASD at earlier ages; for example, while we found significant diagnostic group difference in ReHo pSTS, we did not find age-related changes in our sample starting at 7 years of age. Accordingly, studies examining younger children are of paramount importance to our understanding of the emergence of ASD and to the development of early interventions. Although rs-fMRI has facilitated the study of increasingly younger children, with rare exceptions (Redcay and Courchesne, 2008; Dinstein et al., 2011) development earlier than age 6 years is poorly represented in the neuroimaging ASD literature. A shift toward imaging toddlers and preschoolers is needed to move imaging toward greater clinical utility. In parallel with the cross-sectional nature of this study, information on therapeutic history of the participants was limited. Accordingly, it is difficult to disentangle the impact of ASD on development from the impact of psycho-educational treatments received, potentially inducing compensatory effects. For example, several studies showed that social skills and emotional functioning tend to improve with increasing age with social skills intervention (Shattuck et al., 2007; Farley et al., 2009). Further work, including longitudinal designs, is therefore necessary to explore whether divergent or delayed developmental maturation is paralleled by behavioral improvements later in life.
It should be noted that our study included only male participants. This reflects the higher prevalence of ASD among males as data from only a small number of girls with ASD were available in the ABIDE repository. Yet, sex is increasingly recognized as a source of heterogeneity in psychiatric neuropathophysiology (Cahill, 2006), and the ASD phenotype appears distinct in females (Geschwind and Levitt, 2007; Frazier et al., 2014). This is particularly relevant for pSTS development, as a recent finding showed different developmental trajectories in females and males for the social cognitive processes supported by its circuitry (Anderson et al., 2013). Despite our ability to robustly capture sex differences in TCs (e.g. Biswal et al., 2010), to date, there are no rs-fMRI studies of females with ASD. Given the rarity of girls with ASD, coordinated efforts such as ABIDE are needed to accelerate the discovery process. Further, several participants with ASD presented with psychiatric co-morbidities which are common in ASD (e.g. Simonoff et al., 2008). Co-morbidities were however highly heterogeneous in nature and not equally distributed across the sampled age-range thus preventing examinations of their effect on intrinsic brain properties. Given initial evidence that specific co-morbidities may affect iFC patterns (e.g. Di Martino et al., 2013 for ADHD), future data sharing efforts including deeper phenotyping are needed. Reliance on multi-center datasets may include sources of variance related to site-specific differences (e.g. factors related to participant selection, scan parameters and instructions). However, despite these limitations, results obtained with an aggregate sample may also have higher generalizability by increasing the likelihood that a given effect reflects actual disease properties rather than particulars of a single dataset. Finally, it should be noted that no debriefing was performed regarding the cognitive state of the participants during the scanning session. Therefore, it remains unknown whether ASD patients and TC or children and adults experienced the resting state scan differently and to what extent any related differences in cognitive state affected iFC (see Buckner et al. (2013) for discussion). Collecting debriefing questionnaires about the participants’ cognitive state [e.g. The Amsterdam Resting-State Questionnaire (Diaz et al., 2013) or the New York Cognition Questionnaire (NYC-Q) (Gorgolewski et al., 2014)] can improve interpretation of results. Other strategies, such as the use of movies rather than simple ‘rest’, may also help to constrain participants’ psychological states (Betti et al., 2013). Also methods that capture ‘dynamic functional connectivity’ (e.g. Allen et al., 2014; Yang et al., 2014) will be useful in disentangling the effect of changes in psychological states on functional connectivity.
