Abstract
Burkholderia pseudomallei is the causative agent of melioidosis, a disease with high mortality, which is prevalent in tropical regions of the world. A recent study shows that B. pseudomallei can survive inside mammalian cells because of its ability to actively evade cell autophagy. However, the underlying mechanisms remain unclear. In the present study, based on microarray screening, we found that ATG10 was downregulated following B. pseudomallei infection in A549 human lung epithelial cells. Forced expression of ATG10 accelerated the elimination of intracellular B. pseudomallei by enhancing the process of autophagy. Moreover, MIR4458, MIR4667-5p, and MIR4668-5p were found, by microarray screening, to be upregulated in response to B. pseudomallei infection. These 3 novel miRNAs, MIR4458, MIR4667-5p, and MIR4668-5p, targeted to the 3′-untranslated region of ATG10 in different time-course and spatial manners. Upregulation of these miRNAs reduced the level of ATG10 and inhibited autophagy, leading to increasing survival rate of intracellular B. pseudomallei. Furthermore, the increase of these miRNAs was correlated with the reduced promoter methylation status in A549 cells in response to B. pseudomallei infection. Our results reveal that 3 novel miRNAs regulate autophagy-mediated elimination of B. pseudomallei by targeting ATG10, and provide potential targets for clinical treatment.
Keywords: ATG10, autophagy, Burkholderia pseudomallei, DNA methylation, MIR4458, MIR4667-5p, MIR4668-5p
Abbreviations
- 3-MA
3-methyladenine
- 3′UTR
3′-untranslated region
- 5-Aza-CdR
5-aza-2′-deoxycytidine
- ACTB
actin, β
- ATG4C
autophagy-related 4C, cysteine peptidase
- ATG5
autophagy-related 5
- ATG10
autophagy-related 10
- ATG12
autophagy-related 12
- B. pseudomallei
Burkholderia pseudomallei
- CFU
colony forming unit
- CQ
chloroquine
- DNMT1
DNA (cytosine-5-)-methyltransferase 1
- DRAM2
DNA-damage regulated autophagy modulator 2
- MAP1LC3B
microtubule-associated protein 1 light chain 3 β
- miRNAs
microRNAs
- MOI
multiplicity of infection
- MSP
methylation-specific PCR
- PBS
phosphate-buffered saline
- p.i.
postinfection
- Rapa
rapamycin
- siRNA
small interfering RNA
- SQSTM1
sequestosome 1
- TEM
transmission electron microscopy.
Introduction
Burkholderia pseudomallei, a gram-negative pathogen, is the causative agent of melioidosis, which is classically characterized by pneumonia and multiple abscesses with a mortality rate of up to 40%.1 Melioidosis is endemic in Southeast Asia, northern Australia, and other tropical regions, and is the most common cause of pneumonia-derived sepsis in Thailand.1-3 Because melioidosis carries a high fatality rate, B. pseudomallei is classified as a category B potential bioterrorism agent by the Center for Disease Control and NIAID. It has now been well accepted that a key component of the pathogenesis of B. pseudomallei is its ability to invade and survive intracellularly in both phagocytic and nonphagocytic cells, which explains numerous features of melioidosis including latency, recrudescence, and antibiotic resistance.4-5
Autophagy, as one of the earliest defense responses encountered by intracellular pathogens, is a process that engulfs and delivers intracellular bacteria for lysosomal degradation.6-7 However, the battle between the human host and the infecting pathogens is continuously evolving. Preliminary evidence has indicated that several pathogens, such as Shigella, Salmonella, Mycobacteria, and influenza A virus, have evolved mechanisms that evade the autophagic response.8-11 B. pseudomallei has the capacity to invade a number of cultured mammalian cell lines.4 After entry, B. pseudomallei utilizes numerous strategies that enable it to survive in such a specialized niche as the intracellular environment.5 Recently, Cullinane et al. have reported that B. pseudomallei can evade autophagy through an active mechanism, although autophagy is induced in response to B. pseudomallei infection as early as 2-h infection period.12 Thus, the detailed mechanism by which B. pseudomallei-mediates subversion of autophagy should be further clarified.
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression by sequence-specific binding to mRNA (mRNA).13 miRNAs post-transcriptionally regulate gene expression by specifically targeting mRNAs for cleavage or inhibition of protein synthesis, regulating a wide spectrum of cellular processes such as cancers, immune response, and autophagy. Growing evidence has indicated that dysregulated miRNAs expression is associated with the evasion of autophagy, with several intracellular bacteria.14-17 Thus, understanding the molecular mechanisms of autophagy modulation in host response to B. pseudomallei infection is crucial to current and future therapeutic approaches.
In this report, the relationships between miRNAs, autophagy and B. pseudomallei are investigated in lung epithelial cell lines. We provide evidence showing that MIR4458, MIR4667-5p and MIR4668-5p suppress autophagy-mediated removal of B. pseudomallei in epithelial cells by targeting ATG10.
Results
Intracellular survival and replication of B. pseudomallei in human lung epithelium cells
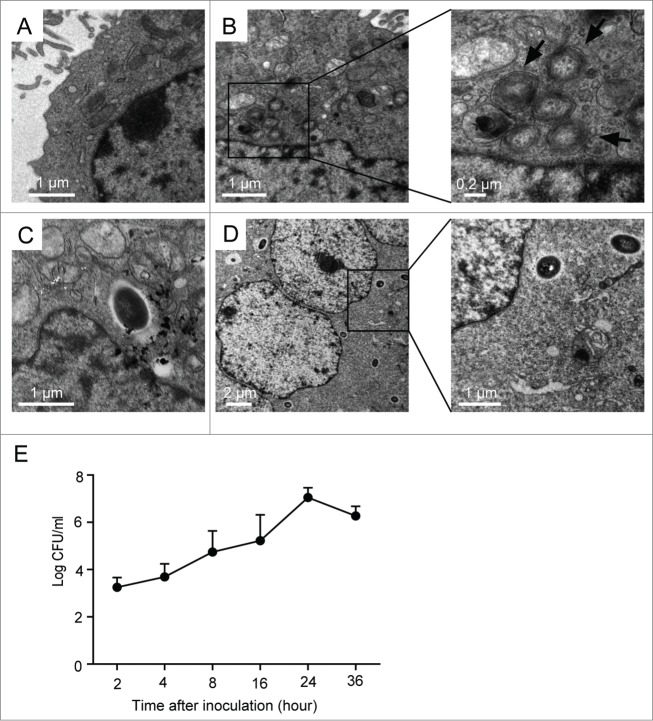
Prior studies have indicated that B. pseudomallei can invade both cultured phagocytic and nonphagocytic cells.4 To investigate the replication of B. pseudomallei in A549 cells, we observed the cells using transmission electron microscopy (TEM) (Fig. 1A–D). As shown in Figure 1B, some double- or multi-membrane compartments (autophagosomes) were observed in the infected cell sections at 2 h postinfection (p.i.), and these autophagosomes did not contain bacteria. Over the infection time, the invading B. pseudomallei were mostly free in the cytosol, and autophagic vacuoles were difficult to be viewed (Fig. 1C and D). Furthermore, the formation of multinucleated giantcells,18 which contains 3 or more nuclei per giant cell, can be induced after B. pseudomallei infection for 24 h (Fig. 1D, left panel).
Figure 1.
Intracellular survival and replication of B. pseudomallei in A549 cells. (A) Representative TEM image of control A549 cells. (B–D) A549 cells were infected with B. pseudomallei at an MOI of 10 for 2, 12, and 24 h, respectively. The scale bars are indicated. (B) Representative TEM images of intracellular autophagosomes within A549 cells at 2 h p.i. Arrows indicate autophagosomes. (C) TEM image of intracellular B. pseudomallei within A549 cells at 12h. (D) TEM images of intracellular B. pseudomallei within A549 cells at 24 h p.i. The left panel shows multinucleated giant cells. Boxed areas are shown as magnified images on the right panel. (E) Multiplication of B. pseudomallei in A549 cells. A549 cells infected with B. pseudomallei (MOI = 10:1) for 2, 4, 8, 16, 24, and 36 h were lysed, and intracellular bacteria were quantified after inoculation. The recovered viable B. pseudomallei were determined as CFU on a LB plate. Results shown are representative of 3 independent experiments.
