Abstract
Purpose
Response to clopidogrel varies widely with nonresponse rates ranging from 4% to 30%. A reduced function of the gene variant of the CYP2C19 has been associated with lower drug metabolite levels, and hence diminished platelet inhibition. Drugs that alter CYP2C19 activity may also mimic genetic variants. The aim of the study is to investigate the cumulative effect of CYP2C19 gene polymorphisms and drug interactions that affects clopidogrel dosing, and apply it into a new clinical-pharmacogenetic algorithm that can be used by clinicians in optimizing clopidogrel-based treatment.
Method
Clopidogrel dose optimization was analyzed based on two main parameters that affect clopidogrel metabolite area under the curve: different CYP2C19 genotypes and concomitant drug intake. Clopidogrel adjusted dose was computed based on area under the curve ratios for different CYP2C19 genotypes when a drug interacting with CYP2C19 is added to clopidogrel treatment. A clinical-pharmacogenetic algorithm was developed based on whether clopidogrel shows 1) expected effect as per indication, 2) little or no effect, or 3) clinical features that patients experience and fit with clopidogrel adverse drug reactions.
Results
The study results show that all patients under clopidogrel treatment, whose genotypes are different from *1*1, and concomitantly taking other drugs metabolized by CYP2C19 require clopidogrel dose adjustment. To get a therapeutic effect and avoid adverse drug reactions, therapeutic dose of 75 mg clopidogrel, for example, should be lowered to 6 mg or increased to 215 mg in patients with different genotypes.
Conclusion
The implementation of clopidogrel new algorithm has the potential to maximize the benefit of clopidogrel pharmacological therapy. Clinicians would be able to personalize treatment to enhance efficacy and limit toxicity.
Keywords: pharmacogenetics, genotype, genetic testing, individualized therapy
Introduction
Clopidogrel is an oral antiplatelet agent and one of the commonly prescribed medications worldwide in the treatment of acute coronary syndrome and in patients undergoing percutaneous coronary intervention to prevent recurrent atherothrombotic events.1 Clopidogrel is also indicated in secondary prevention of stroke in high-risk patients,2 as an alternative for patients who are intolerant to aspirin, with atrial fibrillation and cannot take warfarin.3
Response to clopidogrel varies widely with nonresponse rates ranging from 4% to 30% at 24 hours.4 Interindividual variability is due to the fact that clopidogrel is a pro-drug that requires intestinal absorption followed by enzyme biotransformation to yield its active metabolite, 2-oxoclopidogrel. This active thiol metabolite inhibits adenosine diphosphate (ADP)-induced platelet aggregation by blocking the platelet P2Y12 receptor, resulting in approximately 50% reduction in ADP-mediated platelet aggregation after therapeutically recommended doses.5
Suggested mechanisms for this variability have included under-dosing, intrinsic interindividual differences resulting from genetic polymorphisms, and drug interactions with CYP2C19 substrates and inhibitors.6–8
A reduced function of the gene variant of the CYP2C19 that is located on chromosome 10 has been associated with lower clopidogrel metabolite levels, hence diminished platelet inhibition, and higher rates of adverse cardiovascular events,1,9–11 whereas an increased function of the gene variant of the CYP2C19 has been associated with higher clopidogrel metabolite levels, and consequently an increased risk of bleeding.7
Drug interactions may mimic genetic variants. Drugs can alter CYP2C19 activity, and those drugs are referred to as either inhibitors or inducers. Drugs that inhibit CYP2C19 activity are likely to decrease the plasma concentrations of the active metabolite of clopidogrel. On the other hand, some drugs induce (stimulate) CYP2C19, and they may increase the efficacy of CYP2C19 substrates like clopidogrel since more of the active metabolite is formed. Enzyme inducers tend to be “broad-spectrum”, in that they often induce several CYP450 isozymes. Enzyme induction interactions may be hard to detect clinically, since reduced drug effect may be interpreted as simply a lack of patient drug response.8
Comprehensive information on the effects of CYP2C19 gene polymorphisms and drug–drug interactions on clopidogrel concentrations in patients concomitantly treated with clopidogrel and other drugs that affect CYP2C19 function is unavailable. The aim of the study is to 1) investigate the cumulative effect of CYP2C19 gene polymorphisms and drug interactions that affects the plasma levels of clopidogrel active metabolite dosing, and 2) apply dose adjustment in a new algorithm that can be used in optimizing treatment and stratifying patients for drug response. The algorithm aims at providing clinicians with a guide that helps in dosing patients who are concomitantly treated with clopidogrel and other drugs metabolized by CYP2C19.
