Abstract
Purpose.
We attempt to understand the determinants of self-rated vision status by examining associations with vision tests, self-reported visual function, demographic, and health-status characteristics.
Methods.
Participants included 2467 individuals, aged 65 to 84 years, in a longitudinal, population-based cohort study. Participants rated their vision status from 0 to 10. Visual acuity, contrast sensitivity, stereoacuity, and visual fields were assessed. The Activities of Daily Vision Scale (ADVS) questionnaire was administered. Multivariate ordinal and multinomial logistic-regression models examined the association of demographic, health-status characteristics, vision tests, and ADVS subscales with self-rated vision status score. Odds ratios described the association of these characteristics with reporting better vision status.
Results.
Better visual acuity, contrast sensitivity, stereoacuity, and visual fields were associated with increased odds of reporting better vision status. Among the vision tests, a 2-line increase in visual acuity was most likely to result in an individual reporting better vision status (odds ratio, 1.49; 95% confidence interval [CI], 1.30–1.70). A 5-point increase in the near vision and far vision ADVS subscale scores was associated with increased odds of reporting good versus poor vision status. A 5-point increase in the near vision subscale was most likely to result in an individual reporting good versus poor vision status (odds ratio, 1.38; 95% CI, 1.28–1.50).
Conclusions.
Self-rated vision status is a multidimensional measure. Near-vision visual function, visual acuity, and contrast sensitivity are important determinants of self-rated vision status in an elderly population. This understanding may improve the ability of eye care providers to maximize self-rated vision status among their patients.
Keywords: self-rated vision, visual function, elderly, vision tests
Near-vision visual function, visual acuity, and contrast sensitivity are important determinants of self-rated vision status in an elderly population. This understanding may improve the ability of eye care providers to maximize self-rated vision status among their patients.
Introduction
Self-reported data are used widely in national surveillance surveys, clinical and population based studies, and routine clinical assessments to assess vision status, eye disease, and visual function.1 A number of surveys ask participants to rate the global quality of their vision on a scale from poor to good (self-rated vision status). In routine clinic assessments, patients also are often asked how they would rate their vision. This measure usually is taken as a surrogate for a patient's overall satisfaction with their vision. Understanding which variables are most important to individuals as they rate their vision status is important not only in further understanding the determinants of this measure, but also in ultimately improving an individual's self-rated vision status and their overall satisfaction with their vision.
Previous studies have examined the association between self-rated vision status and vision tests (visual acuity, contrast sensitivity, and visual fields), although some of the results have been inconsistent. The 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25) asks participants their self-rated vision status in the general vision subscale.2 This subscale has been associated significantly with visual acuity in multiple populations.2–8 Other vision tests, such as contrast sensitivity, stereoacuity, and visual fields, also may be important determinants in self-rated vision status.9–13 However, the association among these measures and self-rated vision status has not been well studied. In the populations in which it has been examined the results have been inconsistent. For example, Revicki et al.7 found that the correlation between the general vision subscale of the NEI VFQ-25 and contrast sensitivity was not significant in a population of age-related macular degeneration patients. Noble et al.14 found a similar result in a population of multiple sclerosis patients. In contrast with these findings, other studies have described a significant correlation between self-rated vision status and contrast sensitivity in a population of glaucoma and optic neuritis patients.4,5 Similarly, other reports have been inconsistent regarding the association between self-rated vision status and visual fields.2,4,10,14 Few studies have examined the association between self-rated vision status and stereoacuity. Therefore, it is clear that further study is needed to elucidate more firmly the relationship between self-rated vision status and vision tests.
Vision tests alone do not entirely reflect the degree of visual impairment experienced by an individual.15,16 Measures that evaluate visual function provide additional information about the impact of visual impairment on everyday life, and thus, visual function also may be an important determinant of self-rated vision status. Few studies have examined the association between self-rated vision status and visual function.
The aim of our study is to evaluate the association between self-rated vision status, and tests of visual acuity, contrast sensitivity, stereoacuity, bilateral visual field, as well as self-reported visual function in specific domains of near and far vision. The goal is to understand better which variables are most important when participants rate the quality of their vision.