To estimate the number of live internalized B. pseudomallei in A549 cells, bacterial colony forming units (CFU) were performed in a time-course experiment. The number of CFU assay of B. pseudomallei was gradually increased after infection, indicating that internalized B. pseudomallei underwent intracellular replication; and CFU then decreased after 36 h compared with 24 h of infection (Fig. 1E).
Autophagy is inhibited in response to B. pseudomallei infection
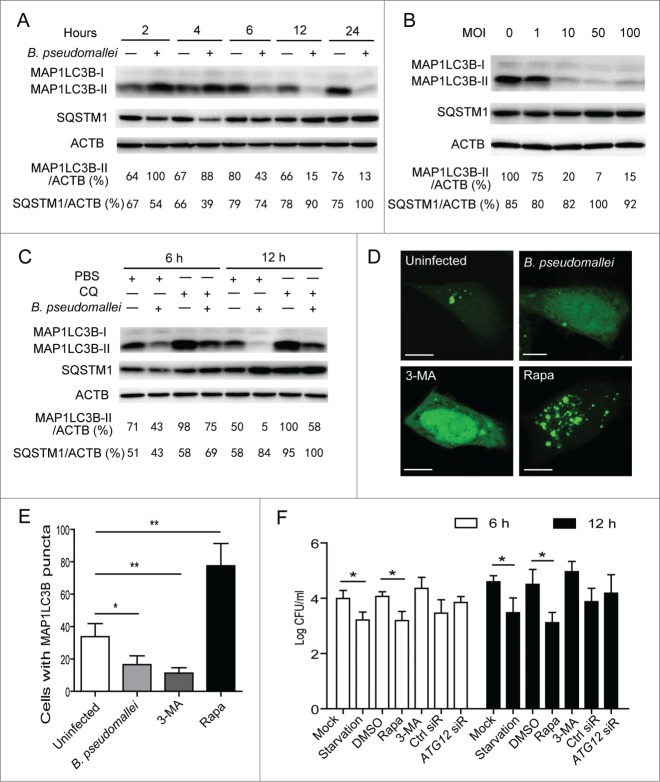
To determine whether B. pseudomallei modulates autophagy, we measured MAP1LC3B levels following B. pseudomallei infection, which is most widely used to monitor autophagy.19 Consistent with the previous study,12 there was an increase at 2 h and 4 h p.i. in the ratio of MAP1LC3B-II to ACTB and a degradation of SQSTM1 with B. pseudomallei infection as compared to uninfected controls in A549 cells (Fig. 2A). However, we observed a subsequent decline in the MAP1LC3B-II protein at over 6 h time points in A549 and BEAS-2B cells, and a delayed accumulation in SQSTM1 protein levels (Fig. 2A; Fig. S1A). Consistent with the observed MAP1LC3B-II ratios and SQSTM1 aggregates in time-course experiments, similar results were observed when dose-dependency was tested (Fig. 2B). Furthermore, the blockade in MAP1LC3B-II generation was more apparent when treated with chloroquine (CQ) to block autophagic flux (Fig. 2C). As further confirmation that MAP1LC3B-II levels were reduced in A549 cells infected with B. pseudomallei, we detected that punctate MAP1LC3B-positive autophagosomes were absent in most cells infected with B. pseudomallei compared with uninfected cells (Fig. 2D and E; Fig. S2).
Figure 2.
Autophagy is inhibited in response to B. pseudomallei infection. (A and B) B. pseudomallei decreased the conversion of MAP1LC3B-I to MAP1LC3B-II in A549 cells. A549 cells were treated with B. pseudomallei (MOI = 10:1) for 2, 4, 6, 12 and 24 h, or at MOI = 0, 1, 10, 50, and 100 for 6 h. (C) A549 cells were treated with B. pseudomallei (MOI = 10:1) for 6 h and 12 h in the presence of CQ (10 μM). (D and E) Confocal images show GFP-MAP1LC3B distribution in A549 cells infected with B. pseudomallei for 6 h, and uninfected cells treated with 3-MA (10 mM) or Rapa (200 nM). Scale bars: 5 μm. The number of GFP-MAP1LC3B puncta in each cell was counted. (F) Stimulation of autophagy suppresses intracellular survival of B. pseudomallei in A549 cells. After pretreatment by DMSO, Rapa and 3-MA, or transfected with ATG12 siRNA (100 nM), A549 cells were infected with B. pseudomallei (MOI = 10:1) for 6 h and 12 h. The bar represents the mean ±SD of 3 experiments. *, P<0.05, **, P<0.01.
To evaluate the effect of autophagy on intracellular multiplication, we infected A549 cells with B. pseudomallei and estimated the CFU. Stimulation of autophagy resulted in an enhanced ability of the cell to control infection, as evidenced by reduced numbers of viable B. pseudomallei in rapamycin (Rapa) or starvation-treated cells (Fig. 2F). In contrast, no significant increases were observed in the presence of 3-MA and ATG12 siRNA compared to the controls (Fig. 2F; Fig. S3). Taken together, we reason that B. pseudomallei may have evolved mechanisms to escape intracellular clearance by subverting autophagy. The exact molecular mechanism underlying this phenomenon is yet to be fully elucidated.
Downregulation of ATG10 expression during B. pseudomallei infection
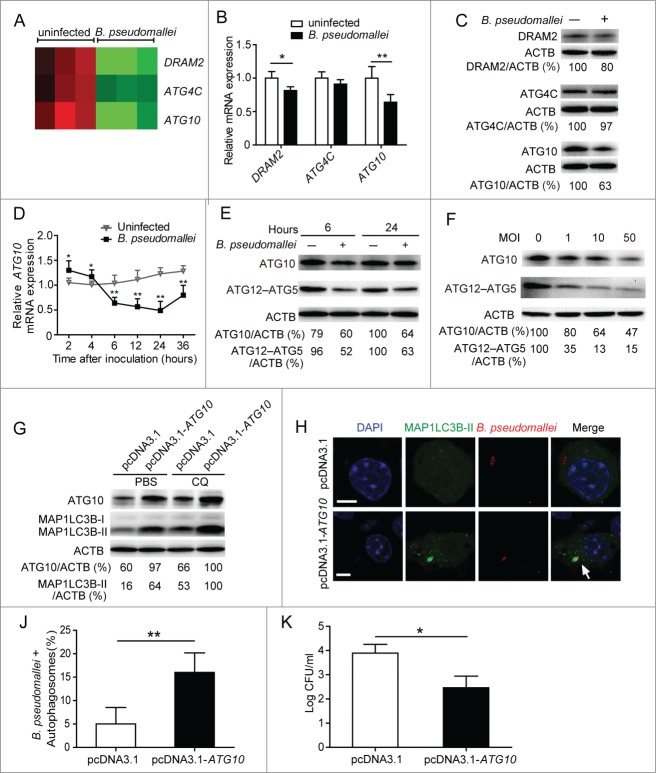
To elucidate the underlying mechanisms that B. pseudomallei uses to inhibit the autophagy pathway to avoid clearance by the host cells, we used Human Affymetrix GeneChips to determine the gene expression profile of A549 cells at 6 h p.i. Analysis of the data revealed that ATG4C, DRAM2, and ATG10 were expressed at decreased levels in response to B. pseudomallei infection compared to controls (Fig. 3A). To confirm the validity of microarray, the expression levels of the selected 3 genes and another 2 additional ATGs, ATG5, and ATG12, were identified with qRT-PCR and western blot analysis (Fig. 3B and C; Fig. S1B and S4A). The results showed that ATG10 was the most significantly downregulated (a 0.6-fold change) among the genes. Obviously, the dynamic expression of ATG10 was increased at 2 h and 4 h p.i., but then decreased at over 6 h p.i. (Fig. 3D). And the protein levels of ATG10 and ATG12–ATG5 conjugate were also decreased at 6 h and 24 h p.i., suggesting that the ATG12–ATG5 change might be caused by the reduced conjugation (Fig. 3E). Consistent with the observations in the time-course experiments, similar results were also obtained when dose-dependency was tested at 6 h p.i. (Fig. 3F). Next, we generated an ATG10-expressing plasmid without a 3′UTR, which markedly increased protein levels of ATG10 and MAP1LC3B-II in the presence of phosphate-buffered saline (PBS) or CQ (Fig. 3G; Fig. S5). As shown in Figure 3H, J and K, enforced expression of ATG10 enhanced the colocalization of intracellular B. pseudomallei with autophagosomes, and significantly decreased the bacterial load of intracellular B. pseudomallei compared to the control vector. Taken together, the above data indicated that B. pseudomallei inhibits autophagy and autophagy-mediated intracellular bacterial elimination by suppressing ATG10 expression.
