Abstract
Engaging social working memory (SWM) during effortful social cognition has been associated with neural activation in two neurocognitive systems: the medial frontoparietal system and the lateral frontoparietal system. However, the respective roles played by these systems in SWM remain unknown. Results from this study demonstrate that only the medial frontoparietal system supports the social cognitive demands managed in SWM. In contrast, the lateral frontoparietal system supports the non-social cognitive demands that are needed for task performance, but that are independent of the social cognitive computations. Moreover, parametric increases in the medial frontoparietal system, but not the lateral frontoparietal system, in response to SWM load predicted performance on a challenging measure of perspective-taking. Thus, the medial frontoparietal system may uniquely support social cognitive processes in working memory and the working memory demands afforded by effortful social cognition, such as the need to track another person’s perspective in mind.
Keywords: social cognition, working memory, fMRI, default network
Introduction
The best mechanic in the factory may fail as a foreman for lack of social intelligence.
Edward L. Thorndike
The capacity to reason, generate solutions to problems and innovate sets humans apart from other primates. Yet, as the quote from Thorndike notes, cognitive intelligence alone is insufficient for human success. In addition to the ability to think intelligently about cognitive information—such as spatial reasoning and logic—humans must be able to think intelligently about people’s states of mind—their perspectives, traits and intentions—to thrive in a social world. For centuries, a dominant view has been that the capacity to understand minds is one of many examples of a general human ability to reason and think abstractly (Goleman, 2007). If this were the case, cognitive and social intelligence would be expected to rise and fall together. Yet, as Thorndike observed, having one kind of intelligence is no guarantee of the other.
A critical component of intelligent thought is working memory, the ability to maintain and manipulate information in mind, without the aid of external resources, such as pen and paper (Miller, 1956; Miyake and Shah, 1999; Andrade, 2001). We recently showed (Meyer et al., 2012) that social working memory (SWM), or the maintenance and manipulation of social cognitive information, recruits two distinct neurocognitive networks: the medial frontoparietal system, often termed the mentalizing system (Frith and Frith, 2006), associated with mental state reasoning (Kampe et al., 2003; Mitchell et al., 2005; Saxe and Wexler, 2005) and the lateral frontoparietal system associated with traditional cognitive working memory (CWM) tasks (D'Esposito et al., 1999; Rypma et al., 1999; Wager and Smith, 2003) and general intelligence (Duncan et al., 1995; Hampshire et al., 2011). Specifically, both networks showed a linear increase in activation as a function of SWM load (i.e. the number of friends considered and ranked along a trait dimension during SWM), which is a response pattern characteristic of working memory systems (Rypma et al., 1999).
One interpretation of these findings is that the mentalizing and lateral frontoparietal systems both support the social cognitive processes that engage as SWM load increases. An alternative interpretation, however, stems from the observation that the previously used SWM task (Figure 1A) requires social and non-SWM operations, both of which increase in difficulty as SWM load level increases. On the one hand, the more friends one must consider on a particular trait dimension, the more social cognitive effort must be expended to rank them on this dimension. This is clearly a social aspect of the task that increases with SWM load. On the other hand, one also must maintain the final reordered list of names (based on the ranking) to answer the probe (i.e. true/false) question following the delay period, and more cognitive effort must be expended with the more names on the list. This is clearly a cognitive aspect of the task that also increases with SWM load. Given that the SWM task simultaneously manipulates social and non-social mental operations, the alternative possibility is that the lateral frontoparietal system increases to support the cognitive operations necessary for task performance, rather than playing a role in the social cognitive processes engaged in SWM.
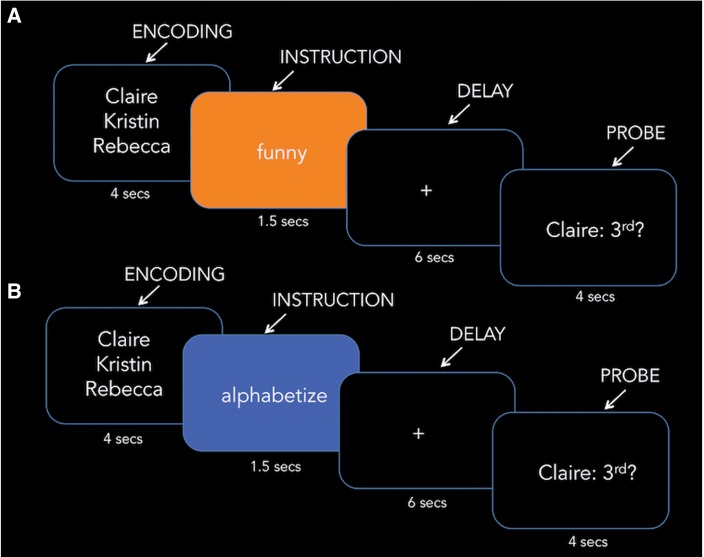
Fig. 1.
Pictorial display of the (A) SWM task and (B) CWM task. Each trial included encoding (4 s), instruction (1.5 s), delay (6 s) and the true/false probe question (4 s).
In this study, we disentangle the roles played by the mentalizing and lateral frontoparietal systems during SWM. While undergoing fMRI scanning, participants performed a SWM task and a CWM task in the scanner. Both tasks include trials in which participants mentally reorder two, three or four names, and maintain the reordered list of names until the probe question at the end of the trial. In the SWM task, the name reordering is based on trait rankings and is social. In the CWM task, the name reordering is based on alphabetical order and is cognitive. Critically, in both tasks, the effort involved in maintaining the new order of names increases with load level. Thus, by comparing the SWM and CWM tasks to one another, the maintenance of the new name order common to both tasks will be controlled for and the remaining differences will isolate the social components of SWM.
