Abstract
Near-miss events are situations in which an action yields a negative result but is very close to being successful. They are known to influence behavior, especially in gambling scenarios. Previous neuroimaging studies have described an ‘anomalous’ activity of brain reward areas following these events. The goal of the present research was to study electrophysiological correlates of near-misses in the expectation and outcome phases. Electroencephalography was recorded while participants were playing a simplified version of a slot machine. Four possible outcomes (gain, near-miss, loss and no-information) were presented in a pseudorandom order to ensure fixed proportions. Results from the time–frequency analysis for the theta (4–8 Hz), alpha (9–13 Hz), low beta (15–22 Hz) and beta-gamma (25–35 Hz) frequency-bands presented larger power increases for wins and near-misses compared with losses. In the anticipation phase, power changes were lower than in the resolution phase. The current results are in agreement with previous studies showing that near-miss events recruit brain areas of the reward network. Likewise, the oscillatory activity in near-misses is very similar to the one elicited in the gain condition. In addition, present findings suggest that oscillatory activity in the expectation phase does not play a crucial role in near-miss events.
Keywords: near-miss, reward, oscillations, theta, beta-gamma
INTRODUCTION
External feedbacks provide information about the appropriateness of our actions, allowing us to learn from our experiences and to adapt our behavior to maximize rewards and minimize punishments. Hence, positive feedbacks or rewards act as reinforcers whereas negative outcomes or punishments favor the avoidance of certain behaviors (Sutton and Barto, 1998). To achieve their goals, people are able to estimate the likelihood of the different options and make predictions about the consequences of their actions. Nevertheless, behavior is not just regulated by the reward-punishment dichotomy, but may also be influenced by other factors such as intuition, implicit rules or beliefs (Tversky and Kahneman, 1981). In addition, some events also have the capacity to produce misleading or biased interpretations. In the gambling domain, near-misses and illusion of control are two of those factors that may influence people’s cognition and performance. Near-misses (or ‘almost a win’ in Skinner's words) are a special type of negative result that are close to the goal of an action, generating an expectation of success that in the end is not fulfilled. In tasks that depend on people skills, near-misses are related to performance, but in games of chance, such as some gambling scenarios, they are also perceived as being closer to wins than to full-misses (Dixon and Schreiber, 2004) even though they do not reflect players’ accuracy. The effect of near-misses on self-confidence is misleading, and it is closely related to the illusion of control, the commonly held but biased belief of gamblers that they can influence chance outcomes (Langer, 1975). Some authors have found maximal effects of near-misses on gambling persistence when they represent around 30% of the total plays (Strickland and Grote, 1967; Kassinove and Schare, 2001; Côté et al., 2003). Participants describe near-misses as more unpleasant but more motivating than full-misses (Qi et al., 2011). Moreover, they are known to influence gamblers’ behavior, showing bigger money bets after near-misses or an increased desire to continue gambling (Griffiths, 1991; Côté et al., 2003). Other studies have also found that near-misses produce similar or even higher physiological arousal (electrodermal activity and heart rate) than wins (Dixon et al., 2012). Thus, near-misses are known to act as reinforcers despite the absence of real reward (Skinner, 1953).
One potential explanation for the reinforcement effect of near-misses in gambling scenarios is that they are actually perceived similarly to positive outcomes, indicating closeness to gain. In agreement with this assumption, previous studies using functional Magnetic Resonance Imaging (fMRI) have found that near-misses activate regions of the reward network such as the bilateral ventral striatum and the right anterior insula (Clark et al., 2009; Chase and Clark, 2010). According to these findings, Clark et al. (2009) proposed that near-misses invigorate gambling through the anomalous recruitment of brain reward-related areas. However, an alternative explanation to these findings could be driven by the expectancy of a reward, so the invigorating effect could emerge as a result of the momentarily increase of expectancy of winning before the occurrence of a near-miss and be masked by the low temporal resolution of the BOLD fMRI response. Indeed, similar brain regions engaged during the delivery of reward (especially ventral striatum) are also recruited in its anticipation (Knutson et al., 2003; Knutson and Cooper, 2005; Yacubian et al., 2006; Diekhof et al., 2012). Expectation of winning might therefore play a key role in near-miss events.