Figure 3.
For figure legend, see page 1298.
MIR4458, MIR4667-5p, and MIR4668-5p are upregulated after B. pseudomallei infection
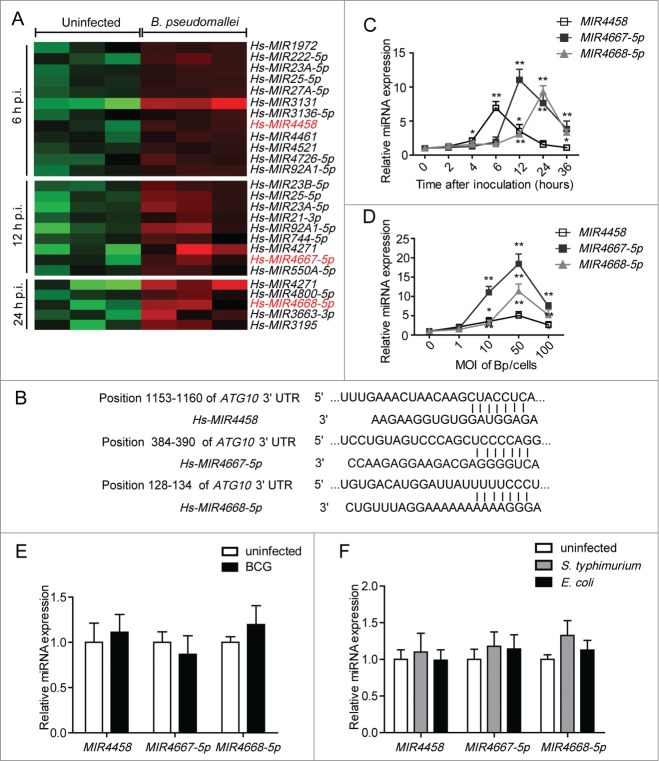
To elucidate the underlying mechanisms for downregulated ATG10 expression, we performed miRNA microarrays in A549 cells at 6 h, 12 h, and 24 h p.i. Hierarchical clustering analysis revealed groups of differentially upregulated miRNAs, respectively (Fig. 4A). To identify upstream regulators of ATG10, we used TargetScan and miRDB to predict its targets, and we focused on MIR4458, MIR4667-5p, and MIR4668-5p (Fig. 4B). In agreement with microarray findings, the results of qRT-PCR showed that MIR4458 was rapidly upregulated in the early infection phase (at 6 h p.i.), then marginally decreased in the later phase; and MIR4667-5p and MIR4668-5p were significantly upregulated at 12 h and 24 h p.i., respectively (Fig. 4C; Fig. S1C). Consistent with the time-course experiments, similar results were also observed when dose-dependency was tested at 12 h p.i. (Fig. 4D). In addition, cell death was measured considering long infection periods and high multiplicity of infection (MOI) (Fig. S6). The results demonstrated that B. pseudomallei could not induce apoptosis of the infected cells even though the incubation time was extended from 12 h to 36 h (Fig. S6A). However, when infected with B. pseudomallei at an MOI of 50 or 100, A549 cells underwent cell death, especially for the MOI of 100 (Fig. S6B).
Figure 4.
MIR4458, MIR4667-5p, and MIR4668-5p are upregulated after B. pseudomallei infection. (A) Total RNA from A549 cells infected with B. pseudomallei for 6, 12, and 24 h to perform microarray assay. Hierarchical clustering analysis was performed to show upregulated miRNAs by B. pseudomallei infection. (B) The region of the human ATG10 mRNA 3′UTR predicted to be targeted by MIR4458, MIR4667-5p, and MIR4668-5p, respectively (TargetScan 6.2). (C and D) Confirmation of microarray results by qRT-PCR. qRT-PCR analysis of MIR4458, MIR4667-5p, and MIR4668-5p expression levels in A549 cells infected with B. pseudomallei (MOI = 10) for 0, 2, 4, 6, 12, 24, and 36 h, or at MOI = 0, 1, 10, 50, and 100 for 12 h. (E) Expression of MIR4458, MIR4667-5p, and MIR4668-5p in A549 cells when infected with BCG (MOI = 10) for 12 h. (F) Expression of MIR4458, MIR4667-5p and MIR4668-5p in HeLa cells infected with S. typhimurium and E. coli (MOI = 10). *P < 0.05, **P < 0.01. Experiments performed in triplicate showed consistent results.
To determine whether the upregulation of MIR4458, MIR4667-5p, and MIR4668-5p was specific to B. pseudomallei, we used Mycobacterium tuberculosisvar. bovis BCG (BCG) and Salmonella enterica serovar Typhimurium (S.typhimurium) as another intracellular bacteria controls, and Escherichia coli as an extracellular control. As shown in Figure 4E and F, BCG, S. typhimurium and E. coli did not induce the production of MIR4458, MIR4667-5p, and MIR4668-5p significantly. Taken together, the results above suggest that expression levels of MIR4458, MIR4667-5p, and MIR4668-5p are increased upon B. pseudomallei infection.
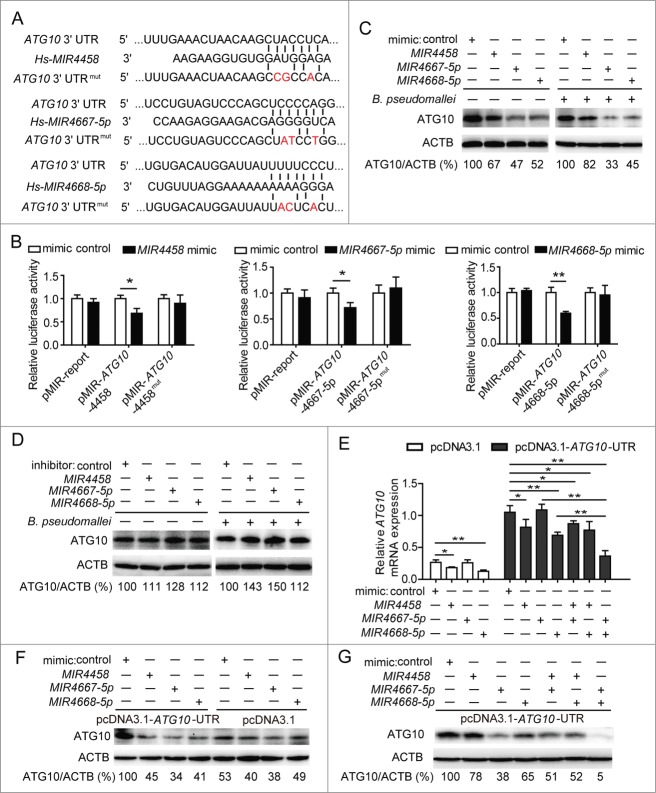
MIR4458, MIR4667-5p, and MIR4668-5p target and suppress ATG10
To identify whether ATG10 is targeted by MIR4458, MIR4667-5p, and MIR4668-5p, we constructed vectors containing the wild-type or mutant 3′UTR of ATG10 mRNA, which were individually fused downstream to the firefly luciferase gene (Fig. 5A). For the luciferase assay, the indicated wild-type or mutant vectors were cotransfected into HEK293 cells with control or mimics of the 3 miRNAs. The relative luciferase activity of wild-type vectors was effectively suppressed by the 3 miRNA mimics, while that of mutant vectors was not significantly changed, suggesting that MIR4458, MIR4667-5p, and MIR4668-5p could bind to the 3′UTR of ATG10, resulting in downregulation of gene expression (Fig. 5B; Fig. S7). In addition, we observed that the protein levels of ATG10 were dramatically decreased by the 3 miRNAs with different inhibition rates, especially for MIR4667-5p, which exhibited the most effective role, but was increased by the miRNA inhibitors compared to the control (Fig. 5C and D).