In summary, pSTS circuitry showed a complex developmental picture, with some functional networks showing atypical development in ASD relative to TC (DMN) and others showing delayed maturation (action perception network). These novel findings of distinct developmental trajectories in different functional circuits in ASD are likely to reflect differential age-related changes in the socio-cognitive processes they underlie. Future developmental investigations should extend to other neural networks relevant to social cognition in ASD and beyond. Further, studies including task-based fMRI investigations can provide a more thorough understanding of how neural developmental trajectories in ASD are paralleled by age-related changes in the behavioral processes that these circuits support. Together, these insights will further advance the quest to develop age-specific interventions for ASD.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by grants from the Flanders Fund for Scientific Research (FWO Krediet aan Navorsers 1521313N, FWO travel grant V417412N, FWO postdoctoral research fellowship grant 1206013N to K.A.), an honorary fellowship of the Belgian American Education Foundation to K.A., as well as by the National Institute of Mental Health (K23MH087770 to A.D.M.), the Leon Levy Foundation awarded to M.P.M., A.D.M. and C.K.; and gifts from Phyllis Green and Randolph Cowen, Joseph P. Healey and the Stavros Niarchos Foundation to the Child Mind Institute (M.P.M.). We would also like to thank all the members of the Autism Brain Imaging Data Exchange Consortium (ABIDE; http://fcon_1000.projects.nitrc.org/indi/abide/) and the INDI team (http://fcon_1000.projects.nitrc.org/) supporting the ABIDE effort. We especially thank the sites whose data were included in these analyses and their funding sources: (i) University of Utah, School of Medicine [National Institute of Health (grant numbers: K08 MH092697, RO1MH080826, P50MH60450, T32DC008553 and R01NS34783), Autism Speaks Mentor-based Predoctoral Fellowship (grant number: 1677), University of Utah Multidisciplinary Research Seed Grant, NRSA Predoctoral Fellowship (grant number: F31 DC010143), Ben B. and Iris M. Margolis Foundation], and (ii) NYU Langone Medical Center [National Institute of Health (K23MH087770; R21MH084126; R01MH081218 and R01HD065282), Autism Speaks, The Stavros Niarchos Foundation, The Leon Levy Foundation and an endowment provided by Phyllis Green and Randolph Cōwen]. We are grateful to F. Xavier Castellanos for helpful discussions, Krishna Somandepalli for technical support, all the members of the Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience at the NYU CSC for their suggestions, as well as R. Cameron Craddock and the Center for the Developing Brain’s team at the CMI for their support with C-PAC use.
REFERENCES
- Akaike H. New look at statistical-model identification. Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- Alaerts K, Woolley DG, Steyaert J, Di MA, Swinnen SP, Wenderoth N. Underconnectivity of the superior temporal sulcus predicts emotion recognition deficits in autism. Social Cognitive and Affective Neuroscience. 2014;9:1589–600. doi: 10.1093/scan/nst156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex. 2014;24:663–76. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, et al. Decreased interhemispheric functional connectivity in autism. Cerebral Cortex. 2011;21:1134–46. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Bolling DZ, Schelinski S, Coffman MC, Pelphrey KA, Kaiser MD. Sex differences in the development of brain mechanisms for processing biological motion. Neuroimage. 2013;83:751–60. doi: 10.1016/j.neuroimage.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, et al. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79:782–97. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The serendipitous discovery of the brain's default network. Neuroimage. 2012;62:1137–45. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network, anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience. 2013;16:832–7. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;6:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain?. A review of the neuroimaging literature. Human Brain Mapping. 2009;30:2313–35. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Di Martino A, Craddock RC, Mehta AD, Milham MP. Clinical applications of the functional connectome. Neuroimage. 2013;80:527–40. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–26. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Craddock RC, Jbabdi S, Yan CG, et al. Imaging human connectomes at the macroscale. Nature Methods. 2013;10:524–39. doi: 10.1038/nmeth.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, et al. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014b;83:1335–53. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biological Psychiatry. 2011;69:847–56. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, et al. The autism brain imaging data exchange, towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry. 2014a;19:659–67. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;74:623–32. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz BA, Van Der Sluis S, Moens S, et al. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Frontiers in Human Neuroscience. 2013;7:446. doi: 10.3389/fnhum.2013.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–25. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6769–74. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research. 2009;2:109–18. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:329–40. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, et al. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6790–5. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders, developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Lurie D, Urchs S, et al. A correspondence between individual differences in the brain's intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One. 2014;9:e97176. doi: 10.1371/journal.pone.0097176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature Neuroscience. 2015;18:302–9. doi: 10.1038/nn.3919. [DOI] [PubMed] [Google Scholar]