In addition to better isolating the social aspects of SWM, this study examined whether the neural regions that increase activation with SWM load predict social cognitive ability. Past working memory research has found that CWM ability is a good predictor of general intelligence and reasoning at the behavioral and neural levels of analysis (Conway et al., 2003; Gray et al., 2003; Metcalfe et al., 2013). Such observations raise the possibility that neural responses in the mentalizing system and/or lateral frontoparietal system during SWM explain variance in social cognitive ability. One social cognitive skill that SWM neural responses are likely to facilitate is perspective-taking ability. Perspective-taking refers to considering the point-of-view, or mental state, of another person. By virtue of the fact that other peoples’ mental states can be distinct from one’s own, perspective-taking skills may benefit from the ability to hold representations of other minds in SWM. However to date, whether neural responses during SWM relate to objective measures of perspective-taking skills [rather than subjective, self-report measures of perspective-taking (Meyer et al., 2012)] remains unknown. To examine this possibility, outside of the scanner, participants completed a computerized task (i.e. a variant of the referential communication task referred to here as the ‘Director Task’; Keysar et al., 2000; Keysar et al., 2003; Dumontheil et al., 2010) designed to measure perspective-taking ability and we examined which, if any, neural responses during SWM predict perspective-taking ability.
Methods
Participants
Twenty-five right-handed participants [15 female, mean age = 21.56, SD = 2.5; sample size based on power analysis for fMRI data (Mumford and Nichols, 2008)] from the University of California, Los Angeles (UCLA) community participated in this study. Participants were paid $100 and provided written informed consent according to the procedures of the UCLA Institutional Review Board.
Procedure
Participants completed a friend trait-rating questionnaire for 10 of their close friends 2 weeks prior to scanning. For each trait, participants rated how much each of their friends possesses the trait on a 1–100 scale (1 being the least and 100 being the most). These ratings were later used to create SWM trials (see Materials). During their scan, participants completed SWM trials in which they encoded two, three or four of their friends’ names, ranked the friends along a trait dimension during the delay period, and answered a probe (true/false) question about their rank order (Figure 1A). Additionally, participants completed CWM trials in which they alphabetized their friends’ names during the delay period (Figure 1B). To keep the format between SWM and CWM trial types as similar as possible, for the true/false probe question, participants read a ranked position and a friend’s name with a question mark (e.g. second: Claire?) and determined if the position was true or false based on their ranking. Thus, SWM and CWM trials only differed in terms of the instruction word shown prior to the delay period. Prior to their scan, participants completed practice SWM and CWM trials (distinct from those shown during the scan) to become familiar with the task. Participants were instructed to answer questions as quickly and accurately as possible and used a button-box in the scanner to record their answers to the probe questions for each trial.
In the scanner, participants completed a structural scan (MP-RAGE) and three functional scans. Each functional scan included 18 SWM trials (6 trials in which two friends were encoded, 6 trials in which three friends were encoded, and 6 trials in which four friends were encoded) and 18 CWM trials (6 trials in which two friends were encoded, 6 trials in which three friends were encoded and 6 trials in which four friends were encoded). Each working memory trial spanned 15.5 s (independent of jitter; see Figure 1). The 18 trials of each working memory type were presented in blocks of 9 trials, with the cue ‘traits’ or ‘alphabetize’ shown prior to each block to ensure that participants knew which trial type they were going to complete next (each task block was ∼3 min). The order of these blocks was counterbalanced across participants. Trials within each block were jittered in timing (within and between trial elements) and ordered according to Optimize Design (Wager and Nichols, 2003; jitter time was randomly chosen and centered around the mean of 1.5 s).
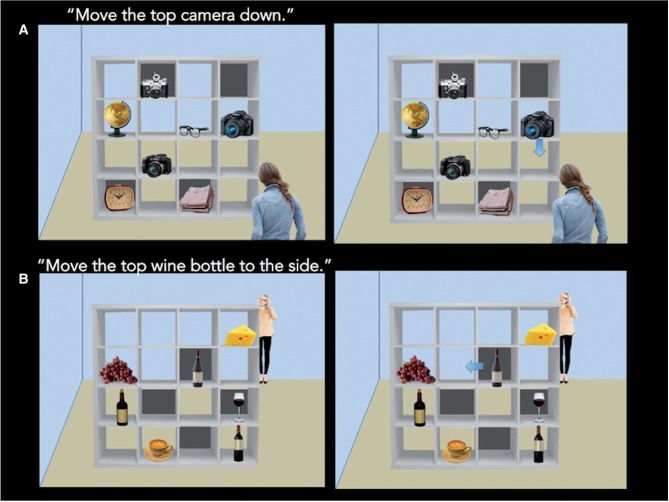
After their scan, participants completed the Director’s Task (Keysar et al., 2000; Dumontheil et al., 2010; Figure 2), a computerized, objective measure of perspective-taking ability, in a quiet testing room outside of the scanner. In this task, participants observe a bookshelf with various objects, and a woman near the bookshelf (the ‘Director’) asks the participant to move one of the objects that appears on the shelf. Some of the shelves on the bookshelf have a wall behind them and others do not. Importantly, for half of the trials (8 trials total), the Director has the same perspective as the participant and can see all of the objects on the bookshelf [first-person perspective-taking (1PP) condition]. For the other half of the trials (eight trials total), the Director is standing on the other side of the bookshelf and therefore cannot see the items on the shelf that are blocked by the shelf walls [third-person perspective-taking (3PP) condition].
Fig. 2.
Pictorial display of the Director’s Task. For each trial, the participant viewed the first slide and heard, via audio recording, the Director ask for one of the objects on the shelf to be moved (2.5 s). One the next screen, the participant saw an arrow indicating one of the objects to be moved and determined whether the arrow indicated the object that the director asked to be moved (up to 5 s).
For each trial, first the participant heard via an audio recording the Director ask for an object to be moved (2.5 s) and next an arrow appeared on the screen indicating one of the objects to be moved. When the arrow appeared on the screen, participants indicated by keyboard button press whether the arrow that appeared on the screen corresponded with the object that the director asked to be moved. Participants had up to 5 s to make their response after which the screen advanced to the next trial.