Figure 5.
MIR4458, MIR4667-5p, and MIR4668-5p target and suppress ATG10. (A) Sequences of MIR4458, MIR4667-5p, and MIR4668-5p, and the potential binding site at the 3′UTR of ATG10. Also shown are nucleotides mutated in the ATG10-3′UTR mutant. (B) Luciferase reporter assay. HEK293 cells were transiently cotransfected miRNA mimics or control with luciferase reporter vectors. Luciferase activity was normalized to the activity of Renilla luciferase. (C and D) Western blot analysis of endogenous ATG10 in uninfected and B. pseudomallei-infected A549 cells after transfected with miRNA control, mimics, or inhibitors. (E and G) The mRNA level of ATG10 and the protein levels of ATG10 were determined by qRT-PCR and western blot. HEK293 cells were cotransfected the ATG10-expressing plasmid containing wild-type 3′UTR or the control plasmid, with miRNA mimics or control for 24 h. Data are representative of at least 3 independent experiments (*P < 0.05, **P < 0.01).
To further determine if there was an inverse correlation between the 3 miRNAs and the target gene ATG10, we expressed the ATG10 cDNA containing all the MIR4458, MIR4667-5p, and MIR4668-5p binding sites in the 3′UTR (Fig. S5). Interestingly, at the mRNA level, expression of ATG10 was significantly reduced by MIR4458 and MIR4668-5p, whereas no significant change was observed with MIR4667-5p (Fig. 5E). In contrast to qRT-PCR analysis, at the protein level, expression of ATG10 protein was significantly repressed by the 3 miRNAs (Fig. 5F). Because miRNAs may downregulate their target genes through mRNA degradation or translation inhibition, the above results suggest that MIR4458 and MIR4668-5p may downregulate ATG10 expression through mRNA degradation, while MIR4667-5p does it through translation inhibition. Next, to determine if there was a cooperative effect among the 3 miRNAs in the regulation of the target gene, we combined 2 miRNAs at a time to detect the expression of ATG10 by qRT-PCR and the protein by western blot. Notably, MIR4667-5p and MIR4668-5p resulted in relatively lower mRNA and protein levels of ATG10 compared to any other miRNA combinations, suggesting that ATG10 is likely to be regulated by a coordinate action of MIR4667-5p and MIR4668-5p (Fig. 5E and G). Together, our results provide evidence for a direct regulation of ATG10 by MIR4458, MIR4667-5p, andMIR4668-5p, and suggest that ATG10 expression is coordinately regulated by MIR4667-5p and MIR4668-5p.
MIR4458, MIR4667-5p, and MIR4668-5p inhibit autophagy and promote the intracellular survival of B. pseudomallei by targeting ATG10
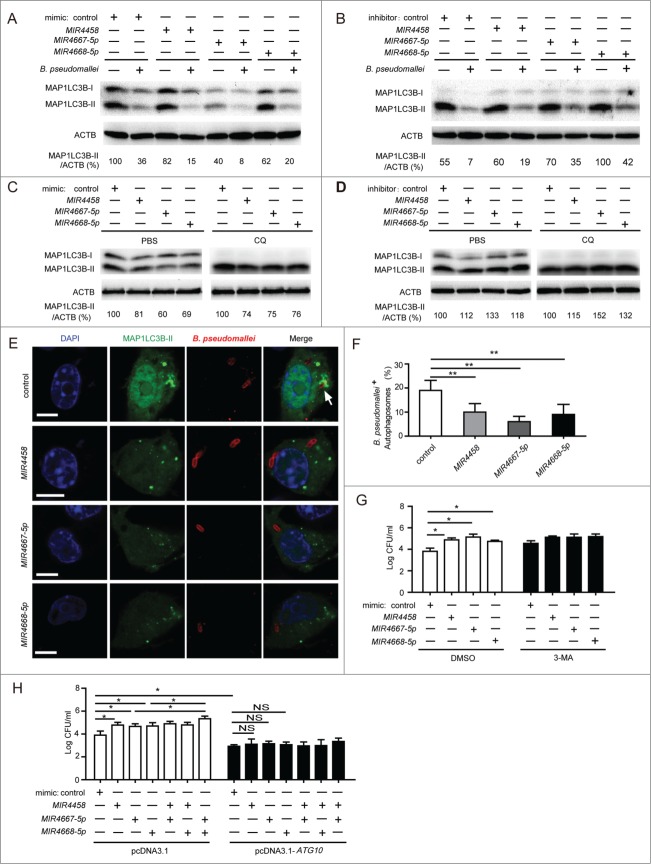
To provide evidence that MIR4458, MIR4667-5p and MIR4668-5p have roles in the negative regulation of autophagy during B. pseudomallei infection, we examined the processing of MAP1LC3B (conversion from MAP1LC3B-I to MAP1LC3B-II) and the number of MAP1LC3B puncta in A549 cells. Transfection with the 3 miRNA mimics decreased MAP1LC3B-II conversion in uninfected and B. pseudomallei infected A549 cells, while transfection with the inhibitor increased the amount of MAP1LC3B-II (Fig. 6A and B). To further confirm the role of the 3 miRNAs on autophagy activity, we employed CQ to block autophagic flux. As expected, in the presence of CQ, MIR4458, MIR4667-5p, and MIR4668-5p mimic decreased the amount of MAP1LC3B-II, respectively, while the inhibitor for the 3 miRNAs increased it (Fig. 6C and D). Furthermore, cotransfection of a GFP-MAP1LC3B vector with the 3 miRNA mimics significantly decreased autophagy in A549 cells, which supports our western blot data (Fig. 6E and F).
Figure 6.
For figure legend, see page 1302.
Then, to investigate whether MIR4458, MIR4667-5p and MIR4668-5p modulate the intracellular survival of B. pseudomallei via autophagy, we detected the effect of the 3 miRNAs on bacterial survival under autophagy inhibition condition. Transfection with MIR4458, MIR4667-5p, and MIR4668-5p mimics increased intracellular survival of B. pseudomallei in A549 cells, but no significant changes were observed when treated with 3-MA (Fig. 6G). Moreover, to further determine whether MIR4458, MIR4667-5p, and MIR4668-5p enhance the intracellular survival of B. pseudomallei by targeting ATG10, we cotransfected the 3 miRNA mimics or control with the ATG10 expression-plasmid without 3′UTR into A549 cells before B. pseudomallei infection. Overexpression of MIR4458, MIR4667-5p, and MIR4668-5p significantly increased the bacterial load of intracellular B. pseudomallei, whereas ATG10 overexpression attenuated miRNA-mediated intracellular survival of B. pseudomallei in A549 cells (Fig. 6H). Notably, MIR4667-5p and MIR4668-5p had a cooperative effect in regulation of intracellular survival of B. pseudomallei, consistent with previous data on MIR4667-5p and MIR4668-5p-mediated ATG10 expression. Collectively, these results suggest that MIR4458, MIR4667-5p and MIR4668-5p inhibit autophagy-mediated elimination of intracellular B. pseudomallei by targeting ATG10.