Methods
The authors confirm there is no need for ethics approval as this study does not deal with any ethical issues. The cumulative effect of CYP2C19 gene polymorphisms and drug interactions that affects clopidogrel dosing was investigated based on the following rationale: clopidogrel is metabolized by CYP2C19; CYP2C19 enzyme activity is altered in subjects with mutated CYP2C19 alleles who may be poor metabolizers, intermediate metabolizers, or ultra-extensive metabolizers as compared to the extensive metabolizers. The activity of the enzyme is also affected by drug inducers and inhibitors. Thus, the cumulative effect influences the patient’s response to clopidogrel. Drugs metabolized by CYP2C19 were identified and classified as enzyme inducers and inhibitors. CYP2C19 gene variants and their effects on the enzyme activity were also determined.
Statistical analysis of data
The cumulative effect was analyzed based on two main parameters that affect clopidogrel area under the curve (AUC): different CYP2C19 genotypes and polypharmacy. The general metrics used is the ratio of altered clopidogrel AUC, where the alteration may be caused by either gene polymorphism or drug interaction, to the reference AUC measured in patients with no mutation or no interaction.12 The study used a software13 to compute the AUC ratio for different CYP2C19 genotypes when a drug interacting with CYP2C19 is added to clopidogrel treatment. The adjusted dose was based on a current dose of 75 mg, taken as an example, and is calculated as follows: interacting drug AUCXM with genotype (*)/AUCEM ×75 mg, where AUC consists of the area under the clopidogrel active metabolite concentration in blood or plasma over the dosing interval at steady state; AUCEM is the AUC of clopidogrel given alone in “extensive metabolizers”; AUCXM is the AUC of the substrate given alone in patients with any other phenotype (ultra-extensive metabolizer to poor metabolizer) as defined by the genotype of the major CYP2C19 involved in the substrate metabolism; and AUCXM* is the AUC of the substrate when given with an inhibitor or an inducer.
To obtain the adjusted dose for clopidogrel based on doses of 150, 300, and 600 mg, the adjusted dose of clopidogrel 75 mg value shall be multiplied by 2, 4, and 8, respectively.
For patients taking more than one CYP2C19 interacting drug along with clopidogrel, adjusted clopidogrel dose = (interacting drug 1 AUCXM*/AUCEM) × (interacting drug 2 AUCXM*/AUCEM) × indicated clopidogrel dose.
Additionally, the study projected an algorithm as a clinical decision model for pharmacogenetic testing. The objective of the algorithm is to assess whether a pharmacogenetic testing is useful for a given patient under clopidogrel treatment. Three scenarios were anticipated: 1) the patient is well-controlled and shows expected clopidogrel effect as per indication, 2) the patient experiences little or no effect of clopidogrel, or 3) the patient presents with clinical features that fit with clopidogrel adverse drug reactions (ADRs). CYP2C19–drug interactions were mainly considered in the algorithm decisions. Patients’ potential medications were explored for CYP2C19 inducers and/or inhibitors that interact with clopidogrel.