For both trial types, the Director asks for one of three objects of the same category to be moved up, down or to the side. For example, for a trial in which there are three cameras on the bookshelf as well as other various objects, the Director may ask to move the top camera down and then the arrow would indicate one of the shown cameras to be moved down (see Figure 2). For each trial, one of the three objects pertinent to the Director’s request is blocked by a shelf wall, ensuring the necessity of perspective-taking to determine a correct answer in the 3PP trials. Additionally, the arrow always pointed in the correct direction (up, down, side). Thus, whether the arrow was referring to the object that the Director wanted to be moved was the only factor influencing whether a trial was correct or incorrect.
To avoid mental set effects, all trials showed different objects on the bookshelf, the shelves that were blocked by walls varied from trial to trial, and the arrow indicated the correct object for 50% of the trials in each condition. For eight additional trials (catch trials), the director asked the participant to move one object not belonging to a set of objects (e.g. move the popcorn to the side). These trials were not included in the analyses because they less clearly isolate perspective-taking. That is, participants can correctly answer 3PP trials with strategies other than perspective-taking when they are not required to assess which of three objects the Director wants to be moved. Prior to the experimental trials, participants completed practice trials to familiarize themselves with the task.
Materials
For each SWM trial, participants encoded the names of 2, 3 or 4 friends selected from a list of their 10 close friends that they provided 2 weeks before the scan. To control for rating distance effects on task difficulty, we selected friends who were ranked no more than 25 points apart (on the 100-point scale) and no closer than 5 points apart from one another for each trait word. These distances served as a rule for friend name selection and were adhered to as closely as possible given the distribution of ratings given by the participants (Mean distance for friend names within a trial = 12.60; SD = 4.00). Each participant was shown his or her own friends’ names on each trial. For both the SWM task and the Director’s task, trials were standardized on brightness, contrast, font and size.
Functional magnetic resonance imaging (fMRI) acquisition
Functional images were acquired on a 3 Tesla (T) Siemans Trio with a T2*-weighted echo-planar plus sequence covering 36 axial slices [TR/TE = 2000/25 ms, flip angle = 90°, 64 × 64 matrix, 3 mm thick, field of view (FOV) = 200]. To aid in fMRI data registration we also acquired a Magnetization Prepared Rapid Gradient Echo scan (MP- RAGE; TR/TE = 2170/4.33 ms, flip angle = 7°, 256 × 256 matrix, 1 mm thick, 192 sagittal slices, FOV = 256).
Data analysis
Imaging data were analyzed in SPM8 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, UK). The following preprocessing steps were performed to prepare the fMRI data for statistical analysis. First, each EPI volume was realigned to the first EPI volume of each run. Second, the T1 structural volume was coregistered to the mean EPI. Third, to normalize the T1 structural volume to a common group-specific space (with subsequent affine registration to MNI space), we used the group-wise DARTEL registration method included in SPM8 (Ashburner, 2007). Fourth, we normalized the EPI volumes to MNI space using the deformation flow fields generated in the previous step, which simultaneously resampled volumes (3 mm isotropic) and applied spatial smoothing (Gaussian kernel of 8 mm, full width at half maximum).
At the first level of analysis, each participant’s preprocessed data was modeled as an event-related design in the general linear model framework. Thus, we modeled regressors for each trial component (encoding, delay, retrieval) separately for SWM and CWM trials and regressors of no interest capturing the portions of the task not related to working memory trials (i.e. the instruction cue preceding each block that notified participants whether the following block would comprise SWM or CWM trials), as well as six motion regressors for each of the motion parameters from image realignment. Encoding and delay periods were modeled as a boxcar spanning their duration. Retrieval was modeled as a boxcar from the true/false probe question onset to the participant’s response. Each event type (encoding, delay and retrieval) also had an associated parametric modulator (regressor) coding for the trial load (two names, three names or four names). We orthogonalized the social load and cognitive load parameters with respect to the main effect of each working memory type to examine the unique effect of social load vs cognitive load, over and above any effects of performing the tasks collapsing across load level.
At the second level of analysis, the linear contrasts computed for each participant as a measure of differential BOLD activation were entered into random effects analyses at the group level for statistical inference. All whole-brain analyses were conducted using a statistical criterion of at least 39 contiguous voxels exceeding a voxel-wise threshold of P < 0.005. This joint voxelwise and cluster-size threshold corresponds to a false-positive discovery rate of 5% across the whole brain as estimated by a Monte Carlo simulation (10 000 iterations) implemented using AlphaSim in AFNI (Cox, 1996). For visual presentation, thresholded t-statistic maps were surface rendered using the SPM Surfrend toolbox Version 1.0.2 (I. Kahn; http://spmsurfrend.sourceforge.net). We performed the following second level analyses on delay period activation to examine our questions regarding SWM. First, to examine the neural processes common to SWM and CWM, we performed whole-brain conjunction analysis, using a factorial repeated-measures ANOVA (within subject factor: parametric modulator coding working memory load; blocking factor: subject) to test the conjunction null (Nichols et al., 2005) of parametric modulation by SWM load level and CWM load level. Second, to identify neural activity specific to SWM, we performed whole-brain analysis contrasting parametric modulation by load for SWM vs CWM trials. Third, we performed linear regression analysis to examine which neural mechanisms predict working memory task accuracy and perspective-taking ability. For each participant, average parametric modulation of activation by load level, during SWM and CWM separately, was computed from the clusters (i) observed in the whole-brain contrast comparing SWM load vs CWM load and (ii) the conjunction analysis showing activity common to both SWM load and CWM load. These values were then used as a predictor variable in a linear regression model (using SPSS Version 22 software) predicting working memory task accuracy and perspective-taking ability (separately). Perspective-taking ability was defined as accuracy on 3PP, controlling for accuracy on 1PP trials (i.e. the unstandardized residuals resulting from the regression of first-person perspective accuracy on third-person perspective accuracy).