Upregulation of MIR4458, MIR4667-5p and MIR4668-5p is correlated with reduced promoter methylation in B. pseudomallei-infected A549 cells
We proceeded to identify the mechanism responsible for the upregulation of MIR4458, MIR4667-5p, and MIR4668-5p during B. pseudomallei infection in A549 cells. In a screen for MIR4458, MIR4667-5p, and MIR4668-5p regulatory regions, we identified a CpG island (or CpG-rich sequence) located within the 3 miRNA promoters using UCSC Genome Bioinformatics. Because it has been recently demonstrated that DNA methylation may be transiently and rapidly regulated on inducible promoters soon after stimulation,20-21 we sought to perform a DNA methylation analysis after B. pseudomallei exposure.
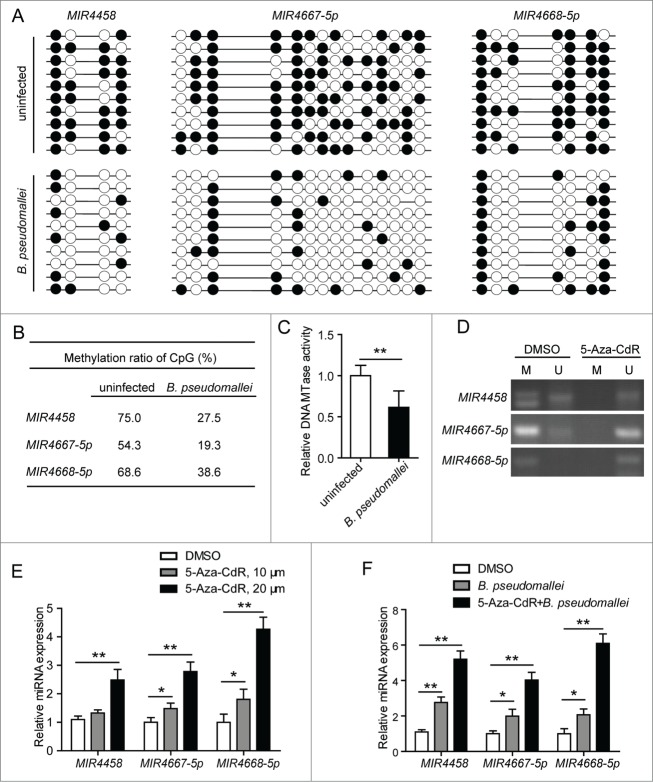
To detect the methylation of the CpGs within MIR4458, MIR4667-5p, and MIR4668-5p promoters, respectively, we first evaluated the methylation status of these regions in uninfected and B. pseudomallei infected cells by using bisulfite sequencing (Fig. 7A). As shown in Figure 7B, the percentages of methylated CpGs were lower in B. pseudomallei-infected A549 cells (for MIR4458, 27.5%; for MIR4667-5p, 19.3%; for MIR4668-5p, 38.6%) than in the uninfected cells (75%, 54.3%, and 68.6%, respectively). To further determine whether B. pseudomallei upregulates the 3 miRNA expression by reducing methylation, we examined the influence of B. pseudomalleion the DNA methyltransferase (DNMT1) activity in A549 cells, which is responsible for the increase in DNA methylation. And we found that B. pseudomallei could reduce the DNMT1 activity in the nuclear extract (Fig. 7C).
Figure 7.
Decrease of promoter methylation and upregulation of MIR4458, MIR4667-5p, and MIR4668-5p in B. pseudomallei-infected A549 cells. (A) Genomic DNA from A549 cells infected with B. pseudomallei for 24 h were subjected to sodium bisulfite sequencing analysis. The frequency of methylated CpGs in the each promoter CpG sites is shown, respectively. Black and white circles correspond to methylated and unmethylated, respectively. (B) The average of methylation at each CpG site within promoters. (C) DNMT1 (DNA MTase) activity assay of nuclear extracts of A549 cells infected with B. pseudomallei for 24 h. (D) Detection of methylation by MS-PCR. A549 cells were treated with DMSO or 5-Aza-CdR (20 μM) for 3 d. M, methylation-specific PCR product; U, unmethylation-specific PCR. (E and F) The expression of MIR4458, MIR4667-5p, and MIR4668-5p was analyzed by qRT-PCR in uninfected or B. pseudomallei-infected A549 cells. A549 cells were treated with 5-Aza-CdR for 3 d at the indicated concentration. Results are represented as mean±SD (*, P < 0.05, **, P < 0.01).
To further confirm the correlation between the DNA methylation status and the 3 miRNA expression levels, we treated cells with 5-aza-2′-deoxycytidine (5-Aza-CdR), an inhibitor of DNA methylation, then detected the methylation level by methylation-specific PCR (MSP) (Fig. 7D). As shown in Figure 7E, inhibition of promoter CpG methylation activates MIR4458, MIR4667-5p and MIR4668-5p expression in A549 cells in a dose-dependent manner. And pretreatment of A549 cells with 5-Aza-CdR, followed by stimulation with B. pseudomallei, clearly resulted in a marked induction of MIR4458, MIR4667-5p, and MIR4668-5p expression (Fig. 7F). Furthermore, to determine whether the upregulation of MIR4458, MIR4667-5p, and MIR4668-5p was specifically activated by DNA methylation modification, we quantified the methylation status of CpGs within another miRNA promoter, MIR23A-5p, from the previous miRNA microarrays data; however, the methylation status of MIR23A-5p CpG positions assessed remained unchanged, of which the expression was also upregulated in response to B. pseudomallei infection (Fig. S8). Taken together, these results suggest that upregulation of MIR4458, MIR4667-5p, and MIR4668-5p is correlated with the B. pseudomallei-induced decrease of promoter methylation in A549 cells.
Discussion
Autophagy has been demonstrated to play an essential role in the host innate immune response against B. pseudomallei infection. This study shows the molecular basis involved in the impaired autophagic clearance of B. pseudomallei in human lung epithelium cells. Specifically, we found that the underlying mechanism for downregulated ATG10 expression after exposure to B. pseudomallei in A549 cells is through increased MIR4458, MIR4667-5p, and MIR4668-5p expression, caused by reducing promoter methylation.
Intracellular pathogens have adopted various strategies to evade host defense mechanisms, including escaping into the cytoplasmic compartment, interfering with host's inflammatory response and autophagy pathways, and developing resistance to lysosomal contents.6 For instance, adherent invasive Escherichia coli upregulates levels of MIR30C and MIR130A to reduce expression of ATG5 and ATG16L1 and inhibit autophagy.14 Our study clearly shows that inhibition of autophagy is mediated by directly targeting ATG10 that results in blocking the conversion from MAP1LC3B-I to MAP1LC3B-II, potentially explaining the impaired autophagic clearance of B. pseudomallei. Previous studies have reported that autophagic evasion of B. pseudomallei is an active process relying on TTSS effector, BopA, which shows 23% amino acid identity to the Shigella TTSS effector, IcsB.12,22 In Shigella, IcsB competitively binds to its surface protein VirG to inhibit the autophagic process; ATG5 recognizes VirG.8 Therefore, in a manner analogous to IcsB, it appears unlikely that BopA is the key factor to interfere with ATG10 expression upon B. pseudomallei infection.
