Abstract
Background
Nuclear factor E2-related factor 2 (Nrf2) plays an anti-oxidative and phase II detoxification function via its up-regulation on various antioxidant response elements (ARE) genes. Nrf2 can protect both normal and cancer cells from damages of cell stress, thereby exerting a critical role in the development of cancer. The expression and significance of Nrf2 in gastric cancer, however, has not been reported. This study thus aimed to investigate the expression of Nrf2 in gastric cancer tissues via immunohistochemical (IHC) staining.
Material/Methods
Gastric carcinoma tissues from a total of 175 patients during surgical resection were examined for Nfr2 expression profiles using IHC staining on paraffin-embedded slides. Between-group-comparisons were performed by chi-square, Fisher’s exact, or Mann-Whitney U test. The correlation between Nfr2 expression and clinical indexes was further analyzed by Kaplan-Meier test, univariate/multivariate analysis, and log-rank test.
Results
Nrf2 is mainly expressed in nuclei of gastric carcinoma tissues, with significant correlation with clinical indexes, including tumor size, invasive depth, lymph node metastasis, and invasion. Patients with Nrf2-positive expression had significantly lower survival rates compared to those in the negative group (p<0.01), with chemo-resistance against 5-fluorouracil (5-FU) (p<0.05).
Conclusions
Nrf2 expression is positively correlated with invasive gastric cancer, suggesting its utility as a predictive index for unfavorable prognosis.
MeSH Keywords: Gastric Acidity Determination; NF-E2-Related Factor 2; Receptors, Tumor Necrosis Factor, Type I
Background
Gastric carcinoma is the fourth most common gastrointestinal cancer and has the second highest mortality rate among all gastrointestinal cancers [1]. Three critical measures improving patient prognosis are early diagnosis, radical surgery, and endoscopic surgery [1,2]. Although radical resection has been widely promoted, the post-surgery recovery is still unfavorable due to TNM stages [3,4]. The prognosis of gastric cancer is also affected by patient biological factors and clinical pathological conditions, necessitating the identification of novel bio-markers for diagnosis and treatment [5]. A widely accepted opinion is that oxidative stress (OS) participates in pathogenesis of gastric cancer because it can damage DNA and cause mucous injuries [6,7]. Nuclear factor E2-related factor 2 (Nrf2), as a basic redox-sensitive bZIP transcription factor, plays a role in intracellular anti-oxidation and phase II detoxification through the up-regulation of various antioxidant response elements (ARE) genes [8,9] via the Keap1-Cul3-dependent E3 ubiquitination pathway [10,11]. Under oxidative stress or chemical stimulus, Nrf2 translocates into the nucleus, where it forms heterodimers with Maf and binds onto the ARE sequence on the chromosome to activate the transcription of downstream genes, including antioxidants and phase II detoxification enzymes [12–14]. The importance of Nrf2 resides in its protective roles against various human disease and pathological conditions, including cancer, neurodegenerative disease, cardiovascular dysfunction, inflammation, and pulmonary fibrosis, along with ameliorating aging [15–19], making Nrf2 a “beneficial” transcriptional factor protecting the body from oxidative stress [20]. In tumor tissues, Nrf2 has also been reported to have abnormal activation [21]. Therefore, Nrf2 has a dual role in both preventing oncogenesis and protecting tumor cells from stress injury, making it a candidate factor for tumor growth and invasion. Currently there is no available study regarding either expression or clinical significance of Nrf2 in gastric carcinoma. This study therefore aimed to address this issue and further elucidate the clinical implication of anti-oxidative stress-related Nrf2 expression.