Methods
Subjects
The study sample consisted of 2467 individuals enrolled in the Salisbury Eye Evaluation (SEE) Study. The Johns Hopkins Institutional Review Board approved all protocols. Data collection began in 1993. All subjects gave written informed consent before participation. All subjects were treated in accordance with the Declaration of Helsinki. A detailed description of the sampling procedure has been described previously.17,18 Briefly, the sample was selected from the Health Care Financing Administration (HCFA) Medicare eligibility list, and included only individuals between 65 and 84 years of age as of July 1, 1993 living in the Salisbury, Maryland metropolitan area. This sample included 100% of identified African American residents and a random age-stratified sample of 58% of identified white residents. No other ethnic groups were on the list. To be eligible for the study, the participant had to be able to travel to the clinic for vision tests and score greater than a 17 on the Mini-Mental Status Exam (MMSE).19 Eligible participants participated in a 2-hour in-home interview followed by a 4- to 5-hour clinic examination. Of those who were eligible, 65% participated. Details on the differences between participants and refusals have been described previously.18
Self-Rated Vision Status and Baseline Variables
Demographic characteristics were collected from the Social Security Administration records. Participants were asked how they would rate their current vision with glasses on, if they wore glasses, on a scale of 0 = blindness to 10 = excellent vision (self-rated vision status). The MMSE also was administered, as well as a standardized home questionnaire that included sections on years of education received, and self-reported visual function on a variety of visual tasks (Activities of Daily Vision Scale [ADVS]). The clinical examination included an assessment of depression using the depression scale of the General Health Questionnaire and vision testing.
Visual Acuity
Detailed descriptions of the vision tests have been reported previously.17 Trained technicians using strict forced-choice testing procedures administered all vision tests.
Distance visual acuity was tested using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts.20 The acuity charts were transilluminated with a light box (The Lighthouse, New York, NY) that maintains chart luminance at 130 cd/m2. Acuity was measured monocularly and binocularly, with habitual refractive correction and best correction after subjective refraction. Visual acuity was scored as the total number of letters read correctly and converted to log10 minimum angle resolution (logMAR).21 Participants who failed to read any letters were assigned arbitrarily an acuity of 1.7 logMAR (20/1000).
Contrast Sensitivity
Contrast sensitivity was measured with the Pelli-Robson letter sensitivity test.22 The test was administered at 1 m under controlled room illumination (∼100 cd/m2). Contrast sensitivity was scored letter by letter.23
Stereoacuity
Stereoacuity was tested with the Randot Circles test (Stereo Optical, Inc., Chicago, IL). The test consists of a series of 10 panels that form a graded disparity series from a maximum of 457 to a minimum of 17 seconds of arc when viewed at a distance of 36 cm. The panels were tested in order, beginning with the largest disparity and continuing until there was an incorrect response. The participant's score was the disparity (in log10 seconds of arc visual angle) of the panel before the first incorrect response.
Visual Fields
Visual fields were tested separately for each eye using the 81-point, single-intensity screening test strategy on a field analyzer (Humphrey Systems, San Leandro, CA). This strategy tests points in a 60° (radius) field with a single target intensity of 24 dB. If the fixation losses, false-negative responses, or false-positive responses exceeded 20%, the test was stopped and the participant reinstructed before undertaking a new test. Field tests were scored by counting separately the number of points missed in the central 30° and peripheral 30°. The square root of the number of points missed was used for analysis.
Visual Disability Questionnaire
Visual function was assessed during the home interview with the ADVS, a forerunner to the NEI-VFQ.24 The original ADVS is a 22-item questionnaire used to assess difficulty performing a range of vision tasks that were judged to be important to patients with cataract. It includes 5 subscales, including near vision, far vision, day driving, night driving, and glare. Trained interviewers administered the ADVS as originally published, excluding one question on the use of bus service, which was not available in Salisbury. For each item, it was determined whether the participant had done the activity within the past 3 months and, if not, whether it was because of vision problems. Activities that had not been engaged in recently for reasons unrelated to vision were not scored. The remaining items were scored according to level of difficulty: 1, unable to perform because of vision problems; 2, extreme difficulty; 3, moderate difficulty; 4, a little difficulty; and 5, no difficulty. For each subscale, all recorded activity scores were averaged eliminating activities not participated in for reasons other than vision. Then, the average subscale scores were rescaled to a range of 0 to 100, with 0 denoting responses of “unable to do due to vision” on all scored activities and 100 denoting responses of “no difficulty” on all scored activities.