Electroencephalography (EEG) technique might help in disentangling the activations of the two phases and in determining the exact time-course of the brain activity in near-misses as it provides fine-grain temporal tracking of neural processes with millisecond time resolution. Previous studies have shown that the P300 event-related potential (ERP) is modulated by the valence (larger for gains compared with losses; Wu and Zhou, 2009; Luo et al., 2011), the magnitude (Yeung and Sanfey, 2004) and probability (Spencer and Polich, 1999) of monetary outcomes. With respect to near-miss events, ERPs present contradictory results, showing similar P300 ERP amplitudes for near-miss and full-miss trials (Luo et al., 2011), larger amplitudes for near-misses compared with full-misses (Qi et al., 2011) or, contrarily, showing larger amplitudes for full-misses compared with near-misses (Ulrich and Hewig, 2014). Moreover, time–frequency decomposition of EEG data allows the study of the oscillatory components related to gains and losses. Increased medial–frontal theta activity (4–8 Hz) has been found after negative feedback or error performance (Luu et al., 2004; Yordanova et al., 2004; Cohen et al., 2007, 2009; Marco-Pallares et al., 2008; Cavanagh et al., 2010; Cunillera et al., 2012; De Pascalis et al., 2012; HajiHosseini et al., 2012) and also following unexpected positive feedbacks (Cavanagh et al., 2010; Bunzeck et al., 2011; Doñamayor et al., 2011; Cunillera et al., 2012; Mas-Herrero and Marco-Pallarés, 2014). In addition, beta-gamma power increase (20–35 Hz) has been found after monetary rewards or positive feedbacks (Cohen et al., 2007; Marco-Pallares et al., 2008; van de Vijver et al., 2011; Leicht et al., 2013).
The goal of the current research was to study the electrophysiological correlates (ERPs and oscillatory activity) related to near-miss events in both anticipation and outcome phases of a slot machine gambling task. We hypothesized that if the activation of brain reward areas found in previous neuroimaging studies took place in the expectancy of reward, the presentation of the final (negative) feedback would produce a fast decrease of activity. In this case, oscillatory power changes associated with reward processing would take place in the expectation phase for near-misses. Alternatively, if the effects of near-miss events were produced, as previously proposed, by an anomalous recruitment of reward areas, similar oscillatory responses to near-misses and gains after the outcome presentation would be expected.
MATERIALS AND METHODS
Participants
Twenty-four healthy volunteers (15 women, 24.12 ± 4.8 years old) participated in the experiment. All participants were informed that they would receive a monetary reward at the end of the task, one fixed part and another variable part depending on their performance. Informed consent was obtained from all participants. The ethics committee of the Biomedical Research Institute of Bellvitge (IDIBELL) approved all the procedures of the experiment.
Task
We designed a simplified version of a slot machine with two reels, inspired by the experimental task used by Clark et al. (2009) and adapted for EEG recording (Figure 1).
Fig. 1.
Experimental design showing the steps for each trial. 1. In the first part of each trial, the participants could select to bet 1, 5 or 25 points. 2. In half of the trials (N = 175), the participants chose the image they wanted to play with, and in the other half of the trials the figure was selected by the computer. 3. After the image selection, the second reel spun for 2.5–3.5 s. 4. The second reel made a random number of 1-s movements (2, 3 or 4) before stopping. 5. Final outcome (gain, loss, near-miss and no-information) was presented for 1 s.
At the beginning of the task, each participant had 1000 points, equivalent to 10 Euros. In each trial (out of a total of 350), the subjects chose to bet 1, 5 or 25 points. These points were added or subtracted to the total points depending on the result of each game. In half of the trials, the participants could choose the image of the first reel they wanted to play (unforced selection condition to generate illusion of control). This was indicated by a blue arrow. In the other half of the trials, the computer randomly selected the image, and it was indicated by a red arrow (forced selection condition). Once the picture was selected, the second reel began to spin for 2.5–3.5 s, and it stopped after performing a random number of 1-s movements (2, 3 or 4) to prevent the anticipation of the final result before the appearance of a red rectangle to indicate the outcome of that trial.
There were four different conditions: gain, loss, near-miss and no-information. Unknown to the participants, all conditions were presented in a pseudorandom ratio to ensure the proportion of each outcome. ‘Gains’ occurred in P = 1/7 of the total (N = 50), when the two central pictures were the same. ‘Near-misses’ occurred P = 2/7 of the total (N = 100), consistent with previous studies showing that the optimal percentage of near-miss outcomes to increase the player’s desire to continue playing is around 30% (Kassinove and Schare, 2001). Near-misses were divided into two different possibilities: ‘near-miss pre’ (N = 50) happened when the winning figure was one movement before the central position, and ‘near-miss post’ (N = 50) when the winning figure advanced one position after passing through the center. Near-misses differed from ‘full-misses’ (‘Loss’ condition: P = 3/7 of the total; N = 150), which occurred without expectation of winning: the winning figure was more than two steps from (or to) the center. The ‘no-information’ condition (P = 1/7 of the total; N = 50) was designed as a control condition. In the outcome of no-information trials, the second reel displayed question marks replacing all the images without previous movements (no anticipation phase), and bet points were neither added nor subtracted from total points.
When a game was lost, the bet amount was subtracted from the total points (loss and near-miss conditions). When a game was won, the bet amount was multiplied by 4 and then added to the total. This was decided upon to encourage the participants and with the aim of offsetting the much higher percentage of games in which they were losing money. Before the register, participants had six training trials to habituate to the task, and the outcomes were presented in fixed proportions (two gains, two near-misses, one loss and one no-information trial).