Material and Methods
Western blotting for Nrf2 expression in gastric carcinoma cell lines
Gastric cancer cells MKN74, MNK45, KATOIII, and NUGC4 (Cell Institute of Chinese Academy of Science, China) were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 u/mL penicillin, and 100 μg/mL streptomycin. Cells collected by centrifugation were rinsed in phosphate-buffered saline (PBS) and extracted for total proteins by lysis buffer. Cell nucleus/cytoplasmic fractions were separated using a test kit (BioVision, USA) according to the manufacturer’s instructions. Denatured proteins were separated on 10% SDS-PAGE and transferred to PVDF membrane, which was blocked in 5% defatted milk powders overnight. Mouse monoclonal anti-Nrf2 antibody (1:500, Santa Cruz, USA) was used to incubate the membrane overnight. After rinsing in Tris-buffered saline-Tween 20 (TBST), anti-mouse IgG conjugated with horseradish peroxidase (RD systems, US) was used for a further 15-min incubation. The signal was finally developed using ECL-Plus reagents and was visualized on X-ray films. The nucleus and cytoplasm fractions were labelled by anti-lamin B1 (1:1 000, Abcam, USA) or anti-α tubulin antibody (1:1000, Calbiochem, USA).
Patients and samples
A total of 175 gastric adenocarcinoma patients were included in this study. All patients underwent surgical resection of stomach (57 cases of distal, 14 cases of proximal, 99 cases of total, and 5 cases of partial stomach resection) with lymph node clearance between Jan 2004 and Dec 2013 in our hospital. There were 116 males and 59 females in the patient group, ages 31–84 years (average, 66 years). Among all patients, 47 were at stage I when diagnosed, 33 were at stage II, and 95 were at stage III. Histopathological typing showed 71 cases of differentiated (papillary, high-differentiated, and moderate differentiated tubular adenocarcinoma) tumors and 104 undifferentiated tumors (including low differentiated adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma). No patients underwent chemotherapy before the surgery. This study was pre-approved by the ethics committee of our hospital and we obtained written consent from all patients and their families.
Nrf2 IHC staining
Tumor tissues were first fixed in 10% formalin-PBS buffer, embedded in paraffin, sectioned (4-μm thickness), and was mounted on glass slides. After de-waxing in xylene and re-hydration in gradient ethanol, endogenous peroxidase activity was quenched by 3% hydrogen peroxide in methanol. After gentle washing in PBS, antigen retrieval was performed using heated citrate buffers for 10 min. Non-specific binding sites were further blocked in 10% FBS. Anti-Nrf2 antibody (1:200, Santa Cruz, USA) was then used for overnight incubation. Further detection was accomplished by streptavidin-biotin peroxidase (SP) kit (Nichirei, Japan) and was visualized by diaminobenzidine incubation. Counter-staining was further performed using hematoxylin. A parallel positive control for Nfr2 was performed on normal placenta tissues.
The expression level of Nrf2 was evaluated by number of positive tumor cells. Two independent blinded observers evaluated staining images. In brief, 10 representative regions within the tumor samples were selected. In each region, the expression was observed in 100 cells under a high-magnification microscope (×400). The undifferentiated region was preferred to differentiate tissues on the same slide. Averaged Nrf2 index was calculated based on the number of positive cells in each region. The expressional profile was described from both the positive rate and staining intensity, the latter classified as 0, 1+, and 2+. The staining score was calculated as intensity X positive rates (in percentage).
Relationship between Nrf2 expression and chemo resistance
We compared the Nrf2 expression level in patients who underwent 5-fluorouracil (5-FU) treatment. As only stage II/III patients were qualified for chemotherapy and patients with severe adverse effects were excluded, only 72 patients were recruited for the analysis of Nrf2 expression and tumor chemoresistance.
Statistical analysis
All data were collected and analyzed in SPSS 16.0 software. Between-group comparisons were performed by chi-square, Fisher’s exact, or Mann-Whitney U test. The correlation between Nrf2 expression and clinical indexes was analyzed by Kaplan-Meier curve, univariate/multivariate analysis, and log-rank test. Statistical significance was defined at p<0.05.