Results
Behavioral results
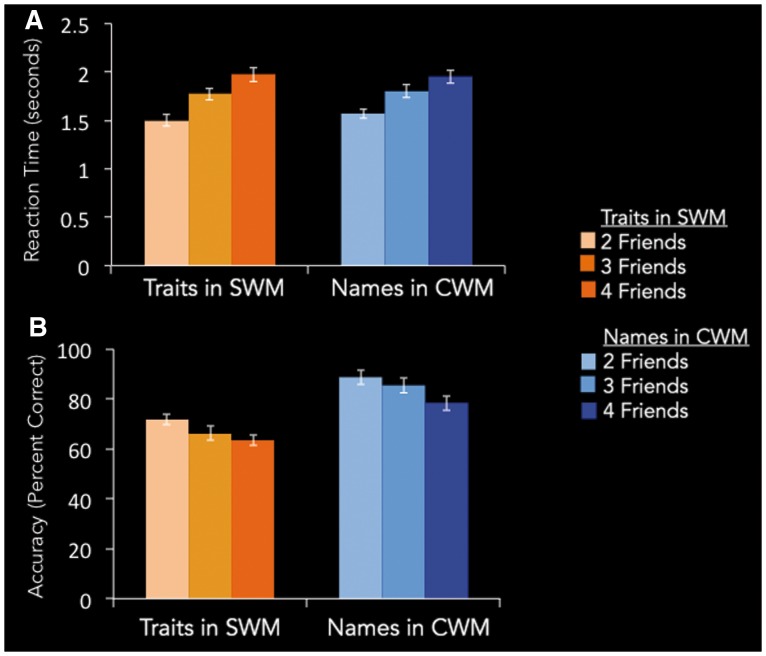
Before examining neural effects across the two forms of working memory, it is important to verify that parametric changes in neural activity as a function of WM load do not reflect differences in task difficulty, rather than differences in working memory mechanisms. Thus, we performed a 2 (SWM vs CWM) × 3 (load level: 2 friends, 3 friends, 4 friends) repeated-measures ANOVA on participants’ mean reaction times (RTs). Results showed no significant interaction of each load level across the SWM and CWM trials [F(2, 24) = 1.25, P = 0.30, Figure 3A], suggesting that observed parametric changes in neural activation as a function of load did not reflect differences in task difficulty across the two kinds of WM trials. There was also no significant main effect for WM type collapsed across load level [F(2, 24) = 0.67, P = 0.42]. There was a main effect of load, however [F(2, 24) = 103.62, P < 0.0001, η2 = 0.27]. Looking at SWM and CWM RTs separately, each working memory task showed significant increases in RT as a function of load, suggesting the more friends considered during each working memory task, the greater demands to SWM or CWM, respectively [SWM F(2, 24) = 62.80, P < 0.0001, η2 = 0.29; CWM F(2, 24) = 41.21, P < 0.0001, η2 = 0.19].
Fig. 3.
SWM and CWM task performance. (A) Reaction Time, in seconds. (B) Accuracy, in percent correct.
We also examined if there were differences in task accuracy across the two WM tasks. For the SWM task, a trial was considered accurate if the participant’s answer to a trial was consistent with his or her original trait ranking from the online questionnaire. Again, there was no significant interaction of load level for SWM vs. CWM trials [F(2, 24) = 0.54, P = 0.58; Figure 3B] Thus, although there was a main effect of WM type on task accuracy collapsing across load [F(2, 24) = 56.5, P < 0.001, η2 = 0.40], SWM and CWM tasks did not statistically differ in their pattern of increasing difficulty as a function of load. As with RT, there was also a main effect of load on accuracy across all trials [F(2, 24) = 13.4, P < 0.0001, η2 = 0.20], as well as separately for each WM type [SWM F(2,24) = 3.39, P < 0.05, η2 = 0.08; CWM F(2,24) = 10.36, P < 0.0001, η2 = 0.09], again suggesting performance decrements for both WM tasks as a function of load.
In summary, performance changes due to working memory load were similar for the two working memory tasks. While on average, the accuracy data suggests the SWM task is harder than the CWM task, our focus is on neural changes as a function of load level, and thus the interaction analyses—in terms of accuracy and RT—suggest the two tasks do not vary from each other on this dimension.
Neural results
Whole-brain analyses
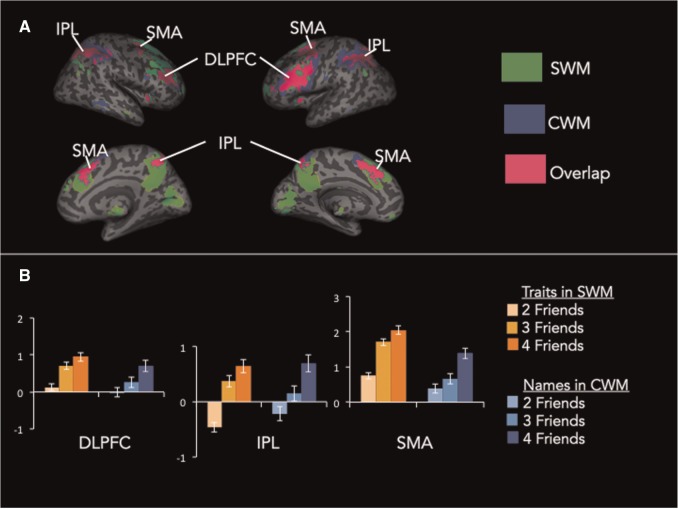
First, we identified regions of the brain that increase with working memory load, regardless of whether the content dealt with in working memory is social or cognitive. At the first level of analysis, we modeled linear changes in neural activity as a function of working memory load (i.e. parametric modulation analysis) separately for SWM and CWM tasks. At the second level of analysis, we then performed a conjunction analysis of SWM and CWM parametric modulation analyses. Results from the conjunction analysis showed that regions of the lateral frontoparietal system (bilateral dorsolateral prefrontal cortex (DLPFC), bilateral inferior parietal lobule (IPL), middle frontal gyrus (MFG) and supplementary motor area (SMA), Figure 4, Table 1), previously associated with working memory, parametrically increase activity with load, regardless of whether the working memory processes engaged were social or cognitive.