- Hoff GE, Van den Heuvel MP, Benders MJ, Kersbergen KJ, De Vries LS. On development of functional brain connectivity in the young brain. Frontiers in Human Neuroscience. 2013;7:650. doi: 10.3389/fnhum.2013.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews, Neuroscience. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism, evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Pelphrey KA. Disrupted action perception in autism, behavioral evidence, neuroendophenotypes, and diagnostic utility. Developmental Cognitive Neuroscience. 2012;2:25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Social Cognitive and Affective Neuroscience. 2012;9:98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Uddin LQ, Kenet T, Chugani D, Müller RA. Brain connectivity in autism. Frontiers in Human Neuroscience. 2014;8:349. doi: 10.3389/fnhum.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–57. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome, promise and pitfalls. Trends in Cognitive Science. 2012;16:181–8. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, et al. Naturalistic fMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in Human Neuroscience. 2012;6:233. doi: 10.3389/fnhum.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents, insights from anatomical magnetic resonance imaging. Neuroscience and Biobehaviour Reviews. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, et al. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. American Journal of Neuroradiology. 2008;29:1883–9. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Service; 1999. [Google Scholar]
- Lord C, Rutter M, Le CA. Autism diagnostic interview-revised, a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1233–51. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems, exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–23. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–9. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism, functional brain imaging studies. Current Opinion in Neurology. 2010;23:124–30. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties, a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews. 2012;36:341–9. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Samson AC, et al. Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism, a multimodal MRI study. PLOS One. 2013;8:e67329. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity mri studies in autism spectrum disorders. Cerebral Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, Müller RA. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Human Brain Mapping. 2014;35:4035–48. doi: 10.1002/hbm.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long XY, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Research. 2010;1321:169–79. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Lynn A, Foran W, Luna B, O_Hearn K. Age related changes in striatal resting state functional connectivity in autism. Frontiers in Human Neuroscience. 2013;7:814. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Wyk BCV. Research review, constraining heterogeneity, the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2012;36:901–42. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? Journal of Neuroscience. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception, Implications for the emergence of autism. Neuroscience and Biobehavioral Reviews. 2008;32:123–42. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biological Psychiatry. 2008;64:589–98. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37:1735–47. doi: 10.1007/s10803-006-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gogtay N, Rapoport J. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping. 2010;31:917–25. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Muller RA. Functional differentiation of posterior superior temporal sulcus in autism, a functional connectivity magnetic resonance imaging study. Biological Psychiatry. 2011;70:270–7. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Muller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–9. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Regional homogeneity of fMRI time series in autism spectrum disorders. Neuroscience Letters. 2010;476:46–51. doi: 10.1016/j.neulet.2010.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–9. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, et al. Salience network-based classification and prediction of symptom severity in children with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2013b;70:869–79. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks, insights from resting-state FMRI. Frontiers in Systems Neuroscience. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience. 2013a;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Fischi-Gomez E, Huppi PS. Multimodality evaluation of the pediatric brain, DTI and its competitors. Pediatric Radiology. 2013;43:60–8. doi: 10.1007/s00247-012-2515-y. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism, a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews. 2012;36:604–25. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, et al. Dysmaturation of the default mode network in autism. Human Brain Mapping. 2013;35:1284–96. doi: 10.1002/hbm.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wiggins JL, Bedoyan JK, Peltier SJ, et al. The impact of serotonin transporter (5-HTTLPR) genotype on the development of resting-state functional connectivity in children and adolescents, a preliminary report. Neuroimage. 2012;59:2760–70. doi: 10.1016/j.neuroimage.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Ashinoff S, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Research. 2011;1380:187–97. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76C:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Craddock RC, Margulies DS, Yan CG, Milham MP. Common intrinsic connectivity states among posteromedial cortex subdivisions: insights from analysis of temporal dynamics. Neuroimage. 2014;93:124–37. doi: 10.1016/j.neuroimage.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neurosciences. 2006;29:359–66. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain, complex and reliable. Neuroimage. 2010a;49:1432–45. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, et al. Growing together and growing apart, regional and sex differences in the lifespan developmental trajectories of functional homotopy. Journal of Neuroscience. 2010b;30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Xing XX. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience and Biobehavioral Review. 2014;45:100–18. doi: 10.1016/j.neubiorev.2014.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.