Results
Expression of Nrf2 in gastric carcinoma cell lines
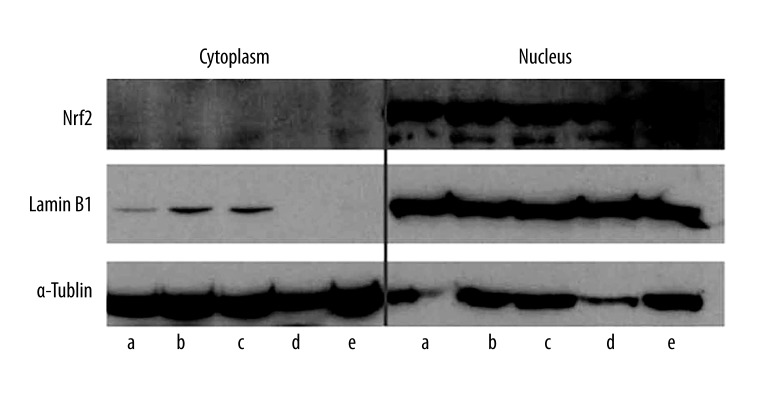
As shown by Western blotting in both nucleus and cytosolic fractions, Nrf2 protein is mainly expressed in the nucleus, with minimal expression in the cytoplasm (Figure 1).
Figure 1.
Western blotting of Nrf2 expression. Representative bands from cytoplasmic (left) or nucleus (right) fractions were aligned with their markers, lamin or tubulin. Note that Nrf2 is prominently expressed in the nucleus of all cell lines. a, MKN45; b, MKN74; c, KATOIII; d, NUGC4; e: MKN7.
The correlation between Nrf2 expression and clinical indexes
Table 1 lists general information and pathological indexes of all 175 patients. IHC staining obtained results consistent with those in the abovementioned Western blotting, as the Nrf2 signal localizes in the nucleus (Figure 2). Further examination revealed higher Nrf2 immune reactivity in undifferentiated cells. We calculated the staining scored as described in the methods section, and classified those with scores higher than 100 into the Nrf2-positive group. In all 175 patients, 108 were classified into the positive group (61.7%) and the other 67 were classified into the negative group (38.3%). Further correlation analysis between Nrf2 expression and clinical indexes are tabulated in Table 2. Significant correlations were discovered between Nrf2-positive expression and patient sex, tumor volume, infiltrative depth, lymph node metastasis, lymph tube invasion, and TNM staging.
Table 1.
General information of patients.
| Parameters | N |
|---|---|
| Sex | |
| Male | 116 |
| Female | 59 |
| Age | |
| Average | 66 (years old) |
| Range | 31–84 (years old) |
| Surgical types | |
| Complete resection | 99 |
| Distal resection | 56 |
| Proximal resection | 14 |
| Partial resection | 6 |
| TNM stage | |
| IA/IB | 47 |
| IIA/IIB | 33 |
| IIIA/IIIB/IIIC | 95 |
| Tissue differentiation | |
| Differentiated | 71 |
| Undifferentiated | 104 |
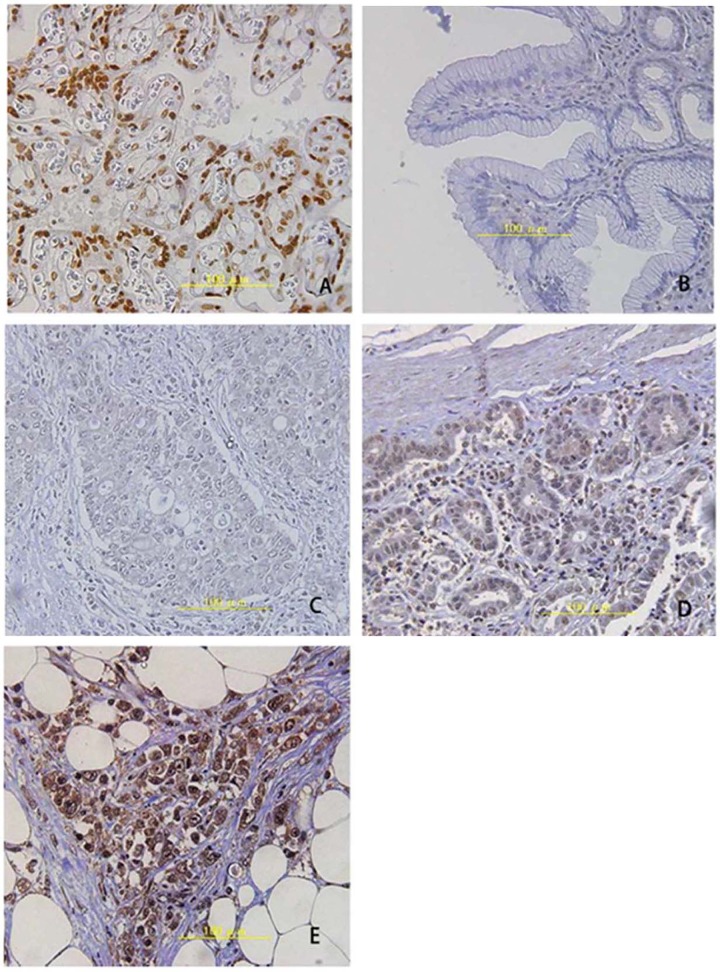
Figure 2.