We have published previously an evaluation of the psychometric properties of the ADVS based on data from the SEE study.25 Our study determined that the overall ADVS scale, and night-driving, near-vision, and far-vision subscales exhibit adequate content validity, internal consistency, and discriminability. Our validation study determined that the original day-driving and glare subscales had insufficient internal consistency and were limited by items that were not applicable to a large proportion of our participants. These two subscales were not used in the analyses.
Furthermore, the measurement properties and construct validity of the ADVS for low-vision patients has been examined previously using Muraki's IRT-based rating scale model and Andrich's Rasch-based rating scale model. The ADVS makes valid functional ability measures in low vision patients.26,27
Data Analysis
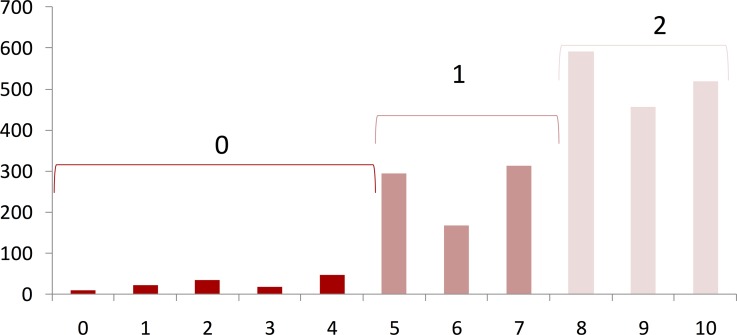
The distribution of the self-rated vision status score was highly skewed. A histogram of the self-rated vision status score is shown in Figure 1. For analysis purposes, the score was transformed into an ordinal variable. Visual inspection of the score distribution suggested cutoff values of <5 and >7, creating three grade score categories of 0 to 4 (poor vision status), 5 to 7 (fair vision status), and 8 to 10 (good vision status). Ordinal logistic regression models, using these 3 categories as the outcome, were used to examine the relationship between self-rated vision status, health status and demographic characteristics, and vision tests. The proportional odds assumption was checked using the self-rated vision status score χ2 test to ensure that the models were appropriate. However, the proportional odds assumption did not hold when the activities of daily vision subscales were used as explanatory variables. In this case multinomial logistic regression models were used instead. The strength of the association is presented as odds ratios and 95% confidence intervals (CI). A change of 1 SD was chosen to report the magnitude when the data on an explanatory variable were continuous. The models were built using a backward elimination strategy: we started with models including all the prespecified variables that potentially were related to the outcome. That is, in addition to the vision-related variables, the initial models always included age, sex, race, education, presence of symptoms of severe depression, MMSE score, and number of comorbid conditions. Among variables whose P value was greater than 0.05, we removed from the model the one that had the highest P value. To create a parsimonious model, we proceeded iteratively until all variables in the model had associated P values of 0.05 or less.
Figure 1.
Histogram of self-reported vision status score.
Results
The characteristics of the study population were examined by categories of self-rated vision status score (Table 1). A lower percentage of participants who reported good vision status were female or black compared to participants who reported worse vision status (score < 8). These participants also were on average younger, had more years of education, had a better MMSE score and reported less comorbidities and symptoms of severe depression than those who reported worse vision status. Those who reported good vision status had on average better presenting visual acuity, contrast sensitivity, stereoacuity, and bilateral visual fields than participants who reported worse vision status. They also had on average higher ADVS subscale scores in the near vision, far vision, and night driving domains. The above held true when comparing the characteristics of those who reported fair vision status to those who reported poor vision status.
Table 1.