Results
Clopidogrel dose adjustment
Clopidogrel dose adjustment was calculated based on a patient whose CYP2C19 genotype is known and a drug metabolized by CYP2C19 and interacting with clopidogrel is added to the patient’s treatment. Table 1 includes a simulation for clopidogrel dose of 75 mg. Table 1 differentiates six genotypes classified into four groups: *17*17 and *17*1 identified as ultra-extensive metabolizers; *1*1 extensive metabolizers; *2-3*17 and *1*2-3 intermediate metabolizers; and *2-3*2-3 poor metabolizers.
Table 1.
Proposed dose adjustment of clopidogrel based on a patient whose CYP2C19 genotype is known and a drug metabolized by CYP2C19 and interacting with clopidogrel is added
| Interacting drug | Different CYP2C19 genotypes
|
|||||
|---|---|---|---|---|---|---|
| *17*17 | *1*17 | *1*1 | *2-3*17 | *1*2-3 | *2-3*2-3 | |
| Amiodarone 1,200 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Azithromycin 250–500 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Benzbromarone 100 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Boceprevir 800–2400 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Bosentan 500–1,000 mg/d | 25 (0.33) | 30 (0.4) | 44 (0.59) | 52 (0.7) | 100 (1.33) | 210 (2.8) |
| Bupropion 300 mg | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Carbamazepine 200–600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Cilcosporin 2.5–5 g/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Cimetidine 800–1,200 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Clarithromycin 500–1,000 mg/d | 75 (0.99) | 86 (1.15) | 111 (1.48) | 123 (1.64) | 167 (2.23) | 213 (2.84) |
| Diltiazem 90–270 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Diphenhydramine 150 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Dronedarone 800 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Duloxetine 120 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Efavirenz 600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Erythromycin 1,000–2,000 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Fluconazole 100–400 mg/d | 117 (1.56) | 130 (1.73) | 152 (2.03) | 161 (2.15) | 191 (2.55) | 214 (2.85) |
| Fluoxetine 20–60 mg/d | 69 (0.92) | 81 (1.08) | 105 (1.4) | 117 (1.56) | 164 (2.18) | 213 (2.84) |
| Fluvoxamine 50–200 mg/d | 200 (2.66) | 203 (2.7) | 206 (2.75) | 208 (2.77) | 212 (2.83) | 215 (2.86) |
| Gatifloxacin 400 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Itraconazole 100–200 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Ketoconazole 200–400 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Levomepromazine 10 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Miconazole 125 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Nefazodone 400 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Noscapine 150 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Omeprazole 40–80 mg/d | 68 (0.91) | 80 (1.06) | 104 (1.39) | 116 (1.55) | 163 (2.17) | 213 (2.84) |
| Pantoprazole 80 mg/d | 56 (0.75) | 68 (0.9) | 90 (1.2) | 102 (1.36) | 152 (2.02) | 213 (2.84) |
| Paroxetine 20 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Phenytoin 300–400 mg/d | 25 (0.33) | 30 (0.4) | 44 (0.59) | 53 (0.7) | 100 (1.33) | 210 (2.8) |
| Pioglitazone 45 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Posacpnazole 200–600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Propafenone 675 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Quinidine 50 mg | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Ranitidine 300–600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Rifampicin 450–600 mg/d | 11 (0.14) | 13 (0.17) | 20 (0.27) | 25 (0.33) | 55 (0.73) | 205 (2.73) |
| Ritonavir 800 mg/d >14 d | 6 (0.08) | 7.5 (0.1) | 12 (0.16) | 15 (0.2) | 35 (0.47) | 197 (2.63) |