IHC staining of Nrf2 in stomach tissues. (A) Positive control staining in placenta; (B) Normal stomach tissues; (C) Negative staining for Nfr2; (D) Weak positive Nrf2; (E) Strong positive signals. Magnification: ×400.
Table 2.
Correlation between Nrf2 expression and clinical indexes.
| Clinical parameters | Nrf2 expression | P value | |
|---|---|---|---|
| Negative (N=67) | Positive (N=108) | ||
| Age group | NA | ||
| <65 years old | 22 | 47 | |
| ≥65 years old | 45 | 61 | |
| Sex | <0.05 | ||
| Male | 51 | 65 | |
| Female | 16 | 43 | |
| Tumor size | <0.01 | ||
| <5 cm | 36 | 27 | |
| ≥5 cm | 31 | 81 | |
| Tumor invasive depth | <0.01 | ||
| T1a, T1b, T2 | 31 | 19 | |
| T3, T4a, T4b | 36 | 89 | |
| Lymph node metastasis | <0.05 | ||
| Yes | 35 | 74 | |
| No | 32 | 34 | |
| TNM staging | <0.01 | ||
| IA/B | 28 | 19 | |
| IIA/B | 12 | 21 | |
| IIIA/B/C | 27 | 68 | |
| Lymph tube infiltration | 49 | 90 | <0.05 |
| Yes | 18 | 14 | |
| No | 0 | 0 | |
| Tissue differentiation | <0.01 | ||
| Differentiated | 37 | 34 | |
| Undifferentiated | 30 | 74 | |
Correlation between Nrf2 expression and 5-FU resistance
Among all 72 patients who underwent 5-FU therapy, there were 59 who were Nrf2-positive and 13 who were Nrf2-negative. Among those 59 Nrf2-positive patients, a total of 43 patients (72.9%) developed 5-FU resistance, which occurred in only 5 of the 13 Nrf2-negative patients (38.5%).
2 The relationship between Survival rate and Nrf2
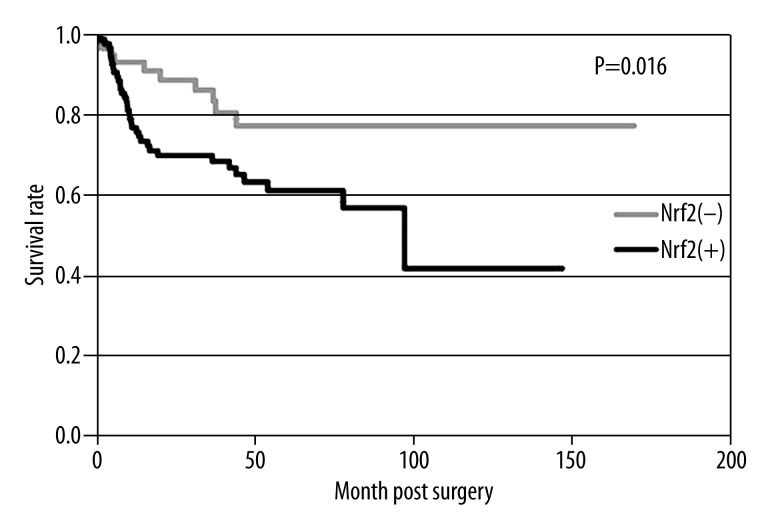
Using Kaplan-Meier method, we found that the overall survival rate in Nrf2-positive patients was significantly lower than that in Nrf2-negative patients (61% vs. 79%, p<0.01, Figure 3). Significant correlation factors of patient survival rate include invasive depth, tumor size, lymph node metastasis, TNM stage, and lymph tube infiltration, by univariate analysis. A further multivariate analysis indicated lymph node metastasis as an independent prognostic index (Table 3).