Characteristics of the Study Population by Categories of Self-Rated Vision Status Score
|
Vision Status Score |
Age-Adjusted
P
Value* |
|||
|
0–4,
N
= 128 |
5–7,
N
= 774 |
8–10,
N
= 1565 |
||
| Demographics | ||||
| Mean age in y (SD) | 75.9 (5.7) | 74.3 (5.3) | 72.9 (4.8) | <0.001 |
| % Females | 62.5 | 60.1 | 55.5 | 0.05 |
| % Blacks | 32.8 | 26.9 | 24.4 | 0.02 |
| Mean y of education (SD) | 10.0 (3.7) | 10.9 (3.3) | 11.5 (3.4) | <0.001 |
| Health status | ||||
| Mean MMSE (SD) | 25.9 (3.0) | 26.9 (2.7) | 27.5 (2.4) | <0.001 |
| % Severe depression symptoms | 16.7 | 12.6 | 6.9 | <0.001 |
| Mean N of comorbidities (SD) | 2.7 (1.6) | 2.6 (1.7) | 2.2 (1.5) | <0.001 |
| Vision tests | ||||
| Mean logMar presenting visual acuity (SD) | 0.34 (0.49) | 0.07 (0.19) | −0.01 (0.13) | <0.001 |
| Mean contrast sensitivity, no. of letters (SD) | 28.7 (10.0) | 34.0 (3.9) | 35.5 (3.0) | <0.001 |
| Mean log stereo acuity (SD) | 2.4 (0.59) | 2.1 (0.55) | 1.8 (0.44) | <0.001 |
| Mean points missing bilateral visual field (SD) | 37.6 (28.3) | 29.5 (18.6) | 22.4 (14.5) | <0.001 |
| Mean activities of daily vision subscales (SD) | ||||
| Near vision | 62.2 (33.7) | 86.7 (17.1) | 96.0 (7.5) | <0.001 |
| Far vision | 68.7 (30.6) | 88.1 (15.7) | 96.1 (7.8) | <0.001 |
| Night driving | 41.8 (40.8) | 65.7 (32.7) | 82.5 (23.7) | <0.001 |
From an ordinal logistic regression model, including age and the characteristic of interest.
A multivariate ordinal logistic regression model examined the association of demographic, health status characteristics, and vision tests with self-rated vision status, controlling for demographic and nonvision health status characteristics (Table 2). Better visual acuity, contrast sensitivity, stereoacuity, and bilateral visual fields increased the odds of reporting better vision status. When comparing the impact of the various vision tests on vision status score, a 1 SD increase in visual acuity increased the odds of reporting better vision status by almost 50% followed by contrast sensitivity, which increased the odds by 34% per 1 SD increase. This was followed by stereoacuity and bilateral visual field, which increased the odds by 28% and 11% per 1 SD increase, respectively. Interestingly, a number of nonvision-related factors also were independently related to self-rated vision status score. Being female, having symptoms of severe depression, a per unit decrease in MMSE score, and a per unit increase in the number of comorbid conditions increased the odds of reporting worse vision status. Race, age, and years of education received were not associated with self-rated vision status once other factors were in the model.
Table 2.
Demographic, Health Status, and Vision Tests Associated With Self-Rated Vision Status Score
|
Characteristic |
Odds Ratio (95% CI)* |
P
Value |
| Demographic and health status | ||
| Female | 0.82 (0.68–0.98) | 0.025 |
| Symptoms of severe depression | 0.71 (0.53–0.95) | 0.022 |
| MMSE, per unit decrease | 0.96 (0.93–0.99) | 0.024 |
| N of comorbid conditions, per unit increase | 0.89 (0.84–0.94) | <0.0001 |
| Vision tests | ||
| Visual acuity, per 1 SD approximately 2 lines increase† | 1.49 (1.30–1.70) | <0.0001 |
| Contrast sensitivity, per 1 SD approximately 4 letters increase† | 1.34 (1.18–1.54) | <0.0001 |
| Stereo acuity, per 1 SD approximately 0.5 log units increase† | 1.28 (1.16–1.42) | <0.0001 |
| Gaining points bilateral visual field, per 1 SD approximately 17 points† | 1.11 (1.01–1.23) | 0.036 |
Multivariate model from ordinal logistic regression models with vision status score (0–10), categorized as poor (0–4), fair (5–7), and good (8–10). Score test for the proportional Odds Assumption: χ2 = 7.37, degrees of freedom 8, P = 0.50.
For change of 1 SD using standardized scores.
Odds ratio associated with reporting better vision, moving from a vision status score of poor (0–4) to fair (5–7), or fair (5–7) to good (8–10).