Electrophysiological recording
EEG was recorded using an elastic cap with 29 electrode standard positions (Fp1/2, Fz, F7/8, F3/4, FCz, FC1/2, Fc5/6, Cz, C3/4, T7/8, Cp1/2, Cp5/6, Pz, P3/4, P7/8, Po1/2, Oz), left and right mastoids, one electrode placed at the lateral outer canthus of the right eye used as an online reference and one electrode to monitor eye movements placed at the infraorbital ridge of the right eye. Electrode impedances were kept below 5 kΩ. EEG was re-referenced offline to the mean of the activity at the two mastoid electrodes. The images were presented centered in a computer screen on a light background. Participants used left and right mouse buttons to select their responses.
Data analysis
Statistical analysis on the proportion of responses to each of the possible choices (1, 5 or 25) was performed using the arcsine transformation (arcsine of the square root of the proportion (), used to stabilize variances and normalize proportional data.
EEG recordings were analyzed using the EEGLAB toolbox (Delorme and Makeig, 2004) by epoching data from 100 ms before the outcome (baseline) to 600 ms after the appearance of the result. All trials with mean amplitudes higher than 100 μV (EEG and electrooculography) were rejected. Four participants were excluded from the study because they had more than 20% trials rejection (20 participants remained, 12 women). Differences among conditions were assessed by using an analysis of variance (ANOVA) with two factors, the four conditions and midline electrodes Fz, Cz and Pz, for averaged ERP values of 50 ms around the peak of the P300 component.
For the time–frequency analysis, a continuous wavelet transformation on single-trial data for each participant and electrode was performed using a complex Morlet wavelet defined as follows:
Epochs comprised 4000 ms (2000 ms before the outcome and 2000 ms after). Changes in time varying energy were computed by squaring the convolution between wavelet and signal, in the frequencies from 1 to 40 Hz for each trial and participant and then baseline-corrected before performing the grand average. In each trial, Z-scores were computed for each frequency-band in the corresponding time-window [200–500 ms for theta (4–8 Hz), 150–300 ms for alpha (9–13 Hz), 450–750 ms for beta (15–25 Hz) and 200–400 ms for beta-gamma (25–35 Hz)]. Trials with values higher than 2.5 s.d. from the mean were rejected. One participant was excluded from the time–frequency analyses because he presented outlier values in most of the studied frequency-bands. We obtained the mean increase/decrease in power for gain, loss near-miss and no-information conditions. Separate ANOVAs were conducted for the power of each frequency-band between conditions. The Greenhouse–Geisser epsilon was used to correct for possible violations of the sphericity assumption for all statistical effects involving two or more degrees of freedom in the numerator. P-value after the correction is reported.
RESULTS
Behavioral results
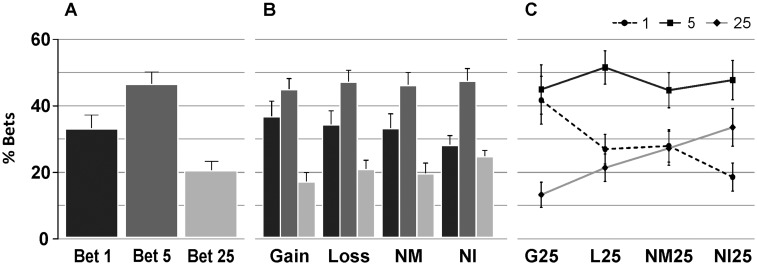
Figure 2A shows the percentage of choices for each of the conditions. Five points was the most chosen bet amount (47% of the total bets) followed by 1 point (33%) and then by 25 points (20%).
Fig. 2.
Behavioral results. (A) Overall percentage of chosen bets. (B) Percentage of selected bets depending on the previous result. (C) Risky bets chosen after each condition with previous 25-point result. Repeated measures (ANOVA) between conditions with risky bets showed a linear pattern (P < 0.05), with the lowest percentage after gains, followed by losses, near-misses and no-information outcomes sequentially.
We also analyzed the bets after each possible outcome (Figure 2B). ANOVA analysis between outcomes and bet amount revealed a significant bet factor [F(2,38) = 6.2, P < 0.01], with no interaction effects [outcome × bet, F(6,114) = 1.15, P > 0.1]. This indicated a clear preference for the 5-point bet independently from previous outcome. However, when selectively analyzing outcomes associated with risky bets, we found differences in the participant’s choices depending on the previous outcome (Figure 2C). The corresponding ANOVA was performed between outcomes with previous 1, 5 or 25-point bet, and risky choice (25 points) selected for the next trial. A significant effect was found in the interaction between condition and bet amount [F(6,114) = 2.63, P < 0.05] and for condition type [F(3,57) = 2.83, P < 0.05], and marginal effect was found for bet amount [F(2,38) = 3.12, P = 0.55]. An ANOVA between trials with 25-point-bet outcomes and 25 points chosen for the next trial was performed in order to accurately identify possible differences in the risky choices depending on high-value previous outcomes. We found significant differences between conditions [F(3,57) = 4.5, P < 0.05], with results following a linear pattern [F(1,19) = 6.1, P < 0.05]. These results show that the participants chose less often a high bet after a winning-25-point-trial, and the proportion of high bet choices increased linearly for loss, near-miss and no-information trials. After a no-information outcome, the main tendency was to repeat the same bet (for risky bets and also for the other bets).