| Roxithromycin 300 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Saquinavir 3,600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Sertraline 150 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| St John Wart 600 mg/d | 29 (0.38) | 35 (0.47) | 51 (0.68) | 86 (1.15) | 110 (1.46) | 210 (2.81) |
| Sulfamethizole 600 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Sulfaphenazole 1,000 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Telaprevir 2.25 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Telithromycin 800 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Terbinafine 250 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Thioridazine 50 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Ticagrelor 90–180 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Ticlopidine 300 mg/d | 75 (1) | 88 (1.17) | 113 (1.5) | 86 (1.15) | 168 (2.24) | 214 (2.84) |
| Valproate 400–800 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Verapamil 240–480 mg/d | 45 (0.6) | 54 (0.72) | 75 (1) | 86 (1.15) | 137 (1.83) | 212 (2.83) |
| Voriconazole 400–800 mg/d | 100 (1.21) | 104 (1.38) | 128 (1.71) | 140 (1.86) | 179 (2.38) | 214 (2.85) |
Notes: Clopidogrel adjusted dose in mg for clopidogrel dose of 75 mg = (interacting drug AUCXM*/AUCEM) ×75 mg. The values shown in bold refer to the dose, and the values in brackets refer to interacting AUC values. List of CYP2C19 inhibitors and inducers. AUC ratio calculation was adopted from Castellan et al.13 AUC consists of the area under the substrate concentration in blood or plasma over the dosing interval at steady state; AUCEM is the AUC of the substrate given alone in “extensive metabolizers”; AUCXM is the AUC of the substrate given alone in patients with any other phenotype (ultra-extensive metabolizer to poor metabolizer) as defined by the genotype of the major CYP2C19 involved in the substrate metabolism; AUCXM* is the AUC of the substrate given with an inhibitor or an inducer. Data from Castellan et al.13
Abbreviations: AUC, area under the curve; d, day.
The study results show that most patients under clopidogrel treatment whose genotypes are different from *1*1 and concomitantly taking other drugs metabolized by inhibiting or inducing CYP2C19 most likely require clopidogrel dose adjustment. If concomitant drugs include but not limited to clarithromycin, fluconazole, fluoxetine, fluvoxamine, omeprazole, ticlopidine, and voriconazole, clopidogrel dose adjustment is required for all patients including *1*1 genotypes. However, patients having the *17*17 genotype and concomitantly taking a CYP2C19 inhibitor or those having the *1*2-3 or *2-3*2-3 and concomitantly taking a CYP2C19 inducer would probably not need dose adjustment. For example, if a patient has the *17*17 genotype, clopidogrel 75 mg as a standard therapeutic dose is required if clarithromycin 500–100 mg/day has been added to the patient treatment. To get a therapeutic effect and avoid ADRs, clopidogrel dose should be lowered to 6 mg and increased to 215 mg in patients taking ritonavir and fluvoxamine, respectively. Commonly, 75 mg is the indicated therapeutic dose for patients with *1*1 genotype; the adjusted doses for patients with genotypes of *17*17 and *17*1 are 45 and 54 mg, respectively, whereas 86, 137, and 212 mg are the adjusted doses for patients with *2-3*17, *1*2-3, and *2-3*2-3 genotypes, respectively.
Clinical-pharmacogenetic algorithm
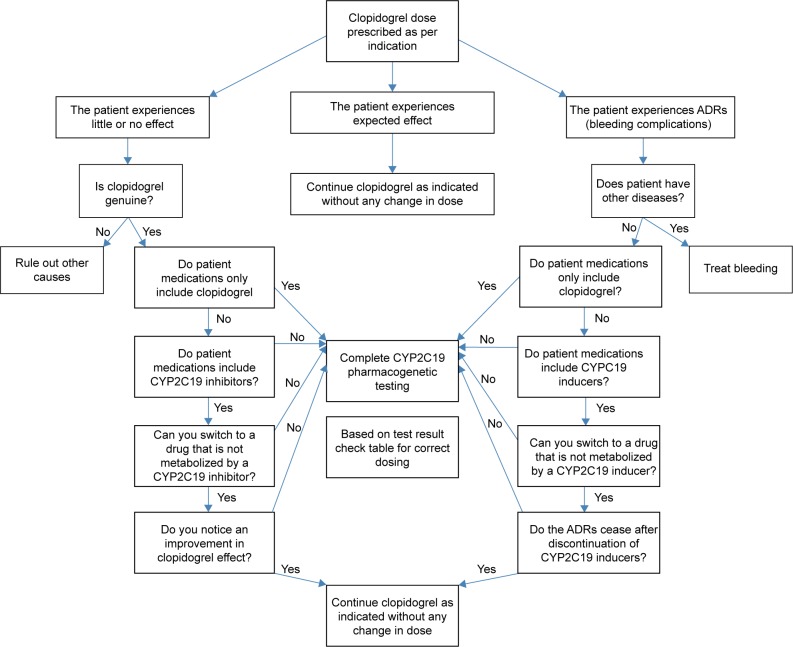
Currently, pharmacogenetic testing is performed in individual cases, and mostly retrospectively, for example, in patients who experience adverse effects or no therapeutic effect. Figure 1 depicts a proposed clinical decision algorithm for the use of pharmacogenetic testing. In brief, dosing patients on clopidogrel usually starts with the therapeutic doses as per indication. Three scenarios may apply.