Next, we examined the association of demographic, health status characteristics, and the functional impact of a decrease in vision, as represented by the ADVS subscales, with vision status score controlling for demographic and nonvision health status characteristics (Table 3). Self-reported difficulties on near and far vision ADVS subscales were associated significantly with self-rated vision status score. A 5-point increase in near and far vision ADVS subscales increased the odds of reporting good versus poor vision status by 38% and 20%, respectively. Furthermore, a 5-point increase in the near vision ADVS subscale also increased the odds of reporting fair versus poor vision status by 11%. The night-driving subscale was not associated significantly with vision status score.
Table 3.
Demographic, Health Status, and Activities of Daily Vision Subscales Associated With Self-Rated Vision Status Score
|
Odds Ratio (95% CI)* |
P
Value† |
||
|
Vision Status Score 8–10 |
Vision Status Score 5–7 |
||
| Demographic and health status | |||
| Age, per y increase | 0.95 (0.91–0.97) | 0.98 (0.94–1.02) | 0.004 |
| MMSE, per unit decrease | 0.89 (0.82–0.96) | 0.96 (0.89–1.03) | 0.0001 |
| Activities of daily vision subscales | |||
| Near vision subscale, per 5 points increase | 1.38 (1.28–1.50) | 1.11 (1.04–1.18) | <0.001 |
| Far vision subscale, per 5 points increase | 1.20 (1.11–1.30) | 1.06 (0.99–1.10) | <0.001 |
Multivariate model from multinomial logistic regression using self-reported vision score 0 to 4 as the reference category.
Wald χ2 for global testing (type 3 analysis).
In this model, a per unit increase in age and a per unit decrease in MMSE score increased the odds of reporting poor versus good vision status. However, unlike the vision test model, being female, a per unit increase in the number of comorbid conditions, and being depressed did not contribute to self-rated vision status, once the model included self-reported visual function.
Discussion
The purpose of our study was to understand further the determinants of self-rated vision status and what variables are most important to individuals as they rate the global quality of their vision. What we found is that multiple components are related to this simple measure. First, multiple vision tests are significant determinants of self-rated vision status. Consistent with previous studies, better visual acuity, contrast sensitivity, and bilateral visual fields were associated significantly with reporting better vision status even when demographic factors, cognitive status, depression, and a number of other chronic medical conditions were considered.2–8,10,14 Few studies had previously examined the association between stereoacuity and self-rated vision status. In our study population, stereoacuity also was associated significantly with self-rated vision status. Although better performance on all the vision tests was associated independently with reporting better vision status, better distance visual acuity followed by contrast sensitivity were most important in increasing the odds of reporting better vision status.
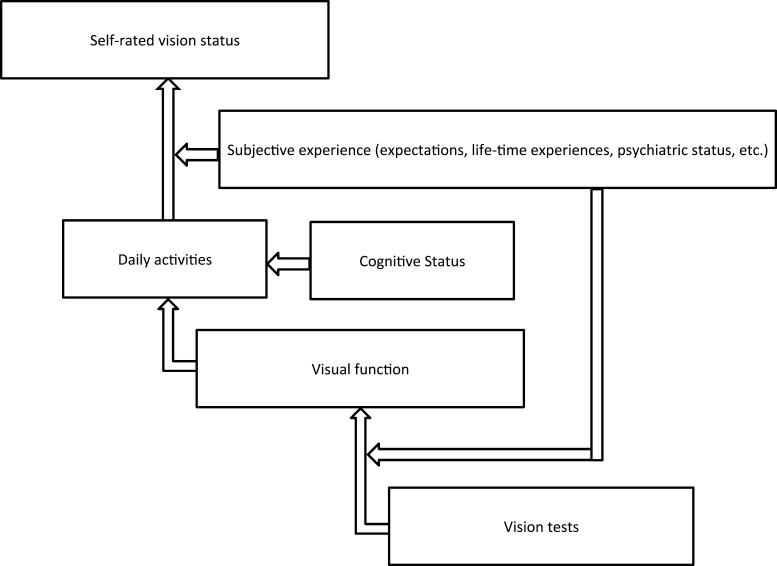
When thinking about why visual acuity and contrast sensitivity were the most important determinants for self-rated vision status, it is important to recognize that previous studies have demonstrated the importance of visual acuity and contrast sensitivity in visual function.11–13,28–31 Furthermore, studies have emphasized particularly the importance of visual function in an individual's perception of vision status.32–36 Therefore, it is likely that the association of vision tests and self-rated vision status is, in part, by way of visual function (Fig. 2). It has been demonstrated previously in our study population that improvements in all of the vision tests were associated with an increase in the odds of being high functioning versus low functioning.37 Consistent with other studies, better visual acuity and contrast sensitivity were most important in increasing the odds of being high functioning. This further supported that idea that visual acuity and contrast sensitivity are important determinants of self-rated vision status because they are important determinants of visual function. Visual function is, in turn, a driving determinant of self-rated vision status.