Event-related brain potentials
ERPs elicited by gain, near-miss, loss and no-information outcomes are shown in Figure 3. Main responses were found in P300 component. Gains presented the largest amplitude, followed by near-misses and losses (near-miss responses were in between gains and losses). The no-information condition had a similar response pattern to the loss condition. The maximal amplitude was reached around 360 ms after the outcome for gains in the central electrodes, being largest at Cz electrode (Figure 3B).
Fig. 3.
ERP results. ERP results from 100 ms before the outcome (baseline) to 600 ms after the appearance of the result. (A) P300 component analysis (340–388 ms after the outcome) showed statistically significant differences between gain, near-miss and loss conditions (P < 0.001 for all comparisons). Near-misses presented an intermediate activity between gains and losses. Loss and no-information conditions presented similar amplitudes (P > 0.05). (B) Topographical graphs of the potential distribution of gain, near-miss, loss and no-information conditions in the time window from 340 to 388 ms after the outcome. Note the difference in the scales between the different conditions.
An ANOVA with condition type and midline electrodes (Fz, Cz and Pz) was performed in the range between 340 and 388 ms after the outcome. The results showed a significant effect for condition type [F(3,57) = 78.4, P < 0.001], electrode type [F(2,38) = 19.7, P < 0.001], and also for the interaction between conditions and electrodes [F(6,114) = 11.76, P < 0.001]. Further pairwise comparisons revealed significant differences among all conditions (amplitude of gain > near-miss > loss > no info) at all three electrodes [t(19) > 5, P < 0.001], except between loss and no-information, which presented significant differences at Pz [t(19) = 2.17, P < 0.05] and Cz [t(19) = 2.76, P < 0.05], but only marginal at Fz [t(19) = 2.02, P < 0.1].
Time–frequency analysis
The time–frequency analysis showed power enhancements in theta, alpha, low beta and beta-gamma frequency-bands for gains and near-misses compared with loss and no-information conditions (Figure 4). Time windows were selected by visual inspection and according to prior knowledge, and the selected electrodes for each frequency corresponded to the maximal activations shown in the scalp topographies.
Fig. 4.
(A) Time–frequency plots for spectral power differences for gain over loss outcomes at CZ and FC6 locations and topographical maps of power distribution in theta (4–8 Hz, 200–500 ms window), alpha (9–13 Hz, 150–300 ms), low beta (15–25 Hz, 450–750 ms) and beta-gamma (25–35 Hz, 200–400 ms window) frequency-bands. (B) Time–frequency analysis for all frequencies showed larger power increases with respect to the baseline for gains and near-misses compared with losses and no-information. No differences were found between gain and near-miss outcomes (P > 0.05 for all frequencies), but there were significant differences between them and loss and no-information outcomes.
Theta band power increase (4–8 Hz) was analyzed in the time window from 200 to 500 ms after the outcome. The ANOVA with condition type and right-parietal electrodes (C4, Cz, CP2, CP6) showed condition type effect [F(3,54) = 23.34, P < 0.001], no electrode effect [F(3,54) = 3.16, P > 0.1] and no interaction effect [condition × electrode, F(9,162) = 0.96, P > 0.1]. Post hoc t-test revealed no significant differences between gain and near-miss [t(18) = 1.55, P > 0.1] nor between loss and no-information [t(18) = 1.55, P > 0.05]. Statistically significant differences were found between gain and loss [t(18) = 5.13, P < 0.001], gain and no-information [t(18) = 5.6, P < 0.001], near-miss and loss [t(18) = 6.6, P < 0.001] and between near-miss and no-information [t(18) = 6.1, P < 0.001]. These results indicate that both gains and near-misses elicited significantly increased theta activity compared with losses at right-parietal region. Although no differences were found between gains and near-misses, the ANOVA for condition type revealed a linear power increase for the different outcomes [F(1,18) = 40.48, P < 0.001; Figure 4B].
For alpha frequency-band, power changes from 9 to 13 Hz were obtained in the time window from 150 to 300 ms after the outcome. The ANOVA with condition type and five central electrodes (C4, Fz, Cz, FC1 and FC2) showed significant effect of condition type [F(3,54) = 9.95, P < 0.001] with a significant linear increase of alpha power [F(1,18) = 21.65, P < 0.001], no effect of electrode location [F(4,72) = 1.52, P > 0.1] and no interaction effect [F(12,216) = 1.79, P > 0.1]. Post hoc t-test analysis showed no statistical differences between gains and near-misses [t(18) = 0.49, P > 0.1] nor between loss and no-information outcomes [t(18) = 1.64, P > 0.1]. Statistical differences were found between gains and losses [t(18) = 2.85, P < 0.005], gains and no-information [t(18) = 4.37, P < 0.001], near-misses and losses [t(18) = 3.6, P < 0.005] and between near-miss and no-information [t(18) = 3.94, P < 0.005]. Therefore, near-misses and gains presented similar power increases at the alpha band, which were significantly larger than loss and no-information outcomes.