Mounting evidence indicates that the post-transcriptional and translational controls mediated by miRNAs contribute significantly to autophagy.23-24 Importantly, several bacteria can make use of miRNAs to participate in modulating autophagy by directly targeting autophagy-related genes, such as BECN1, ATG12, RHEB, ATG5, and ATG16L1.14-16,25 Therefore, involvement of miRNAs in regulating autophagy is undoubtedly worth further investigation. In our model, B. pseudomallei infection increased 3 novel miRNAs, MIR4458, MIR4667-5p and MIR4668-5p, by different time course and spatial expression patterns. Furthermore, synergistic regulations among multiple miRNAs are important to understand the mechanisms of complex post-transcriptional regulations in humans.26-27 Complex diseases are affected by several miRNAs rather than a single miRNA. Azra Krek et al. have shown that MIR375, MIR124, and MIRLET7B jointly regulate MTPN (myotrophin), a 12-kDa soluble protein that can initiate cardiac hypertrophy, providing evidence for coordinate miRNA control in mammals.27 Notably, in our study, the synergistic MIR4667-5p and MIR4668-5p have the same expression tendency, and we provide experimental validation of ATG10 as the mammalian autophagy-related gene that is regulated coordinately by the 2 miRNAs involved in B. pseudomallei infection. Our results thus provide an experimental model for studying translational gene regulation by multiple miRNAs and a glimpse at the complexity of translational gene regulation executed by miRNAs in B. pseudomallei infection.
Previous studies demonstrate that B. pseudomallei stimulation can lead to the activation of NFKB1 and p38-MAPK pathways in A549 cells,28-29 and NFKB1, SP1, and JUN/c-Jun were predicted as mutual promoters of MIR4458, MIR4667-5p, and MIR4668-5p by using UCSC Genome Bioinformatics. However, our unpublished data showed that only the NFKB1 inhibitor had a slight blockage effect on MIR4458 induction. Jixin Cui et al. have reported that another TTSS effector, CHBP, from B. pseudomallei is a general inhibitor of the ubiquitination pathway and has a negative effect on NFKBIA/IκBα degradation and NFKB1 nuclear translocation,30 in accord with the previous studies that NFKBIA in B. pseudomallei-infected cells was degraded after 30 min and then reappeared after 2 h of infection.28-29 These findings may suggest that the NFKB1 and MAPK pathways are unlikely to be involved in the regulation of MIR4458, MIR4667-5p, and MIR4668-5p expression in response to B. pseudomallei infection. Thus, we sought to investigate an alternative regulatory mechanism.
Epigenetic mechanisms play important roles in many biological events. There have been many studies focusing on the epigenetic modification of host genes induced by viral and bacterial infection.31 Influenza virus specifically regulates the expression of IL6 and IL32 by promoter demethylation.32-33 Helicobacter pylori has also been found to regulate PTGS2 gene activation by dynamic changes of CpGs methylation at the PTGS2 promoter.34 In addition, H. pylori-associated DNA methylation alteration occurs to CpG islands of miRNA genes, which leads to change in expression of a subset of host miRNAs.35 Promoter hypomethylation is a feature of gene activation, whereas hypermethylation is associated with gene silencing. Our bisulfite sequencing analysis suggested B. pseudomallei itself was a potent factor in directly causing alterations in methylation of epithelial DNA by reducing DNMT1 activity. According to our data, although detectable, the basal expression levels of MIR4458, MIR4667-5p, and MIR4668-5p were quite low in A549 cells. But there was a significant, 5- to 20-fold enhancement of the expression of all 3 miRNAs, when A549 cells were infected with B. pseudomallei. Moreover, 5-Aza-CdR treatment clearly facilitated MIR4458, MIR4667-5p, and MIR4668-5p expression, and this effect was more marked when cells were pretreated with 5-Aza-CdR and then stimulated with B. pseudomallei. This synergistic effect may result from removal of the inhibitory effect of promoter methylation coupled with transcriptional activation by transcription factors, like influenza virus.32,36 Since our results did not reveal changes in MIR23A-5p promotor CpG methylation, expression of this miRNA may be regulated by other mechanisms, rather than epigenetic alteration in response to B. pseudomallei infection. Taken together, we concluded that the increased expression of MIR4458, MIR4667-5p, and MIR4668-5p observed in A549 cells was attributed to specific alteration in methylation of promoter CpG islands caused by B. pseudomallei infection.
Collectively, this study reveals that 3 novel miRNAs, MIR4458, MIR4667-5p, and MIR4668-5p, are induced by B. pseudomallei infection, and inhibit autophagy in A549 cells by targeting ATG10, conferring bacterial subversion of host innate immune pathways. Further, it reveals insights into the regulation of the methylation status of promoter CpG islands by B. pseudomallei. These insights increase our understanding of the host-pathogen relationship, lay the groundwork for strategies aimed at combating infectious diseases, and may provide useful information for developing potential therapeutic interventions against pathogens.
Materials and Methods
Antibodies and reagents
The GFP-MAP1LC3B plasmid was kindly provided by Dr. Tamotsu Yoshimori (Osaka University, Japan). 3-methyladenine (3-MA, M9281), rapamycin (R8781), chloroquine (CQ, C6628) and 5-Aza-2′-deoxycytidine (5-Aza-CdR, A3656) were purchased from Sigma; antibodies against MAP1LC3B (L7543), ATG10 (A9356), ATG5 (A0731), DRAM2 (HPA018036) and ATG4C (A9482) were obtained from Sigma. The antibodies against ATG12 (2010) and SQSTM1 (5114) were obtained from Cell Signaling Technology. The antibody against ACTB (sc-10731) was obtained from Santa Cruz Biotechnology.
Cell lines and bacterial strains
A549 and HeLa cells were grown in DMEM medium (Gibco, 11965-092) containing 10% fetal bovine serum (Gibco, 10099-141) and 100 U/ml penicillin/streptomycin (Gibco, 15140-122). The normal lung bronchial epithelial BEAS-2B cells and human embryonic kidney HEK293 cells were cultured in RPMI 1640 medium (Gibco, 11875-093) supplemented with 10% fetal bovine serum and penicillin/streptomycin. All the above cell lines were cultured at 37°C in 5% CO2.
For all experiments, the B. pseudomallei strain used in all experiments is BPC006, a virulent clinical isolate from a melioidosis patient in China.37 And E. coli K12 (29425), S. typhimurium (14028) and BCG (19274) were purchased from ATCC.
Plasmid construction
The DNA oligonucleotides containing wild-type or mutant 3′UTR of ATG10 were synthesized with flanking Spe I, Apa I, and Hind III restriction enzyme digestion sites, respectively. The DNAs and pMIR-REPORTTM luciferace vectors (Ambion, AM5795) were used to build the luciferase report vectors. The resulting constructs were named pMIR-ATG10-4458 and pMIR-ATG10-4458mut, pMIR-ATG10-4667-5p and pMIR-ATG10-4667-5pmut, pMIR-ATG10-4668-5p and pMIR-ATG10-4668-5pmut. The mutant 3′UTRs of ATG10 were served as a control. All of the sequences are shown in Table S1.
The plasmids pcDNA3.1-ATG10-UTR and pcDNA3.1-ATG10, encoding an ATG10 protein, were constructed by subcloning the human ATG10 coding sequence with or without 3′UTR into the BamH I and Xho I site of the expression vector pcDNA3.1 (Invitrogen, V795-20). The primers were shown in Supplementary information, Table S2.
Generation of rabbit antiserum
B. pseudomallei was heat-killed as described previously,38 then used to generate polyclonal antibodies as follows: 250 μg of heat-killed bacteria mixed with Freund's complete adjuvant was administered to a New Zealand White female rabbit by subcutaneous injection, followed by 2 similar boosters with 200 μg, 3 and 4 weeks later. Serum was collected 5 wk after the initial immunization. Anti-B. pseudomallei-titers of serum were determined by enzyme-linked immunosorbent assay (data not shown).