Fig. 4.
Whole-brain results from the conjunction of SWM and CWM load effects. (A) Green indicates regions associated with SWM load effects, blue indicates regions associated with CWM load effects and pink indicates regions associated with load effects for both SWM and CWM. (B) Parameter estimats of each load level plotted separately for the SWM and CWM tasks.
Table 1.
Clusters showing significant activation in the conjunction of working memory load across SWM and CWM tasks
| Region | x | Y | z | t | k |
|---|---|---|---|---|---|
| DLPFC | 45 | 36 | 27 | 6.64 | 283 |
| Inferior parietal lobe | −33 | −54 | 45 | 5.9 | 1298 |
| Angular gyrus | 33 | −66 | 48 | 5.33 | - |
| Inferior parietal lobule | 45 | −33 | 45 | 5.19 | - |
| DLPFC | −45 | 30 | 24 | 5.73 | 1310 |
| DLPFC | −51 | 9 | 39 | 5.62 | - |
| Supplementary motor area | −3 | 15 | 48 | 5.62 | - |
| Supplementary motor area | 30 | 3 | 54 | 5.57 | 224 |
| Insula | −30 | 21 | −3 | 5.09 | 70 |
| Cerebellum | 12 | −75 | −24 | 4.69 | 168 |
| Cerebellum | −6 | −78 | −27 | 4.00 | |
| Cerebellum | 33 | −60 | −33 | 4.53 | 92 |
| Cerebellum | −3 | −66 | −30 | 3.81 | 72 |
| Insula | 33 | 21 | 0 | 3.81 | 44 |
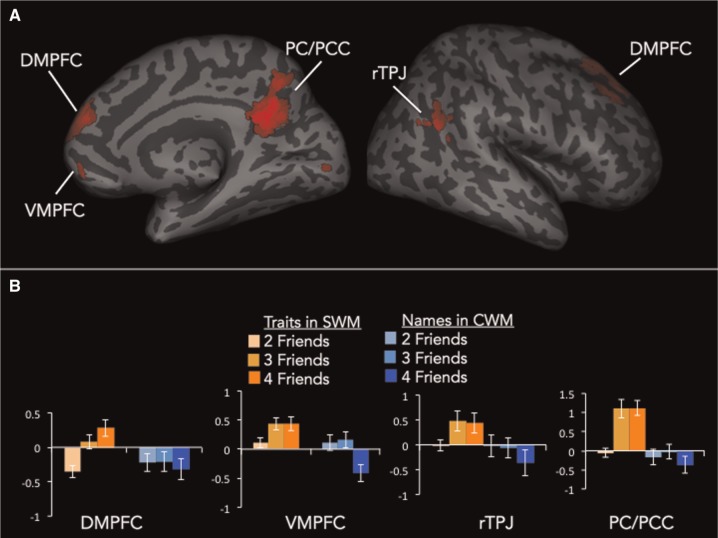
To isolate the neurocognitive mechanisms that specifically support SWM, we directly compared parametric increases as a function of load (i.e. parametric modulation analyses) between SWM and CWM trials. This analysis identified regions that were more strongly increasing with SWM load level than CWM load level. Results revealed robust activation of the mentalizing system (dorsomedial prefrontal cortex (DMPFC), precuneus/posterior cingulate cortex (PC/PCC), right tempoparietal junction (rTPJ) as well as ventromedial prefrontal cortex (VMPFC); Figure 5A, Table 2). The only other region of the brain that showed activation in this analysis was a cluster of lingual gyrus in the visual cortex. In Figure 5B, we illustrate this effect in mentalizing regions by plotting parameter estimates from the four mentalizing clusters observed in this analysis for each working memory load level separately for SWM and CWM trials. This illustrates the results shown in the brain images, that the mentalizing regions increase with SWM load, but decrease with CWM load.
Fig. 5.
(A) Whole-brain results from the comparison of SWM load effects vs CWM load effects. (B) Parameter estimates from the whole-brain results broken down by trial type (SWM vs CWM) and trial difficulty (2/3/4 friends).
Table 2.
Brain regions showing differential parametric increases as a function of SWM load vs CWM load
| Region | x | y | z | t | k |
|---|---|---|---|---|---|
| DMPFC | 15 | 39 | 54 | 4.91 | 173 |
| 6 | 54 | 24 | 4.26 | 96 | |
| 12 | 66 | 15 | 3.17 | - | |
| −9 | 54 | 39 | 3.7 | 53 | |
| VMPFC | −9 | 51 | −18 | 4.19 | 72 |
| 9 | 57 | −9 | 3.31 | - | |
| −12 | 42 | −6 | 3.22 | - | |
| PC/PCC | −3 | −60 | 27 | 5.37 | 507 |
| −3 | −54 | 21 | 5.35 | - | |
| 3 | −54 | 36 | 4.58 | - | |
| rTPJ | 42 | −54 | 24 | 3.73 | 50 |
| 54 | −66 | 27 | 3.37 | - | |
| Lingual gyrus | −6 | −90 | 0 | 3.73 | 105 |
The reverse contrast comparing increases with CWM load vs increases with SWM load revealed a cluster of left DLPFC [x = −57, y = 6, z = 15], as well as insula [x = −45, y = 3, z = 6], middle temporal gyrus [x = −48 y = −57 z = 3] and putamen [x = 33, y = −12, z = 0].