Low beta frequency-band was also studied from 15 to 25 Hz in the time-window between 450 and 750 ms. The main power increase for this frequency-band after the outcome was in F4, FC2 and FC6 electrodes. The ANOVA showed differences between conditions [F(3,54) = 9.99, P < 0.001] with a linear power increase [F(1,18) = 13.1, P < 0.005], no electrode effect [F(2,36) = 0.95, P > 0.1] and marginal interaction effect [F(6,108) = 2.7, P = 0.053]. Post hoc t-test analysis showed no differences between gains and near-misses [t(18) = 0.42, P > 0.1]. Statistical differences were found for all the other comparisons: gains and losses [t(18) = 2.74, P < 0.05], gains and no-information [t(18) = 3.49, P < 0.05], near-misses and losses [t(18) = 2.13, P < 0.05], near-misses and no-information [t(18) = 3.72, P < 0.05] and, only in this frequency-band, differences were also found between loss and no-information [t(18) = 3.02, P < 0.05]. Thus, gains and near-misses presented larger power increases than losses and no-information outcomes.
Finally, we also analyzed beta-gamma band (25–35 Hz) in the time window from 200 to 400 ms. The power increase was significantly greater for gains and near-misses, followed by loss and no-information outcomes. The results from the ANOVA with condition type and right-central electrodes (C4, F4, FC2 and FC6) showed condition effect [F(3,54) = 5.18, P < 0.01], no electrode effect [F(3,54) = 0.19, P > 0.5], and no significance for the interaction between electrodes and conditions [F(9,162) = 0.46, P > 0.5]. Further analysis between the four conditions presented a linear pattern in the power increases [F(1,18) = 7.9, P < 0.05]. Post hoc t-test results between conditions showed no significant differences between gains and near-misses [t(18) = 1.78, P = 0.092] nor between loss and no-information [t(18) = 0.3, P > 0.5]. Differences were found between gains and losses [t(18) = 3.27, P < 0.005], gains and no-information [t(18) =2.46, P < 0.005], and also between near-misses and losses [t(18) = 2.9, P < 0.05] and between near-misses and no-information [t(18) = 2.2, P < 0.05].
Possible differences in power increase depending on the image selection type (automatic or manual) were also analyzed. Different ANOVA analyses with selection type and condition type were performed for all studied frequency-bands in the corresponding electrodes. We found no differences manual and automatic selection (P > 0.05 for all comparisons).
In summary, the results of the time–frequency analysis for gain, loss, near-miss and no-information outcomes reveal a common tendency over the different oscillatory frequency-bands, with greater induced activity after gains and near-misses compared with loss and no-information trials, following a linear pattern although differences between gain and near-miss do not reach significance.
Expectation phase
One of the hypotheses for this study was that the brain activity related to near-miss events could be increased in the expectation phase but, after the outcome, those activations would decrease and be similar to the ones found after losses. Hence, the oscillatory activity in the phase preceding the final result was also investigated by analyzing the power increases for the studied frequency-bands 1 s before the outcome (anticipation phase; Figure 5).
Fig. 5.
Power of the different components analyzed in the anticipation (n − 1) and outcome phases for the different outcomes. In the anticipation phase (1 s before the outcome), power changes for theta, alpha, beta and beta-gamma frequency-bands in gain and near-miss conditions were lower than in the resolution phase.
ANOVA analyses in the anticipation phase revealed differences among conditions in theta and alpha frequency-bands, and no differences among conditions for low beta and beta-gamma frequency-bands [F(2,38) = 3.19, P > 0.05 and F(2,38) = 0.36, P > 0.1, respectively]. Further pairwise comparisons of the power increases showed significant differences among all conditions for theta frequency-band (gain > near-miss > loss; t(18) > 3, P < 0.005). For alpha band, t-test analysis showed no differences between gain and near-miss [t(18) = 0.9, P > 0.1] and differences between them and loss (gain and near-miss > loss; t(18) = 2.5, P < 0.05).
Pairwise comparisons between the power increases 1 s before and in the outcome for the theta band revealed significant differences for gains [t(18) = 5.1, P < 0.001], near-misses [t(18) = 5.5, P < 0.001] and losses [t(18) = 4.6, P < 0.001], being greater in the outcome than in the expectation phase. For the alpha and low beta bands, t-test analyses between the anticipation and outcome phases also showed significantly increased power for gains [t(18) = 2.9, P < 0.05], near-misses [t(18) = 2.8, P < 0.05] and losses [t(18) = 2.3, P < 0.05] in the outcome compared with the expectation phase for alpha band, and gains [t(18) = 3.4, P < 0.05], near-misses [t(18) = 3.1, P < 0.05] and losses [t(18) = 2.6, P < 0.05] for the low beta band.