Figure 3.
Postoperative survival rates of Nrf2-positive and Nrf2-negative patients.
Table 3.
Univariate and multivariate analysis of prognostic factors in gastric carcinoma.
| Clinical factors | P value under univariate analysis | P value under multivariate analysis | Risk ratio | 95% confidence interval (CI) |
|---|---|---|---|---|
| Age | 0.42 | – | – | – |
| Sex | 0.50 | – | – | – |
| Invasive depth | <0.01 | 0.25 | 2.44 | 0.52–11.44 |
| Tumor size | <0.01 | 0.35 | 1.58 | 0.61–4.09 |
| Lymph node metastasis | <0.01 | 0.03 | 5.02 | 1.18–21.41 |
| Lymph tube invasion | <0.05 | 0.27 | 0.35 | 0.05–2.26 |
| TNM staining | <0.05 | 0.17 | 1.62 | 0.81–3.24 |
| Nrf2 expression | <0.05 | 0.40 | 1.37 | 0.66–2.87 |
Discussion
As an antioxidant, Nrf2 can protect normal cells from oxidative stress injuries, thereby forming a self-protective mechanism. The over-expression of Nrf2 and its downstream genes in various tumors, however, raised the potency of Nrf2 in facilitating survival and proliferation of cancer cells [20–26]. Our study for the first time reports that a close relationship exists between Nrf2 expression in gastric carcinoma cell nucleus and patient clinical features.
The prominent expression of Nrf2 in nucleus of gastric cancer cells from both in vivo and in vitro samples suggests a persistent expression of Nrf2 may cause production of antioxidants, which further endow cancer cells with elevated anti-reactive oxygen species (ROS) activity. This proposed mechanism has been reported by Ma et al., who found higher nuclear Nrf2 levels and downstream anti-oxidative proteins in more advanced cervical cancer tissues [23]. Therefore, it is likely that gastric cancer cell nuclear Nrf2 level is related with tumor malignancy.
IHC staining results showed elevated Nrf2 expression in gastric cancer tissues. Among all patients, 61.7% were Nrf2-positive, higher than those in non-small cell lung cancer (NSCLC) (26%) or gall bladder cancer (23%). The survival rate-related factors include tumor invasive depth, tumor size, lymph node metastasis, TNM stage, and lymph tube infiltration, in agreement with a gall bladder cancer study [24]. Recent study in gastric carcinoma found consistent effects of Nrf2 expression on the prognosis, but only with immune reactivity in cytoplasm rather than the nucleus, probably due to the use of different antibodies [27]. Various studies have confirmed the importance of cytoplasmic-nuclear translocation of Nrf2 for exerting its anti-oxidative activity [28]. Western blotting results in our study found prominent expression of Nrf2 in nuclear fraction. Persistent over-expression of Nrf2 may protect cancer cells from ROS injuries because it can work as an anti-oxidant. Stronger Nrf2 expression was also found in tumors with higher invasiveness. Therefore, it is necessary to quantify nuclear but not cytoplasmic Nrf2 proteins in gastric cancer tissues.
We also evaluated the prognostic utility of Nrf2 expression by univariate analysis. Although Nrf2-positive is not included in multivariate analysis as an independent prognostic evaluating factor, our results showed that lymph node metastasis can significantly affect Nrf2-positive rates as a marker for unfavorable prognosis. Previous studies in NSCLC and gall bladder cancer all supported existence of relationships between Nrf2 positive expression and unfavorable prognosis [22,24], suggesting the utility of Nrf2 as a prognostic index for evaluating the postoperative survival rate of patients.