Predicting SWM task accuracy from SWM neural responses
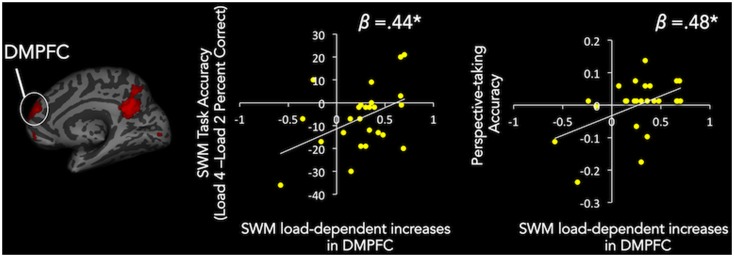
To further understand the roles played by the mentalizing and lateral frontoparietal networks in SWM, we also examined whether neural responses in the observed clusters relate to individual differences in SWM task accuracy. To examine this possibility, we extracted parameter estimates from the SWM parametric modulation by load contrast from the mentalizing clusters observed in the comparison of SWM load vs CWM load (DMPFC, PCC, rTPJ and VMPFC). These values were then each entered as a predictor variable in separate linear regressions with SWM load 4 vs SWM load 2 task accuracy entered as the dependent variable. Using the SWM load 4 vs SWM load 2 task accuracy as our performance variable allowed us to examine accuracy specific to SWM load, beyond simply mentalizing processes more generally. Load dependent increases during SWM in DMPFC (β = 0.44, P = 0.03; Figure 6) and marginally VMPFC (β = 0.38, P = 0.07) predicted SWM accuracy. In contrast, load-dependent increases during SWM in the lateral frontoparietal clusters identified by the conjunction analysis (which revealed regions that commonly increase with SWM and CWM load level) showed no relationship with SWM task accuracy (P’s > 0.83), further suggesting lateral frontoparietal regions are not critical to SWM processes. Performing the same regression analyses with CWM parametric modulation parameter estimates showed that DMPFC (β = −0.50, P = 0.01), and marginally lateral frontoparietal regions [DLPFC (β = −.35, P = .09) and IPL (β = −0.39, P = 0.05)], negatively predicted CWM task accuracy.
Fig. 6.
Scatter plots of linear regression results. Activation Increases with SWM load in DMPFC predict SWM task accuracy and perspective-taking accuracy. X axes are DMPFC cluster parameter estimates and Y axes are SWM task accuracy and perspective-taking accuracy.
Predicting perspective-taking accuracy from SWM neural responses
Past working memory research has found that individual differences in lateral frontoparietal system activation during working memory corresponds with individual differences in other, related cognitive capacities, such as math and reading ability (Dumontheil and Klingberg, 2011; Ashkenazi et al., 2013; Metcalfe et al., 2013), as well as IQ (Gray et al., 2003). Building off of this prior work, we thus explored whether linear increases in the mentalizing system as a function of SWM load predicted individual differences in social cognitive ability, namely perspective-taking. To examine this possibility, we used the same parameter estimates that were used to predict SWM task performance [i.e. parameter estimates from the SWM parametric modulation by load contrast in the mentalizing clusters identified by the SWM load vs CWM load comparison (DMPFC, PCC, rTPJ and VMPFC)]. These values were then each entered as a predictor variable in separate linear regressions with 3PP accuracy, controlling for 1PP accuracy, as the dependent variable. Results showed that increases in activation with SWM load in DMPFC (β = 0.48, P = 0.02; Figure 6), VMPFC (β = 0.50, P = 0.01, and marginally PCC (β = 0.38, P = 0.06) predict individual differences in perspective-taking.
Again, as in our exploration of predictors of SWM task performance, we also examined whether parameter estimates from regions common to both kinds of working memory would predict perspective-taking ability. Specifically, we pulled parameter estimates from lateral frontoparietal regions observed in the conjunction analysis. Increases with SWM load level in lateral frontoparietal regions did not predict perspective-taking performance (P’s > 0.63). Parametric activation in lDPLFC during CWM showed a negative relationship with perspective-taking that was marginally significant (β = −0.39, P = 0.06); however, IPL showed no relationship with perspective-taking (β = −0.14, P = 0.49). Thus, perspective-taking ability is specifically associated with the ramping up of mentalizing regions during SWM, rather than being associated with lateral frontoparietal regions during SWM, or either the mentalizing or lateral frontoparietal system during CWM.
Finally, we examined whether the relationship between SWM neural responses and perspective-taking may simply be a byproduct of a more general association between social information processing in mentalizing regions and perspective-taking, rather than specifically tied to ramping up these regions’ activity in response to SWM load. Pulling parameter estimates from the same four mentalizing regions from a contrast of all SWM trials, collapsing across load level, vs baseline revealed no significant relationships with perspective-taking performance (DMPFC β = 0.13, P = 0.53; PCC β = 0.04, P = 0.83; rTPJ β = 0.05, P = 0.82 VMPFC β = 0.02, P = 0.94). Thus, the extent to which mentalizing regions increase their activity under SWM load, but not CWM load, is predictive of perspective-taking performance.
Discussion
Past research has found that SWM load is associated with the mentalizing and lateral frontoparietal systems (Meyer et al., 2012). However, the respective roles these systems play in SWM remained unknown because it was unclear which of two competing possibilities reflect the processes supported by the two systems. The first possibility is that both of the systems are needed to carry out challenging social cognitive processing—the more social cognitive processing engaged by working memory, the more mentalizing and lateral frontoparietal system resources may be necessary. The second possibility is that the mentalizing system supports the social cognitive processing that increases with SWM load, whereas the lateral frontoparietal system supports the non-social cognitive processing that facilitates task performance, but is independent of the effortful social cognitive processes engaged during SWM.