In the low beta band, the results also showed larger power increase in the outcome compared with the expectation phase for gain [t(18) = 3.4, P < 0.05], near-miss [t(18) = 3.1, P < 0.05] and loss conditions [t(18) = 2.6, P < 0.05]. Beta-gamma frequency-band showed increased activation for gain outcomes compared with the anticipation phase [t(18) = 2.8, P < 0.05], but there were no differences between expectation and resolution phases for the near-miss and loss conditions [t(18) = 0.34, P > 0.1 and t(18) = −0.9, P > 0.1, respectively]. These results show that the electrophysiological brain responses to near-misses in the anticipation phase for theta, alpha and low beta frequency-bands were lower than the ones elicited after the outcome.
DISCUSSION
The goal of this study was to disentangle possible differences in brain activity along the time course of near-miss events (in the anticipation and resolution phase of a gambling task). First, our ERP results showed differences in P300 component between gain, near-miss and loss conditions. Moreover, time–frequency analysis revealed power increases in theta, alpha, low beta and beta-gamma bands, significantly greater after gain and near-miss outcomes compared with loss and no-information outcomes. These findings provide new evidence on near-miss events, showing that near-miss neural oscillatory responses are similar to the ones elicited by gains. Additionally, the oscillatory activity in the expectation phase in near-misses was lower compared with the outcome, suggesting that near-miss effects cannot be explained by activation in the expectation phase.
The main finding of this study is the similar brain oscillatory activity of win and near-miss outcomes, which resulted in a power increase at all the studied frequency-bands compared with loss and no-information conditions. Theta-band oscillatory activity has been related to reward processing, selective attention, behavior adjustments, cognitive control, working memory and learning among other cognitive processes (Bernat et al., 2007; Palva and Palva, 2007; Cavanagh and Frank, 2014). Current research shows increased theta power for gains, in accordance with previous studies describing increased theta power following positive compared with negative outcomes (Doñamayor et al., 2011), as a result of unexpected or surprising outcomes (Bunzeck et al., 2011), as a consequence of prediction error (the difference between an expected value or result and the real outcome; Cavanagh et al., 2010; Mas-Herrero and Marco-Pallarés, 2014), or after a switch cue and after the first positive outcome (Cunillera et al., 2012). In our task, gains were more unexpected than losses, and our results in theta frequency-band might reflect a response to a positive prediction error. However, theta power was also enhanced after near-misses, with a power increase significantly larger than after full-misses. These results are in accordance with Dymond et al. (2014) who also found a theta power increase following near-misses. However, the right-lateralized theta power increase found in the current experiment [also reported in Marco-Pallares et al. (2008) and Christie and Tata (2009)] might arise from parietal regions, reflecting the involvement of attention in the cognitive processing of reward (Schultz, 2006). The increase in the alpha oscillatory activity after near-misses and gains would also support this interpretation, as this activity plays an important role in focusing attention and suppressing irrelevant information (von Stein and Sarnthein, 2000; Ward, 2003). Therefore, results in theta and alpha frequency-bands might be associated with increased attention, behavior adjustments and strategy switching during reward learning, even when near-misses are not related to the participants’ skill.
Time–frequency analysis also revealed increased activity in higher oscillatory bands following gains and near-misses compared with loss and no-information outcomes. Our results on beta-gamma power following gain outcomes compared with losses are consistent with previous results describing a beta-gamma power increase selectively after positive feedbacks (Cohen et al., 2007, 2009; Marco-Pallarés et al., 2008; Cunillera et al., 2012; Leicht et al., 2013), and after unexpected rewards (HajiHosseini et al., 2012). It has also been suggested that beta oscillatory activity might be related to a motivational signal involved in the frontostriatal coupling after important rewards (Marco-Pallarés et al., 2008). Interestingly, near-miss events also present high-frequency oscillatory activity (in contrast to losses) suggesting that they are treated as relevant outcomes, different from full-misses and very close to gains.
Complementary to the findings described above for feedback-evoked oscillatory responses, one of the goals of this study was to identify electrophysiological correlates related to near-miss events in the expectation phase. Previous fMRI studies had described greater BOLD response in the midbrain (Chase and Clark, 2010; Habib and Dixon, 2010), in the right insula and orbitofrontal cortex (Dymond et al., 2014), and in right insula and ventral striatum (Clark et al., 2009) following near-misses. Nevertheless, because of the low time resolution of the fMRI technique, the participation of these brain reward-related areas in the expectation and outcome phases cannot be easily disambiguated. Indeed, some of the areas of the reward network such as ventral striatum are active in the anticipation of rewards (Knutson et al., 2003; Knutson and Cooper, 2005; Yacubian et al., 2006; Diekhof et al., 2012). Hence, it might be that the BOLD activity observed after near-misses would be associated with the anticipation of a potential win more than with the outcome per se. However, the results of the time–frequency analysis discard the anticipation hypothesis, as near-misses elicited similar brain oscillatory activity to gains in the outcome phase and lower activations were found in the anticipation phase compared with the outcome. Therefore, present results would support previous neuroimaging findings showing that near-misses activate the brain reward system.