Figure 1.
Clinical decision model algorithm for pharmacogenetic testing.
Abbreviation: ADRs, adverse reactions.
Clinical situation one – supratherapeutic clopidogrel active metabolite
If the patient experiences clinical features that fit with clopidogrel ADRs, the clinician should first exclude other diagnoses and investigate the patient’s polypharmacy. If ADRs are not due to any other problem and the patient treatment includes only clopidogrel, a pharmacogenetic testing is indicated.
However, if the patient drug intake includes CYP2C19 inducers, the latter should be discontinued and a switch to a drug not metabolized by CYP2C19 if available should be considered. If the above-mentioned clinical features persist, and/or no alternative to CYP2C19 inducer is available, a pharmacogenetic testing is indicated.
Clinical situation two – subtherapeutic clopidogrel active metabolite
If clopidogrel produces little or no therapeutic effect, the clinician should first exclude the possibility of using clopidogrel counterfeit and investigate the patient’s polypharmacy. If the drug is genuine and the patient treatment includes only clopidogrel, a pharmacogenetic testing is indicated.
However, if the patient drug intake includes CYP2C19 inhibitors, the latter should be discontinued and a switch to a drug not metabolized by CYP2C19 if available should be considered. If the patient lab values do not show improvement, and/or no alternative to CYP2C19 inhibitor is available, a pharmacogenetic testing is indicated.
Clinical situation three – therapeutic clopidogrel active metabolite
If the patient is well-controlled and shows expected clopidogrel effect as per indication, clopidogrel should be continued as indicated without any change in dose.
CYP2C19 pharmacogenetic test interpretation
CYP2C19 genetic test results are provided with a genotype to phenotype interpretation. A normal metabolizer has two copies of the wild-type allele (*1*1) known as extensive metabolizer.11 When a variant allele replaces one or both wild-type alleles, this is interpreted as altered enzyme activity. Different variant alleles affecting CYP2C19 enzyme activity include: *17, *2, and *3. If the genotype is *17*17, and/or *1*17, (known as ultra-metabolizer),11 a higher drug metabolite level is expected, and consequently a lower clopidogrel dose is indicated, whereas if the genotype has one allele *17 or *1 in combination with *2 or *3 (which are known as intermediate metabolizers) a lower drug metabolite level is expected, and consequently a higher clopidogrel dose is respectively indicated. A much higher dose is indicated if the patient genotype is homozygous *2, or *3, or includes *3 and *2 in combination (which are known as poor metabolizers).11
Discussion
Pharmacogenetics and drug interactions share a partnership in predicting patients’ resistance or sensitivity to drugs related effects. Thus, understanding the synergistic/additive/antagonistic/subtractive effects of gene polymorphisms and drug interactions provides better patient care. Clopidogrel remains the thienopyridine drug with the most approved indications for use. CYP2C19 genetic mutations and drug interaction-mediated inhibition/induction cause reduction/enhancement in activity of the enzyme, providing mutually corroborating scientific evidence for the interpretation of clinical consequences. Genetic variation in CYP2C19 has shown to affect plasma levels/effect of active metabolite of clopidogrel and therefore clopidogrel dosing, and patients have revealed different CYP2C19 gene polymorphisms; approximately, 5%–30% of patients are ultra-metabolizers, 18%–45% are intermediate metabolizers, 2%–15% are poor metabolizers, as compared to 35%–50% of patients who are extensive metabolizers.14,15 Thus, dose adjustment would be required for almost more than 50% of patients.