In the present study, we found support for the second possibility. Only the mentalizing system increased activation with SWM load, when controlling for increases to the non-SWM demands manipulated by the task (the number of names needed to be remembered in a new order). In contrast, the lateral frontoparietal system increased activation with the number of names that needed to be remembered in a new order, regardless of whether the order was set based on alphabetical (CWM) or trait (SWM) reordering. Moreover, increases in response to SWM load in mentalizing regions (particularly DMPFC), but not lateral frontoparietal regions, predicted SWM task accuracy and perspective-taking ability. Thus, the working memory demands afforded by challenging social cognitive processing appears to rely specifically on the mentalizing system’s ability to ramp up effectively in the face of SWM load.
These results revise the understanding of mentalizing system function. Traditionally, many areas of research consider the medial frontoparietal regions associated with mentalizing as regions that support non-effortful forms of cognitive processing (McKiernan et al., 2003; Greicius and Menon, 2004). Indeed, past working memory research using CWM paradigms finds that medial frontoparietal region activity during CWM actually interferes with CWM performance (Anticevic et al., 2010). However, none of this prior work engaged working memory with mentalizing-related content. In contrast, we observed that increases in medial frontoparietal system activity, particularly DMPFC, positively predict SWM task performance. Our results suggest that these regions may have controlled-processing properties specifically for social cognitive information processing.
That said, it is notable that a large literature finds that these medial frontoparietal regions also highly engage during periods of idle rest (Gusnard et al., 2001; Raichle and Snyder, 2007; Spreng et al., 2009). In fact, research in cognitive neuroscience often refers to the functional network that comprises mentalizing regions as the ‘default network,’ because the regions appear to engage ‘by default’ when participants are resting. Interestingly, all of the mentalizing regions active during three-friend and four-friend SWM trials showed activation higher than the fixation baseline (which can be considered brief periods of rest). This observation is important because prior work using easier social cognitive tasks have found increased activation in mentalizing regions relative to a control condition, but nonetheless decreased activation in mentalizing regions relative to a fixation baseline (Mitchell et al., 2002; Kelley et al., 2002). Such findings made it difficult to rule out the possibility that medial frontoparietal regions are more sensitive to ‘easy’ cognitive processing than social cognitive processing per se. Finding that medial frontoparietal regions are more active than fixation for more challenging SWM trials is inconsistent with this alternative interpretation. Thus, these regions may be more sensitive to the degree of social cognitive processing, rather than ‘easy’ cognitive processes engaged during rest.
Considering the SWM findings and the default network literatures side-by-side raises the question of why the same regions that support externally generated (e.g. stimuli-induced) social cognitive challenges also engage automatically when individuals are not required to attend to externally-generated, non-social stimuli. One possibility is that, given the importance of understanding the social world around us, engaging medial frontoparietal regions during rest may facilitate the ability to effortfully engage these regions during and/or consolidate information learned after externally induced social cognitive challenges. Future research linking default network activation and/or functional connectivity during rest to neural responses during SWM may shed light on these possibilities and clarify the psychological function of medial frontoparietal regions’ activity during rest.
In addition to clarifying our understanding of mentalizing system function, these results also speak to psychological theories of effortful social cognition. That is, it has been assumed that in processes ranging from attribution to self-regulation, poor social cognitive performance caused by cognitive load reflects the taxing of a single pool of cognitive resources. For example, if participants are required to simultaneously engage CWM when encoding a person’s behavior, they are more likely to commit the fundamental attribution error, automatically attributing behaviors to enduring dispositions rather than situational causes (Gilbert et al., 1988). If effortful social and cognitive processes relied on a shared pool of resources, then SWM trials should have only increased lateral frontoparietal regions generically associated with working memory. Instead, SWM load was associated with activation increases in the mentalizing system, whereas CWM was associated with activation decreases in these same regions. Critically, in the parametric modulation analysis used in the present study, a regressor in the model controlled for the fact that all of the SWM trials involved social processing and thus the observed effects are specifically linked to increasing SWM load, rather than social processing in general. Thus, poor social cognitive performance caused by cognitive load may partially reflect the shared recruitment of the lateral frontoparietal system, which is consistent with past theories of shared resources. However, it is also possible that some effects of cognitive load on social cognition may be due to decreases in mentalizing regions required to handle effortful social cognition. Moving forward, social psychologists interested in whether their construct of interest exhausts cognitive resources may gain more traction by asking whether their construct exhausts cognitive resources, social cognitive resources or both.
Finally, these results have potential implications for understanding social cognitive deficits in clinical disorders. For certain clinical disorders, such as schizophrenia, social cognitive deficits are evident in tandem with non-social cognitive deficits (Goldman-Rakic, 1994; Pickup and Frith, 2001; Couture et al., 2006). In other disorders, such as autism spectrum disorder (ASD), social cognitive deficits are evident (Baron-Cohen et al., 1985; Dawson and Fernald, 1987) even though non-social cognitive ability can be spared (Bennetto et al., 1996; Ozonoff and Strayer, 2001). Importantly, most prior social cognitive tasks assessing brain function in these populations are very easy, in terms of mental effort. For example, the false-belief task used to assess ‘theory of mind’ is passed by most typically developing 5-year olds (Wimmer and Perner, 1983; Sommer et al., 2007). Incorporating SWM paradigms may help clarify mentalizing system function, as well as its relationship with the lateral frontoparietal system, in these populations.
Conclusion
In conclusion, these findings suggest that contrary to prior assumptions, working memory for social cognitive information is not simply an instance of generic working memory mechanisms applied to social information. SWM engaged the mentalizing system previously thought to thwart working memory processes. Moreover, engaging the mentalizing system, but not lateral frontoparietal system associated with generic forms of working memory, predicted SWM and perspective-taking ability. Together, these findings suggest that the working memory demands afforded by challenging social cognition rely on distinct neural mechanisms.
Acknowledgements
We thank Elizabeth Pierce, Joselyn Ho, and the UCLA Ahmanson-Lovelace Brainmapping Center for their assistance with data collection.