Finally, our results in the ERPs analysis also provide evidences that near-misses are processed differently from standard losses. Significant differences were found in the P300 ERP amplitude among conditions, being larger after gains, followed by near-misses and then by losses. The P300 component is an ERP that appears as a positive deflection in voltage around 300 ms after the stimulus. P300 has been related to attention, decision making and reward processing and is modulated by the motivational significance (Nieuwenhuis et al., 2005), the probability (Hajcak et al., 2005; von Borries et al., 2013) and the magnitude of the rewards (Yeung and Sanfey, 2004; Sato et al., 2005). Our results are in agreement with previous findings showing greater P300 amplitude for gains compared with losses (Hajcak et al., 2005; Yeung et al., 2005; Wu and Zhou, 2009; Zhou et al., 2010). It is important to note that in our design, no-information outcomes were presented with the same probability as gains, so they could be considered equally unexpected. However, the P300 amplitude for no-information trials was similar to the one elicited by losses (the most probable outcome), and smaller than the P300 elicited by gains, discarding the possibility that the higher amplitude for gains may be explained exclusively by its low probability of appearance. In addition, P300 was not modulated by the reward magnitude, as no differences were found between low, medium and high bets trials. Finally, our ERP results for near-misses are in accordance with Qi et al. (2011), who also described higher P300 for gains, followed by near-misses and then by full-misses.
The significant differences found in the P300 analysis between near-misses and gains contrast with the lack of differences between these two conditions in the time–frequency analysis. However, it cannot be ruled out that the absence of statistical differences in the power activity may be due to low statistical power of the present data. Indeed, a linear pattern in the ANOVA for the condition type was found for all the studied frequency-bands, with the largest power increases in the gain condition. This linear trend would suggest that, although brain oscillatory mechanisms might be similar in the gain and near-miss conditions, the latter could present a reduced activity. A similar result was found in the original fMRI study by Clark et al. (2009), where near-misses recruited less brain areas than gain condition, and with reduced activity. More studies are needed to determine whether oscillatory activity associated with near-misses involves exactly the same oscillatory mechanisms than gains.
In conclusion, present findings are important in understanding the neurophysiological mechanisms associated with reward processing and the cognitive biases influencing gambling behavior. Activations in the brain reward areas after near-misses have been found to correlate with gambling biases and severity (Chase and Clark, 2010; Habib and Dixon, 2010), and differences in theta oscillations have been proposed as a biomarker of vulnerability to pathological gambling (Dymond et al., 2014). Future research in patients with addictions or subclinical behaviors could help in understanding neural mechanisms associated with the vulnerability to addiction and other reward-related pathologies.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by Spanish Government grants to J.M.P. (PSI2012-37472) and predoctoral FPI grants to E.M.H. (BES-2010-032702) and H.A. (BES-2013-067440).
REFERENCES
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Guitart-Masip M, Dolan RJ, Düzel E. Contextual novelty modulates the neural dynamics of reward anticipation. The Journal of Neuroscience. 2011;31:12816–22. doi: 10.1523/JNEUROSCI.0461-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014;18:414–21. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clark L. Gambling severity predicts midbrain response to near-miss outcomes. The Journal of Neuroscience. 2010;30:6180–7. doi: 10.1523/JNEUROSCI.5758-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie GJ, Tata MS. Right frontal cortex generates reward-related theta-band oscillatory activity. Neuroimage. 2009;48:415–22. doi: 10.1016/j.neuroimage.2009.06.076. [DOI] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–90. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback. The Journal of Neuroscience. 2009;29:7591–8. doi: 10.1523/JNEUROSCI.5335-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35:968–78. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté D, Caron A, Aubert J, Desrochers V, Ladouceur R. Near wins prolong gambling on a video lottery terminal. Journal of Gambling Studies. 2003;19:433–8. doi: 10.1023/a:1026384011003. [DOI] [PubMed] [Google Scholar]
- Cunillera T, Fuentemilla L, Periañez J, et al. Brain oscillatory activity associated with task switching and feedback processing. Cognitive, Affective & Behavioral Neuroscience. 2012;12:16–33. doi: 10.3758/s13415-011-0075-5. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, Rotonda M. EEG oscillatory activity associated to monetary gain and loss signals in a learning task: effects of attentional impulsivity and learning ability. International Journal of Psychophysiology. 2012;85:68–78. doi: 10.1016/j.ijpsycho.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–66. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, MacLaren V, Jarick M, Fugelsang JA, Harrigan KA. The frustrating effects of just missing the jackpot: slot machine near-misses trigger large skin conductance responses, but no post-reinforcement pauses. Journal of Gambling Studies. 2012;29(4):661–74. doi: 10.1007/s10899-012-9333-x. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Schreiber JE. Near-miss effects on response latencies and win estimations of slot machine players. The Psychological Record. 2004;54:335–48. [Google Scholar]