To adjust clopidogrel dose and get the desired response in patients with loss of functional CYP2C19 allele, one option is to double the dose: the loading dose can be increased from 300 to 600 mg, and the maintenance dose from 75 to 150 mg. The benefit has been evident in several studies,16–24 whereas contradictory results have been shown in other studies.25,26 Another option is to switch from clopidogrel to a different P2Y12 antagonist. Several agents, such as prasugrel, ticagrelor, elinogrel, and cangrelor, have been approved for this purpose. Although the new drugs share a similar molecule or same mechanism of action to that of clopidogrel, and do not require CYP2C19 to biotransform into the active agent, patients have shown to be more prone to bleeding complications.27–29 The study provides an accurate dosing of clopidogrel as an alternative. Considering alternative dosing of clopidogrel, dose adjustment has to be accurately computed as shown in the study results; however, a major limitation is that clopidogrel is only available in 75 mg and 300 mg dosage strengths. Additionally, clopidogrel tablets are available as pills that cannot be split. Hence, if the patient requires a dose lower than 75 mg, clinician should refer to a clopidogrel substitute.
When patients are extremely sensitive or resistant to clopidogrel effects at normal doses, the clinician should verify if the physical product is genuine if possible, exclude drug–drug interactions and then search for genetic variation in CYP2C19 metabolism as the underlying cause. The availability of such an alternative is integral to the process of integrating the use of the genetic biomarker into clinical decision-making, offering solutions to nonresponders or under-responders to therapy as well as to patients experiencing side effects from the drug.
Conclusion
In conclusion, the study integrates clopidogrel–drug interaction and pharmacogenetic studies in a clinical algorithm and provides an accurate dosing recommendation of clopidogrel. The implementation of such pharmacogenetic–drug interaction approach has the potential to maximize the benefit of clopidogrel pharmacological therapy and to refine the choice of pharmacological agent that may be administered along with clopidogrel in patients on polypharmacy. Clinicians would be able to personalize treatment to enhance efficacy and/or limit toxicity.
Acknowledgments
We would like to acknowledge the Lebanese American University for the financial support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Binazon O, Dubois-Gauche A, Nanau RM, Neuman MG. Efficacy and safety of platelet inhibitors. J Pharm Pharm Sci. 2013;16(1):1–39. doi: 10.18433/j3mp4z. [DOI] [PubMed] [Google Scholar]
- 2.Gouya G, Arrich J, Wolzt M, et al. Antiplatelet treatment for prevention of cerebrovascular events in patients with vascular diseases: a systematic review and meta-analysis. Stroke. 2014;45(2):492–503. doi: 10.1161/STROKEAHA.113.002590. [DOI] [PubMed] [Google Scholar]
- 3.Hankey GJ. Replacing aspirin and warfarin for secondary stroke prevention: is it worth the costs? Curr Opin Neurol. 2010;23(1):65–72. doi: 10.1097/WCO.0b013e328334e67b. [DOI] [PubMed] [Google Scholar]
- 4.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 5.Fareed J, Jeske W, Thethi I. Metabolic differences of current thienopyridine antiplatelet agents. Expert Opin Drug Metab Toxicol. 2013;9(3):307–317. doi: 10.1517/17425255.2013.749238. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration FDA Drug safety communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. [Accessed January 31, 2015]. Available form: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm (March 12, 2010)
- 7.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 8.Ma TKW, Lam YY, Tan VP, Yan BP. Variability in response to clopidogrel: how important are pharmacogenetics and drug interactions? Br J Clin Pharmacol. 2011;72(4):697–706. doi: 10.1111/j.1365-2125.2011.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster V, Sweeny JM. Clopidogrel and the reduced-function CYP2C19 genetic variant: a limited piece of the overall therapeutic puzzle. JAMA. 2010;304:1839–1840. doi: 10.1001/jama.2010.1566. [DOI] [PubMed] [Google Scholar]