Funding
This research was supported by a National Institute of Mental Health Pre-doctoral Ruth L. Kirschstein National Research Service Award awarded to M.L.M.
Conflict of interest. None declared.
References
- Andrade J. (2001). Working Memory in Context. Sussex: Hove. [Google Scholar]
- Anticevic A., Repovs G., Shulman G.L., Barch D.M. (2010). When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage, 49(3), 2638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Black J.M., Abrams D.A., Hoeft F., Menon V. (2013). Neurobiological underpinnings of math and reading learning disabilities. Journal of Learning Disabilities, 46(6), 549–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. (1985). Does the autistic child have a “theory of mind"? Cognition, 21(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Bennetto L., Pennington B., Rogers S. (1996). Intact and impaired memory functions in autism. Child Development, 67, 1816-35. [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. (2006). The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin, 32, S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway A.R., Kane M.J., Engle R.W. (2003) Working memory capacity and its relation to. general intelligence. Trends in Cognitive Science, 7, 547–52. [DOI] [PubMed] [Google Scholar]
- Cox R. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research, 29, 162–73. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Postle B.R., Ballard D., Lease J. (1999). Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain and Cognition, 41(1), 66–86. [DOI] [PubMed] [Google Scholar]
- Dawson G., Fernald M. (1987). Perspective-taking ability and its relationship to the social behavior of autistic children. Journal of Autism and Developmental Disorders, 17(4), 487–98. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Klingberg T. (2011). Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereberal Cortex, 22(5), 1078–85. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Küster O., Apperly I.A., Blakemore S.J. (2010). Taking perspective into account in a communicative task. Neuroimage, 52(4), 1574–83. [DOI] [PubMed] [Google Scholar]
- Duncan J., Burgess P., Emslie H. (1995). Fluid intelligence after frontal lobe lesions. Neuropsychologia, 33(3), 261–8. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–4. [DOI] [PubMed] [Google Scholar]
- Gilbert D.T., Pelham B.W., Krull D.S. (1988). On cognitive busyness when person perceivers meet persons perceived. Journal of Personality and Social Psychology, 54(5), 733–40. [Google Scholar]
- Goldman-Rakic P.S. (1994). Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences, 6(4), 348–57. [DOI] [PubMed] [Google Scholar]
- Goleman D. (2007). Social Intelligence. New York, NY: Random House Inc. [Google Scholar]
- Gray J.R., Chabris C.F., Braver T.S. (2003). Neural mechanisms of general fluid intelligence. Naure Neuroscience, 6(3), 316–22. [DOI] [PubMed] [Google Scholar]
- Greicius M., Menon V. (2004). Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience, 16(9), 1484–92. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Thompson R., Duncan J., Owen A.M. (2011). Lateral prefrontal cortex subregions make dissociable contributions during fluid reasoning. Cereberal Cortex, 21(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe K.K., Frith C.D., Frith U. (2003). “Hey John": signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. Journal of Neurosciences, 23(12), 5258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14(5), 785–94. [DOI] [PubMed] [Google Scholar]
- Keysar B., Barr D.J., Balin J.A., Brauner J.S. (2000). Taking perspective in conversation: the role of mutual knowledge in comprehension. Psychological Sciences, 11(1), 32–8. [DOI] [PubMed] [Google Scholar]
- Keysar B., Lin S., Barr D.J. (2003). Limits on theory of mind use in adults. Cognition, 89, 25–41. [DOI] [PubMed] [Google Scholar]
- McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. (2003). A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience, 15(3), 394–408. [DOI] [PubMed] [Google Scholar]
- Metcalfe A.W., Ashkenazi S., Rosenberg-Lee M., Menon V. (2013). Fractionating the neural correlates of individual working memory components underlying arithmetic problem solving skills in children. Developmental Cognitive Neuroscience, 6, 162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Spunt R.P., Berkman E.T., Taylor S.E., Lieberman M.D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 109(6), 1883–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A. (1956). The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review, 63(2), 81–97. [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. (2005). General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage, 28(4), 757–62. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Heatherton T.F., Macrae C.N. (2002). Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences of the United States of America, 99(23), 15238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Shah P. (1999). Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. New York: Cambridge University Press. [Google Scholar]
- Mumford J.A., Nichols T.E. (2008). Power calculation for group fMRI studies for arbitrary design and temporal autocorrelation. NeuroImage, 39(1), 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.B. (2005). Valid conjunction inference with the minimum statistic. Neuroimage, 25(3), 653–60. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Strayer D.L. (2001). Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders, 31(3), 257–63. [DOI] [PubMed] [Google Scholar]
- Pickup G.J., Frith C.D. (2001). Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychological Medicine, 31(2), 207–20. [DOI] [PubMed] [Google Scholar]
- Raichle M., Snyder A. (2007). A default mode of brain function: a brief history of an evolving idea. Neuroimage, 37(4), 1083–90; discussion 1097–89. [DOI] [PubMed] [Google Scholar]
- Rypma B., Prabhakaran V., Desmond J.E., Glover G.H., Gabrieli J.D. (1999). Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage, 9(2), 216–26. [DOI] [PubMed] [Google Scholar]
- Saxe R., Wexler A. (2005). Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia, 43(10), 1391–99. [DOI] [PubMed] [Google Scholar]
- Sommer M., Dohnel K., Sodian B., Meinhardt J., Thoermer C., Hajak G. (2007). Neural correlates of true and false belief reasoning. NeuroImage, 35, 1378–84. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Wager T. D., Nichols T.E. (2003). Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage, 18(2), 293-309. [DOI] [PubMed] [Google Scholar]
- Wager T. D., Smith E.E. (2003). Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(4), 255–74. [DOI] [PubMed] [Google Scholar]
- Wimmer H., Perner J. (1983). Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition, 13, 103–28. [DOI] [PubMed] [Google Scholar]