Conclusions
Various studies have shown that Nrf2 can decrease the survival rate via its facilitation on tumor cell resistance against radio-/chemo-therapy [29–34]. This study found a higher rate of 5-FU resistance in Nrf2-positive tumors; therefore, the evaluation of Nrf2 in gastric cancer patients may help to optimize the chemotherapy plan. The genetic silencing or functional inhibition can suppress activity of Nrf2-modulated oxidase, including glutathione, thioredoxin, and mercaptan sulfur, leading to the recovery of tumor cell sensitivity to chemo-/radio-therapy. There have been clinical trials supporting this mechanism, such as the sensitization of cancer cells against alkylation drugs by Nrf2 inhibition [32] or the inhibition of tumor growth by the combination of Nrf2 gene knockdown and cisplatin application [23]. Therefore, the next generation of chemotherapy plans involving modulating Nrf2-related anti-oxidative functions may provide more satisfactory treatment efficacy and improve patient survival and prognosis. This study demonstrated a positive relationship between Nrf2 expression and invasiveness of gastric carcinoma, suggesting the utility of Nrf2 as a prognostic index.
Footnotes
Source of support: Research supported by the Guangxi scientific research and technology development projects (NO.1355005-3-6)
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 3.Warneke VS, Behrens HM, Hartmann JT, et al. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol. 2011;29:2364–71. doi: 10.1200/JCO.2010.34.4358. [DOI] [PubMed] [Google Scholar]
- 4.Lagarde SM, ten Kate FJ, Reitsma JB, et al. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2006;24:4347–55. doi: 10.1200/JCO.2005.04.9445. [DOI] [PubMed] [Google Scholar]
- 5.Gao G, Sun Z, Wenyong L, et al. A preliminary study of side population cells in human gastric cancer cell line HGC-27. Ann Transplant. 2015;20:147–53. doi: 10.12659/AOT.892197. [DOI] [PubMed] [Google Scholar]
- 6.Ni J, Mei M, Sun L. Oxidative DNA damage and repair in chronic atrophic gastritis and gastric cancer. Hepatogastroenterology. 2012;59:671–75. doi: 10.5754/hge12177. [DOI] [PubMed] [Google Scholar]
- 7.Engin AB, Karahalil B, Engin A, Karakaya AE. DNA repair enzyme polymorphisms and oxidative stress in a Turkish population with gastric carcinoma. Mol Biol Rep. 2011;38:5379–86. doi: 10.1007/s11033-011-0690-9. [DOI] [PubMed] [Google Scholar]
- 8.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–65. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata T, Saito S, Kokubu A, et al. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DD, Lo SC, Cross JV, et al. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–29. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 13.Katoh Y, Itoh K, Yoshida E, et al. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–68. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 14.Banning A, Deubel S, Kluth D, et al. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–23. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta T, Iijima K, Miyamoto M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–9. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 16.Kim HR, Kim S, Kim EJ, et al. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47–56. doi: 10.1016/j.lungcan.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Bogner PN, Ramnath N, et al. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:3875–82. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- 18.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 19.Stacy DR, Ely K, Massion PP, et al. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–18. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, Kokubu A, Gotoh M, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–68. 1368.e1351–54. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 21.Lau A, Villeneuve NF, Sun Z, et al. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–70. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solis LM, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–53. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Zhang J, Liu S, et al. Nrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancer. Cancer Chemother Pharmacol. 2012;69:485–94. doi: 10.1007/s00280-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Zhang M, Zhang L, et al. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res. 2010;164:e99–105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Kim YR, Oh JE, Kim MS, et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–51. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 26.Grogan TM, Fenoglio-Prieser C, Zeheb R, et al. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–81. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- 27.Hu XF, Yao J, Gao SG, et al. Nrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancer. Asian Pac J Cancer Prev. 2013;14:5231–35. doi: 10.7314/apjcp.2013.14.9.5231. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Singh A, Yegnasubramanian S, et al. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9:336–46. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homma S, Ishii Y, Morishima Y, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 31.Wang X-J, Sun Z, Villeneuve NF, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–43. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho J-M, Manandhar S, Lee H-R, et al. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Jungsuwadee P, Vore M, et al. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–56. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]