Figure 2.
Proposed relationship between vision tests, visual function, and self-rated vision status.
Also, we found that a number of visual function domains as represented by the ADVS subscales were significant determinants of self-rated vision status. Better function on the near and far vision ADVS subscales was associated significantly with increased odds of reporting good versus poor vision status. For our population, better functioning in the near vision subscale was most important for reporting better vision status.
These results demonstrated that different domains of visual function as represented by the ADVS subscales differ in their degree of impact on self-rated vision status. Our results are consistent with previous studies that have demonstrated the importance of near vision to an elderly population. Poor near vision–related function was found to be associated consistently and independently with poorer quality of life and increased risk of being disabled in all areas of instrumental activities of daily living in an elderly population.38 These findings may explain why near vision–related function may have been a particularly important determinant of self-rated vision status in our population. However, the impact of each domain of visual function on self-rated vision status likely is dependent on the activities that individuals favor as well as their expectations and lifetime experiences, and may be population-dependent (Fig. 2). In support of this point, another study in a different population found that distance vision–related function was more important than near vision–related function for overall quality of life.39
In our study population, the night-driving subscale was not significantly associated with self-rated vision status. There are two possible reasons for this finding. One reason is that, for our study population, driving might not have been an important enough activity to affect our population's perception of their vision status. Indeed, nearly 20% of SEE participants never drove or did not drive during the year before the interview. Along those same lines, if poor vision did have a significant role in the self-restriction of driving, and by treating the driving-related items as missing data, we may underestimate the strength of the association between reporting better vision status and driving-related vision disability.
We also examined a number of demographic and nonvision health status characteristics, and their association with self-rated vision status. Interestingly, a decrease in MMSE status was associated independently with reporting worse vision status when examining the association between vision tests and self-rated vision status, and when examining the association between self-reported visual function and self-rated vision status. Previous studies have shown that the MMSE is a strong and independent predictor of activities of daily living (ADL) and instrumental activities of daily living (IADL).40 We suggest that cognitive impairment, as represented by a decrease in MMSE status, globally and negatively affects everyday activity and function. This, in turn, might be interpreted and perceived by an individual as poor visual function and negatively affect self-rated vision status.
When examining the association between vision tests and self-rated vision status, we found that age was not an independent contributor to self-rated vision status. However, when examining the association between self-reported visual function and self-rated vision status, an increase in age increased the odds of reporting poor versus good vision. It is well established that performance on vision tests decreases with age, and we have reported that previously in the SEE population.17 Therefore, the independent contribution of age to self-rated vision status most likely was captured by the decrease in performance on vision tests seen with increasing age. However, age still was important in the model relating self-rated vision status and self-reported visual function, since vision tests were not included in that model.
We had reported previously that race and years of education were significantly correlated with discrepancies between self-rated vision and visual acuity.41 However, we note here that they were not correlated independently with self-rated vision status directly.
Limitations in our study include the potential for same-source bias when attempting to correlate self-report data as we did with the ADVS subscales and self-rated vision status. We see evidence of this in the associations observed with depression, for example. Depression was related to self-rated vision status in the model where vision tests were included, suggesting depression was related independently to self-rated vision status. However, in the model where the predictors were self-reported visual function (ADVS scale scores), depression was not related to self-rated vision status. This suggests that depressed persons tend to report their vision status and difficulty doing vision tasks similarly in a way that is not based on any correlation between the two measures, but rather is a function of the depression.