Luciferase assay
The HEK-293 cells were transfected with 0.8 μg of indicated wide-type or mutant firefly luciferase reporter vectors, 100 nM of the indicated miRNA mimics (Ambion, 4464066) and mimic control (Ambion, 4464058), inhibitors (Ambion, 4464084) and inhibitor control (Ambion, 4464076), and 0.04 μg of Renilla luciferase control vector (pRL-TK-Promega, E2241) using Lipofectamine 2000 (Invitrogen Life Technologies, 11668-019). After transfection for 24 h, all of the cells were lysed via dual luciferase reporter assay system (Promega, E1910), and then the fluorescence activity was detected via a GloMax 20/20 Luminometer (Promega, E5311). Firefly luciferase activity was normalized to Renilla luciferase activity.
Quantitative RT-PCR (qRT-PCR)
qRT-PCR assays for MIR4458, MIR4667-5p, and MIR4668-5p were performed by using TaqMan miRNA assays (Ambion, 4465407) in a Bio-Rad IQ5 (Bio-Rad Laboratories, Inc.). The reactions were performed using the following parameters: 95°C for 2 min followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. U6 small nuclear RNA was used as an endogenous control for data normalization. Relative expression was calculated using the comparative threshold cycle method.
Quantitative RT-PCR analyses for the mRNA of ATG10, ATG4C, DRAM2, ATG5, and ATG12 were performed by using the PrimeScript RT-PCR kit (Takara, RR037A). The mRNA level of ACTB was used as an internal control. The primers were shown in Table S2.
Intracellular survival of bacteria
Bacterial invasion of A549 cells was investigated by using the method described by Elsinghorst,39 except for the following modifications: A549 cells were infected with B. pseudomallei at an MOI of 10. Two h after infection, cells were washed twice with PBS (Beyotime, ST476), and 2 ml of fresh culture medium containing 250 μg of kanamycin per ml was added, and the preparation was incubated to kill the extracellular bacteria. Then cells were washed 3 times with PBS and lysed with 1 ml of 0.1% Triton X-100 (Sigma, T8787) after infection. Diluted cell lysates were plated on Luria broth plates. Colonies were counted after 36 h. Experiments were performed at least 3 times in triplicates.
Transmission electron microscopy
A549 cells were collected and fixed in a solution containing 2.5% glutaraldehyde in 0.1 M sodiumcacodylate for 2 h, postfixed with 1% OsO4 for 1.5 h, and washed and stained in 3% aqueous uranyl acetate for 1 h. The samples were then washed again, dehydrated with a graded alcohol series, and embedded in Epon-Araldite resin (Canemco, 034). Ultrathin sections were cut using a Reichert ultramicrotome (Reichert, United States), counterstained with 0.3% lead citrate, and examined on a Philips EM420 electron microscope (Philips, United Kingdom).
Confocal microscopy
A549 cells were cotransfected the indicated miRNA mimics, inhibitors or control (100 nM) with the GFP-MAP1LC3B plasmid for 24 h, then cells were infected with B. pseudomallei for the indicated time. After infection, cells were washed with PBS, fixed for 10 min at 37°C in 4% paraformaldehyde, permeabilized with 0.1% (vol/vol) Triton X-100. Coverslips were incubated with rabbit anti-B. pseudomallei serum for 1 h, washed extensively with PBS buffer, and incubated with Alexa Fluor 647-conjugated donkey anti-rabbit for 1 h (Molecular Probes, A31573). All steps were carried out at room temperature. After staining, we mounted cover slips using Vectashield (Vector Laboratories, H1200). We used a Radiance 2000 laser scanning confocal microscope (Bio-Rad, San Francisco, CA) for confocal microscopy followed by analysis with LaserSharp 2000 software (Bio-Rad). We acquired images in sequential scanning mode.
Western blot analysis
Cells were washed 3 times with ice-cold PBS and then lysed in lysis buffer (P0013, Beyotime) containing 1% Triton X-100 and protease inhibitors (Beyotime, ST506). After centrifugation at 5000 g for 15 min at 4°C, the protein concentration was measured using a BCA protein assay kit (Pierce, 23227). The lysates were separated using SDS-PAGE and were transferred to PVDF membranes. The membranes were blocked with 5% nonfat dry milk in TBS (AR0031, BOSTER) containing 0.05% Tween 20 (Sigma, P1379) and were incubated with incubated overnight with the respective primary antibodies at 4°C. The membranes were incubated at room temperature for 1 h HRP-conjugated secondary antibodies (Jackson Immuno Research Laboratories, 115-035-003, 111-035-003) according to the manufacturer's instructions. The protein of interest was visualized using the SuperSignal® West Dura Duration substrate reagent (Thermo, 34080).
Bisulphite sequencing and methylation-specific PCR
Methyl Primer Express Software v1.0 was used to design bisulfite sequencing PCR and MSP primers. Bisulfite modification of 500 ng of genomic DNA was performed by using the EZ DNA Methylation-GoldTM Kit (Zymo Research Corp, D5005). PCR products were recovered using QIAGEN Gel DNA Kits (51206) and sequenced at Sangon Biotech (Shanghai, China) and Applied Biosystems 3130 Genetic Analyzers (Foster, CA) in our laboratory. The lengths of the amplification fragments in MSP and bisulphite sequencing PCR for MIR4458, MIR4667-5p, MIR4668-5p, and MIR23A-5p are provided in Supplementary Information, Table S3 and S4.
siRNA assay
The ATG12 (human; sc-72578) siRNAs and control siRNA (sc-44230) were purchased from Santa Cruz Biotechnology. All siRNA transfections were performed using the Dharmafect 1 transfection reagent (Thermo Scientific, T-2001-03). A549 cells were transfected with 50 nM siRNA for 24 h, followed by treatments; protein knockdown was assessed using western blot analysis.
DNMT1 activity assay
Protein samples (nuclear extracts) from A549 cells were prepared according to the manufacturer's protocol (Epigentek, OP-0002), and the measurement of DNMT1 activity was performed with the EpiQuik DNA Methyltransferase Activity/Inhibition AssayKit (Epigentek, P-3001) according to the manufacturer's protocol.
Cell death assay
A549 cells were seeded at 1 ×106 cells per well in a 6-well plates. After infected with B. pseudomallei at the indicated MOI, the cells were washed and further incubated in the presence of 250 μg/ml of kanamycin for the indicated hours. The cells were trypsinized with 0.5 ml of 0.25% trypsin for 2 min, collected, and resuspended in 100 μl of PBS. And the measurement of cell death was performed according to the manufacturer's protocol (BD Biosciences, 556547). Finally, fluorescence of the stained cells was measured by flow cytometry (BD FACScan Flow cytometer, BD Biosciences, San Jose, CA).
Statistical analysis
The results are expressed as the mean ±SD of at least 3 separate experiments performed in triplicate. The differences between the groups were determined with the SPSS 13.0 software. The Student t test was used to analyze the data. The differences were considered significant at P < 0.05. Statistically significant differences are indicated by asterisks (*P < 0.05, **P < 0.01).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Prof. Jiqing Lian (Third Military Medical University, Chongqing, China) for critical reading and editing of the manuscript. We also thank Dr. Tamotsu Yoshimori for providing the GFP-MAP1LC3B plasmid.