- Doñamayor N, Marco-Pallarés J, Heldmann M, Schoenfeld MA, Münte TF. Temporal dynamics of reward processing revealed by magnetoencephalography. Human Brain Mapping. 2011;32:2228–40. doi: 10.1002/hbm.21184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Lawrence NS, Dunkley BT, et al. Almost winning: induced MEG theta power in insula and orbitofrontal cortex increases during gambling near-misses and is associated with bold signal and gambling severity. Neuroimage. 2014;91:1–9. doi: 10.1016/j.neuroimage.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Griffiths M. Interdisciplinary and applied psychobiology of the near-miss in fruit machine gambling. The Journal of Psychology. 1991;125(3):347–57. doi: 10.1080/00223980.1991.10543298. [DOI] [PubMed] [Google Scholar]
- Habib R, Dixon MR. Neurobehavioral evidence for the “Near-Miss” effect in pathological gamblers. Journal of the Experimental Analysis of Behavior. 2010;93:313–28. doi: 10.1901/jeab.2010.93-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–70. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- HajiHosseini A, Rodríguez-Fornells A, Marco-Pallarés J. The role of beta-gamma oscillations in unexpected rewards processing. Neuroimage. 2012;60:1678–85. doi: 10.1016/j.neuroimage.2012.01.125. [DOI] [PubMed] [Google Scholar]
- Kassinove JI, Schare ML. Effects of the ‘near miss’ and the ‘big win’ on persistence at slot machine gambling. Psychology of Addictive Behaviors. 2001;15:155–8. doi: 10.1037//0893-164x.15.2.155. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Langer EJ. The illusion of control. Journal of Personality and Social Psychology. 1975;32(2):311–28. [Google Scholar]
- Leicht G, Troschütz S, Andreou C, et al. Relationship between oscillatory neuronal activity during reward processing and trait impulsivity and sensation seeking. PLoS One. 2013;8:e83414. doi: 10.1371/journal.pone.0083414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Wang Y, Qu C. The near-miss effect in slot-machine gambling: modulation of feedback-related negativity by subjective value. Neuroreport. 2011;22:989–93. doi: 10.1097/WNR.0b013e32834da8ae. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–35. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J, Cucurell D, Cunillera T, et al. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–8. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Mas-Herrero E, Marco-Pallarés J. Frontal theta oscillatory activity is a common mechanism for the computation of unexpected outcomes and learning rate. Journal of Cognitive Neuroscience. 2014;26:447–58. doi: 10.1162/jocn_a_00516. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends in Neurosciences. 2007;30:150–8. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Qi S, Ding C, Song Y, Yang D. Neural correlates of near-misses effect in gambling. Neuroscience Letters. 2011;493:80–5. doi: 10.1016/j.neulet.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16:407–11. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Science and Human Behavior. New York: Macmillan; 1953. [Google Scholar]
- Spencer KM, Polich J. Poststimulus EEG spectral analysis and P300: attention, task, and probability. Psychophysiology. 1999;36:220–32. [PubMed] [Google Scholar]
- Strickland LH, Grote FW. Temporal presentation of winning symbols and slot-machine playing. Journal of Experimental Psychology. 1967;74:10–3. doi: 10.1037/h0024511. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge: MIT Press; 1998. [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–8. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Ulrich N, Hewig J. A miss is as good as a mile? Processing of near and full outcomes in a gambling paradigm. Psychophysiology. 2014;51:819–23. doi: 10.1111/psyp.12232. [DOI] [PubMed] [Google Scholar]
- van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal oscillatory dynamics predict feedback learning and action adjustment. Journal of Cognitive Neuroscience. 2011;23:4106–21. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- von Borries AKL, Verkes RJ, Bulten BH, Cools R, de Brujin ERA. Feedback-related negativity codes outcome valence, but not outcome expectancy, during reversal learning. Cognitive, Affective & Behavioral Neuroscience. 2013;13:737–46. doi: 10.3758/s13415-013-0150-1. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology. 2000;38:301–13. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences. 2003;7:553–9. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–22. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gla J, Schroeder K, Sommer T, Braus DF, Bu C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. The Journal of Neuroscience. 2006;26:9530–7. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15:535–44. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. The Journal of Neuroscience. 2004;24:6258–64. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22:590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Yu R, Zhou X. To do or not to do? Action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia. 2010;48:3606–13. doi: 10.1016/j.neuropsychologia.2010.08.010. [DOI] [PubMed] [Google Scholar]