Also, we attempted to compare the impact of the different vision tests with self-rated vision status by comparing standard deviation units. The standard deviations depend on the distribution of the individual variables, and we recognize that such comparisons may not be equivalent clinically. Finally, near-vision acuity was not evaluated in the Salisbury Eye Study. We would fully expect it to be independently and significantly correlated with self-rated vision status given the importance of near-vision function to self-rated vision status in our population.
Self-rated vision status is a multidimensional measure. In an elderly population of African American and Caucasian participants, a number of demographic and health status characteristics, vision tests, and different domains of visual function proved important determinants of self-rated vision status. However, the most important determinants of self-rated vision status in this population were visual acuity, contrast sensitivity, and near-vision visual function. This understanding may improve the ability of eye care providers to maximize self-rated vision status among their patients.
Acknowledgments
Presented at the annual meeting for the Association for Research in Vision and Ophthalmology, Seattle, Washington, May 2013.
Supported by National Institute on Aging Grant AG02513 and by a Senior Scientific Investigator award from Research to Prevent Blindness (SKW). The authors alone are responsible for the content and writing of the paper. We have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure: M. El-Gasim, None; B. Munoz, None; S.K. West, None; A.W. Scott, None
References
- 1. Enhancing Public Health Surveillance of Visual Impairment and Eye Health in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2010: 27–43. Available at: http://www.cdc.gov/visionhealth/pdf/surveillance_background.pdf. Accessed June 25, 2011. [Google Scholar]
- 2. Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire field test investigators development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 3. Owen CG, Rudnicka AR, Smeeth L, et al. Is the NEI-VFQ-25 a useful tool in identifying visual impairment in an elderly population? BMC Ophthalmology. 2006; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richman J, Lorenzana LL, Lankaranian D, et al. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010; 128: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 5. Cole SR, Beck RW, Moke PS, Gal RL, Long DT. The National Eye Institute Visual Function Questionnaire: experience of the ONTT; Optic Neuritis Treatment Trial. Invest Ophthalmol Vis Sci. 2000; 41: 1017–1021. [PubMed] [Google Scholar]
- 6. Age-Related Eye Disease Study Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS report No. 14. Arch Ophthalmol. 2005; 123: 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Revicki DA, Rentz AM, Harnam N, et al. Reliability and validity of the National Eye Institute visual function questionnaire-25 in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010; 51: 712–717. [DOI] [PubMed] [Google Scholar]
- 8. Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute Visual Function Questionnaire in the Macular Telangiectasia (MacTel) Project. Invest Ophthalmol Vis Sci. 2008; 49: 4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubin GS, Bandeen-Roche K, Huang GH, et al. The association of multiple visual impairments with self-reported visual disability: SEE Project. Invest Ophthalmol Vis Sci. 2001; 42: 64–72. [PubMed] [Google Scholar]
- 10. Patino CM, Varma R, Azen SP, et al. The impact of change in visual field on health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011; 118: 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrahamsson M, Sjostrand J. Impairment of contrast sensitivity function (CSF) as a measure of disability glare. Invest Ophthalmol Vis Sci. 1986; 27: 1131–1136. [PubMed] [Google Scholar]
- 12. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of “real-world” targets. Br J Ophthalmol. 1987; 71: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owsley C, Sekuler R, Boldt C. Aging and low contrast vision: face perception. Invest Ophthalmol Vis Sci. 1981; 21: 362–365. [PubMed] [Google Scholar]
- 14. Noble J, Forooghian F, Sproule M, Westall C, O′Connor P. Utility of the National Eye Institute VFQ-25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol. 2006; 142: 464–468. [DOI] [PubMed] [Google Scholar]
- 15. Genensky SM. Acuity measurements: do they indicate how well a partially sighted person functions or could function? Am J Optom Physiol Opt. 1976; 53: 809–812. [PubMed] [Google Scholar]