Funding
This study was supported by grants from National Natural Science Foundation of China (NSFC, No.81471914), Science Research Foundation of Third Military Medical University (2012XJY03) and Key projects of the science and technology development plan of Hainan province (ZDXM2014143).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012; 367:1035-44; PMID:22970946; http://dx.doi.org/ 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 2.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005; 18:383-416; PMID:15831829; http://dx.doi.org/ 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 2008; 102 1:S1-4; PMID:19121666; http://dx.doi.org/ 10.1016/S0035-9203(08)70002-6 [DOI] [PubMed] [Google Scholar]
- 4.Jones AL, Beveridge TJ, Woods DE. Intracellular survival of Burkholderia pseudomallei. Infect Immun 1996; 64:782-90; PMID:8641782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allwood EM, Devenish RJ, Prescott M, Adler B, Boyce JD. Strategies for intracellular survival of burkholderia pseudomallei. Front Microbiol 2011; 2:170; PMID:22007185; http://dx.doi.org/ 10.3389/fmicb.2011.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial subversion of host innate immune pathways. Science 2013; 340:697-701; PMID:23661751; http://dx.doi.org/ 10.1126/science.1235771 [DOI] [PubMed] [Google Scholar]
- 7.Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev 2011; 240:92-104; PMID:21349088; http://dx.doi.org/ 10.1111/j.1600-065X.2010.00995.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular shigella from autophagy. Science 2005; 307:727-31; PMID:15576571; http://dx.doi.org/ 10.1126/science.1106036 [DOI] [PubMed] [Google Scholar]
- 9.McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012; 338:963-7; PMID:23162002; http://dx.doi.org/ 10.1126/science.1227037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, et al.. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog 2010; 6:e1001230; PMID:21187903; http://dx.doi.org/ 10.1371/journal.ppat.1001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beale R, Wise H, Stuart A, Ravenhill BJ, Digard P, Randow F. A LC3-interacting motif in the influenza A virus M2 protein is required to subvert autophagy and maintain virion stability. Cell Host Microbe 2014; 15:239-47; PMID:24528869; http://dx.doi.org/ 10.1016/j.chom.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, Wolvetang E, Prescott M, Boyce JD, Devenish RJ, Adler B. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy 2008; 4:744-53; PMID:18483470; http://dx.doi.org/ 10.4161/auto.6246 [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281-97; PMID:14744438; http://dx.doi.org/ 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HT, Dalmasso G, Muller S, Carriere J, Seibold F, Darfeuille-Michaud A. Crohn's disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 2014; 146:508-19; PMID:24148619; http://dx.doi.org/ 10.1053/j.gastro.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Chen J, Xu HG, Zhou X, He Q, Li YL, Jiang G, Shan Y, Xue B, Zhao RX, et al.. MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-mediated autophagy. Gastroenterology 2014; 146:188-99; PMID:24036151; http://dx.doi.org/ 10.1053/j.gastro.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang HG, Fang Y, Yu B, Zhang JY, Xie QH, et al.. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy 2012; 8:1045-57; PMID:22647547; http://dx.doi.org/ 10.4161/auto.20159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng K, Chen DS, Wu YQ, Xu XJ, Zhang H, Chen CF, Chen HC, Liu ZF. MicroRNA expression profile in RAW264.7 cells in response to Brucella melitensis infection. Int J Biol Sci 2012; 8:1013-22; PMID:22904669; http://dx.doi.org/ 10.7150/ijbs.3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun 2000; 68:5377-84; PMID:10948167; http://dx.doi.org/ 10.1128/IAI.68.9.5377-5384.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al.. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445-544; PMID:22966490; http://dx.doi.org/ 10.4161/auto.19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. Transient cyclical methylation of promoter DNA. Nature 2008; 452:112-5; PMID:18322535; http://dx.doi.org/ 10.1038/nature06640 [DOI] [PubMed] [Google Scholar]
- 21.Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, et al.. Cyclical DNA methylation of a transcriptionally active promoter. Nature 2008; 452:45-50; PMID:18322525; http://dx.doi.org/ 10.1038/nature06544 [DOI] [PubMed] [Google Scholar]
- 22.Gong L, Cullinane M, Treerat P, Ramm G, Prescott M, Adler B, Boyce JD, Devenish RJ. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One 2011; 6:e17852; PMID:21412437; http://dx.doi.org/ 10.1371/journal.pone.0017852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Wang Y, Tan X, Jing H. MicroRNAs in autophagy and their emerging roles in crosstalk with apoptosis. Autophagy 2012; 8:873-82; PMID:22441107; http://dx.doi.org/ 10.4161/auto.19629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai H, Fesler A, Ju J. MicroRNA: a third dimension in autophagy. Cell Cycle 2013; 12:246-50; PMID:23255136; http://dx.doi.org/ 10.4161/cc.23273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Yang K, Zhou L, Minhaowu , Wu Y, Zhu M, Lai X, Chen T, Feng L, Li M, et al.. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog 2013; 9:e1003697; PMID:24130493; http://dx.doi.org/ 10.1371/journal.ppat.1003697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Li CX, Li YS, Lv JY, Ma Y, Shao TT, Xu LD, Wang YY, Du L, Zhang YP, et al.. MiRNA-miRNA synergistic network: construction via co-regulating functional modules and disease miRNA topological features. Nucleic Acids Res 2011; 39:825-36; PMID:20929877; http://dx.doi.org/ 10.1093/nar/gkq832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al.. Combinatorial microRNA target predictions. Nat Genet 2005; 37:495-500; PMID:15806104; http://dx.doi.org/ 10.1038/ng1536 [DOI] [PubMed] [Google Scholar]
- 28.Utaisincharoen P, Anuntagool N, Arjcharoen S, Lengwehasatit I, Limposuwan K, Chaisuriya P, Sirisinha S. Burkholderia pseudomallei stimulates low interleukin-8 production in the human lung epithelial cell line A549. Clin Exp Immunol 2004; 138:61-5; PMID:15373906; http://dx.doi.org/ 10.1111/j.1365-2249.2004.02601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utaisincharoen P, Arjcharoen S, Lengwehasatit I, Limposuwan K, Sirisinha S. Burkholderia pseudomallei invasion and activation of epithelial cells requires activation of p38 mitogen-activated protein kinase. Microb Pathog 2005; 38:107-12; PMID:15748812; http://dx.doi.org/ 10.1016/j.micpath.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Cui J, Yao Q, Li S, Ding X, Lu Q, Mao H, Liu L, Zheng N, Chen S, Shao F. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 2010; 329:1215-8; PMID:20688984; http://dx.doi.org/ 10.1126/science.1193844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med 2012; 2:a010272; PMID:23209181; http://dx.doi.org/ 10.1101/cshperspect.a010272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Sun W, Liu L, Yang F, Li Y, Chen Y, et al.. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol 2010; 185:5056-65; PMID:20889550; http://dx.doi.org/ 10.4049/jimmunol.0902667 [DOI] [PubMed] [Google Scholar]
- 33.Tang B, Zhao R, Sun Y, Zhu Y, Zhong J, Zhao G, Zhu N. Interleukin-6 expression was regulated by epigenetic mechanisms in response to influenza virus infection or dsRNA treatment. Mol Immunol 2011; 48:1001-8; PMID:21353307; http://dx.doi.org/ 10.1016/j.molimm.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Pero R, Peluso S, Angrisano T, Tuccillo C, Sacchetti S, Keller S, Tomaiuolo R, Bruni CB, Lembo F, Chiariotti L. et al.. Chromatin and DNA methylation dynamics of Helicobacter pylori-induced COX-2 activation. Int J Med Microbiol 2011; 301:140-9; PMID:20934379; http://dx.doi.org/ 10.1016/j.ijmm.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 35.Ando T, Yoshida T, Enomoto S, Asada K, Tatematsu M, Ichinose M, Sugiyama T, Ushijima T. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer 2009; 124:2367-74; PMID:19165869; http://dx.doi.org/ 10.1002/ijc.24219 [DOI] [PubMed] [Google Scholar]
- 36.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 2002; 16:6-21; PMID:11782440; http://dx.doi.org/ 10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- 37.Fang Y, Huang Y, Li Q, Chen H, Yao Z, Pan J, Gu J, Tang B, Wang HG, Yu B, et al.. First genome sequence of a Burkholderia pseudomallei Isolate in China, strain BPC006, obtained from a melioidosis patient in Hainan. J Bacteriol 2012; 194:6604-5; PMID:23144371; http://dx.doi.org/ 10.1128/JB.01577-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitlock GC, Lukaszewski RA, Judy BM, Paessler S, Torres AG, Estes DM. Host immunity in the protective response to vaccination with heat-killed Burkholderia mallei. BMC Immunol 2008; 9:55; PMID:18823549; http://dx.doi.org/ 10.1186/1471-2172-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol 1994; 236:405-20; PMID:7968625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.