- 10.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goutelle S, Bourguignon L, Bleyzac N, Berry J, Clavel-Grabit F, Tod M. In vivo quantitative prediction of the effect of gene polymorphisms and drug interactions on drug exposure for CYP2C19 substrates. AAPS J. 2013;15(2):415–426. doi: 10.1208/s12248-012-9431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellan C, Charpiat B, Gueyffier F, Kassaï B, Nony P, Genophar II working group DDI-predictor. [Accessed January 31, 2015]. Available from: http://www.ddi-predictor.org/
- 14.Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29(3):192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 16.Simon T, Bhatt DL, Bergougnan L, et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharmacol Ther. 2011;90(2):287–295. doi: 10.1038/clpt.2011.127. [DOI] [PubMed] [Google Scholar]
- 17.Gladding P, Webster M, Zeng I, et al. The antiplatelet effect of higher loading and maintenance dose regimens of clopidogrel: the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc Interv. 2008;1(6):612–619. doi: 10.1016/j.jcin.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Gladding P, White H, Voss J, et al. Pharmacogenetic testing for clopidogrel using the rapid INFINITI analyzer: a dose-escalation study. JACC Cardiovasc Interv. 2009;2(11):1095–1101. doi: 10.1016/j.jcin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Barker CM, Murray SS, Teirstein PS, Kandzari DE, Topol EJ, Price MJ. Pilot study of the antiplatelet effect of increased clopidogrel maintenance dosing and its relationship to CYP2C19 genotype in patients with high on-treatment reactivity. JACC Cardiovasc Interv. 2010;3(10):1001–1007. doi: 10.1016/j.jcin.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Bonello L, Armero S, Ait Mokhtar O, et al. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J Am Coll Cardiol. 2010;56(20):1630–1636. doi: 10.1016/j.jacc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos D, Dimitropoulos G, Davlouros P, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC Cardiovasc Interv. 2011;4(4):403–410. doi: 10.1016/j.jcin.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Collet JP, Hulot JS, Anzaha G, et al. CLOVIS-2 Investigators High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2) JACC Cardiovasc Interv. 2011;4(4):392–402. doi: 10.1016/j.jcin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Hamilos M, Muller O, Ntalianis A, et al. Relationship between peripheral arterial reactive hyperemia and residual platelet reactivity after 600 mg clopidogrel. J Thromb Thrombolysis. 2011;32(1):64–71. doi: 10.1007/s11239-011-0557-x. [DOI] [PubMed] [Google Scholar]
- 24.Mega JL, Hochholzer W, Frelinger AL, 3rd, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306(20):2221–2228. doi: 10.1001/jama.2011.1703. [DOI] [PubMed] [Google Scholar]
- 25.Price MJ, Berger PB, Teirstein PS, et al. GRAVITAS Investigators Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 26.Bonello L, Pansieri M, Mancini J, et al. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58(5):467–473. doi: 10.1016/j.jacc.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Barthélémy O, Silvain J, Brieger D, et al. Bleeding complications in primary percutaneous coronary intervention of ST-elevation myocardial infarction in a radial center. Catheter Cardiovasc Interv. 2012;79(1):104–112. doi: 10.1002/ccd.23164. [DOI] [PubMed] [Google Scholar]
- 28.Caldeira D, Fernandes R, Costa J, et al. Branded versus generic clopidogrel in cardiovascular diseases: a systematic review. J Cardiovasc Pharmacol. 2013;61(4):277–282. doi: 10.1097/FJC.0b013e31827e5c60. [DOI] [PubMed] [Google Scholar]
- 29.Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]