- 16. Cullinan TR. The Epidemiology of Visual Disability: Studies of Visually Disabled People in the Community. Research Report 28. Canterbury, UK: Health Services Research Unit, University of Kent; 1977. [Google Scholar]
- 17. Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans: the SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 1997; 38: 557–568. [PubMed] [Google Scholar]
- 18. Munoz B, West S, Rubin GS, et al. Who participates in population based studies of visual impairment? The Salisbury Eye Evaluation Project experience. Ann Epidemiol. 1999; 9: 53–59. [DOI] [PubMed] [Google Scholar]
- 19. Folsten MF, Folstein SE, McHugh PR. “MMSE state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 20. Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982; 94: 91–96. [PubMed] [Google Scholar]
- 21. Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991; 32: 422–432. [PubMed] [Google Scholar]
- 22. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988; 2: 187–199. [Google Scholar]
- 23. Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli–Robson contrast sensitivity test. Clin Vision Sci. 1991; 6: 471–475. [Google Scholar]
- 24. Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale”: a measure of visual functional status. Med Care. 1992; 30: 1111–1126. [DOI] [PubMed] [Google Scholar]
- 25. Valbuena M, Bandeen–Roche K, Rubin GS, Munoz B, West SK. Self-reported assessment of visual function in a population-based study. The SEE project: Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1999; 40: 280–288. [PubMed] [Google Scholar]
- 26. Massof RW, Ahmadian L. What do different visual function questionnaires measure? Ophthalmic Epidemiol. 2007; 14: 198–204. [DOI] [PubMed] [Google Scholar]
- 27. Massof RW. Application of stochastic measurement models to visual function rating scale questionnaires. Ophthalmic Epidemiol. 2005; 12: 103–124. [DOI] [PubMed] [Google Scholar]
- 28. West SK, Rubin GS, Broman AT, et al. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002; 120: 774–780. [DOI] [PubMed] [Google Scholar]
- 29. Ross JE, Bron AJ, Clarke DD. Contrast sensitivity and visual disability in chronic simple glaucoma. Br J Ophthalmol. 1984; 68: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lennerstrand G, Ahlström CO. Contrast sensitivity in macular de- generation and the relation to subjective visual impairment. Acta Ophthalmol (Copenh). 1989; 6: 225–233. [DOI] [PubMed] [Google Scholar]
- 31. Pulling NH, Wolf E, Sturgis SP, Vaillancourt DR, Dolliver JJ. Headlight glare resistance and driver age. Hum Factors. 1980; 22: 103–112. [DOI] [PubMed] [Google Scholar]
- 32. Bailis DS, Segall A, Chipperfield JG. Two views of self- rated general health status. Soc Sci Med. 2003; 56: 203–217. [DOI] [PubMed] [Google Scholar]
- 33. Damron-Rodriguez J, Frank JC, Enriquez-Haass VL, Reuben DB. Definitions of health among diverse groups of elders: implications for health promotion. Generations. 2005; 29: 11–16. [Google Scholar]
- 34. Krause NM, Jay GM. What do global self-rated health items measure? Med Care. 1994; 32: 930–942. [DOI] [PubMed] [Google Scholar]
- 35. Bourque LB, Cosand BB, Drews C, et al. Reported satisfaction, fluctuation of vision, and glare among patients one year after surgery in the Prospective Evaluation of Radial Keratotomy (PERK) study. Arch Ophthalmol. 1987; 104: 356–363. [DOI] [PubMed] [Google Scholar]
- 36. Horowitz A. The prevalence and consequences of vision impairment in later life. Topics Geriatr Rehab. 2004; 20: 185–195. [Google Scholar]
- 37. Adamsons IA, Vitale S, Stark WJ, Rubin GS. The association of post-operative subjective visual function with acuity, glare and contrast sensitivity in patients with early cataract. Arch Ophthalmol. 1996; 114: 529–536. [DOI] [PubMed] [Google Scholar]
- 38. Bekibele CO, Gureje O. Impact of self-reported visual impairment on quality of life in the Ibadan study of ageing. Br J Ophthalmol. 2008; 92: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. du Toit R, Palagyi A, Ramke J, et al. The impact of reduced distance and near vision on the quality of life of adults in Timor-Leste. Ophthalmology. 2010; 117: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 40. Ford GR, Haley WE, Thrower SL, et al. Utility of MMSE state exam scores in predicting functional impairment among white and African American dementia patients. J Gerontol. 1996; 51: 185–18 8. [DOI] [PubMed] [Google Scholar]
- 41. El-Gasim M, Munoz B, West SK, Scott AW. Discrepancies in the Concordance of Self-Reported Vision Status and Visual Acuity in the Salisbury Eye Evaluation Study. Ophthalmology. 2012; 119: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]